Umifenovir: Difference between revisions
US FDA clarification, going by existing reference |
m the |
||
| Line 45: | Line 45: | ||
}} |
}} |
||
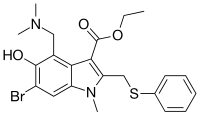
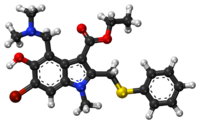
'''Umifenovir'''<ref>[http://apps.who.int/medicinedocs/documents/s18407en/s18407en.pdf Recommended INN: List 65.], WHO Drug Information, Vol. 25, No. 1, 2011, page 91</ref> (trade names '''Arbidol''' {{lang-ru|Арбидол}}, {{zh|c=阿比朵尔}}) is an [[Antiviral drug|antiviral]] treatment for [[influenza]] infection used in [[Russia]]<ref name="pmid19028526">{{cite journal | vauthors = Leneva IA, Russell RJ, Boriskin YS, Hay AJ | title = Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol | journal = Antiviral Research | volume = 81 | issue = 2 | pages = 132–40 | date = February 2009 | pmid = 19028526 | doi = 10.1016/j.antiviral.2008.10.009 }}</ref> and [[China]]. The drug is manufactured by [[Pharmstandard]] ({{lang-ru|Фармстандарт}}). Although some Russian studies have shown it to be effective, it is not approved for use in other countries. It is not approved by [[US FDA]] for the treatment or prevention of influenza.<ref>{{cite web | title = FDA Approved Drugs for Influenza | url = https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information#ApprovedDrugs | work = U.S. Food and Drug Administration }}</ref> Chemically, umifenovir features an [[indole]] core, functionalized at all but one positions with different substituents. The drug is claimed to inhibit viral entry into target cells and stimulate the immune response. Interest in the drug has been renewed as a result of the [[SARS-CoV-2 outbreak]]. |
'''Umifenovir'''<ref>[http://apps.who.int/medicinedocs/documents/s18407en/s18407en.pdf Recommended INN: List 65.], WHO Drug Information, Vol. 25, No. 1, 2011, page 91</ref> (trade names '''Arbidol''' {{lang-ru|Арбидол}}, {{zh|c=阿比朵尔}}) is an [[Antiviral drug|antiviral]] treatment for [[influenza]] infection used in [[Russia]]<ref name="pmid19028526">{{cite journal | vauthors = Leneva IA, Russell RJ, Boriskin YS, Hay AJ | title = Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol | journal = Antiviral Research | volume = 81 | issue = 2 | pages = 132–40 | date = February 2009 | pmid = 19028526 | doi = 10.1016/j.antiviral.2008.10.009 }}</ref> and [[China]]. The drug is manufactured by [[Pharmstandard]] ({{lang-ru|Фармстандарт}}). Although some Russian studies have shown it to be effective, it is not approved for use in other countries. It is not approved by the [[US FDA]] for the treatment or prevention of influenza.<ref>{{cite web | title = FDA Approved Drugs for Influenza | url = https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information#ApprovedDrugs | work = U.S. Food and Drug Administration }}</ref> Chemically, umifenovir features an [[indole]] core, functionalized at all but one positions with different substituents. The drug is claimed to inhibit viral entry into target cells and stimulate the immune response. Interest in the drug has been renewed as a result of the [[SARS-CoV-2 outbreak]]. |
||
Umifenovir is manufactured and made available as [[Tablet (pharmacy)|tablets]], [[Capsule (pharmacy)|capsules]] and syrup. |
Umifenovir is manufactured and made available as [[Tablet (pharmacy)|tablets]], [[Capsule (pharmacy)|capsules]] and syrup. |
||
Revision as of 20:16, 23 March 2020
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Arbidol |
| Pregnancy category |
|
| Routes of administration | Oral (hard capsules, tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Metabolism | Hepatic |
| Elimination half-life | 17–21 hours |
| Excretion | 40% excrete as unchanged umifenovir in feces (38.9%) and urine (0.12%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.800 |
| Chemical and physical data | |
| Formula | C22H25BrN2O3S |
| Molar mass | 477.41 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Umifenovir[2] (trade names Arbidol Template:Lang-ru, Chinese: 阿比朵尔) is an antiviral treatment for influenza infection used in Russia[3] and China. The drug is manufactured by Pharmstandard (Template:Lang-ru). Although some Russian studies have shown it to be effective, it is not approved for use in other countries. It is not approved by the US FDA for the treatment or prevention of influenza.[4] Chemically, umifenovir features an indole core, functionalized at all but one positions with different substituents. The drug is claimed to inhibit viral entry into target cells and stimulate the immune response. Interest in the drug has been renewed as a result of the SARS-CoV-2 outbreak.
Umifenovir is manufactured and made available as tablets, capsules and syrup.
Status
Testing of umifenovir's efficacy has mainly occurred in China and Russia,[5][6] and it is well known in these two countries.[7] Some of the Russian tests showed the drug to be effective[5] and a direct comparison with Tamiflu showed similar efficiency in vitro and in a clinical setting.[8] In 2007, Arbidol (umifenovir) had the highest sales in Russia among all over-the-counter drugs.
Mode of action
Biochemistry
Umifenovir inhibits membrane fusion.[3] Umifenovir prevents contact between the virus and target host cells. Fusion between the viral envelope (surrounding the viral capsid) and the cell membrane of the target cell is inhibited. This prevents viral entry to the target cell, and therefore protects it from infection.[9]
Some evidence suggests that the drug's actions are more effective at preventing infections from RNA viruses than infections from DNA viruses.[10]
As well as specific antiviral action against both influenza A and influenza B viruses, umifenovir exhibits modulatory effects on the immune system. The drug stimulates a humoral immune response, induces interferon-production, and stimulates the phagocytic function of macrophages.[11]
Clinical application
Umifenovir is used primarily as an antiviral treatments for influenza. The drug has also been investigated as a candidate drug for treatment of hepatitis C.[12]
More recent studies indicate that umifenovir also has in vitro effectiveness at preventing entry of Ebolavirus Zaïre Kikwit, Tacaribe arenavirus and human herpes virus 8 in mammalian cell cultures, while confirming umifenovir's suppressive effect in vitro on Hepatitis B and poliovirus infection of mammalian cells when introduced either in advance of viral infection or during infection.[13][14]
Research
In February 2020, Li Lanjuan, an expert of the National Health Commission of China, proposed using Arbidol (umifenovir) together with darunavir as a potential treatment during the 2019–20 coronavirus pandemic.[15] Chinese experts claim that preliminary tests had shown that arbidol and darunavir could inhibit replication of the virus.[16][17] So far without additional effect if added on top of recombinant human interferon α2b spray.[18]
Side effects
Side effects in children include sensitization to the drug. No known overdose cases have been reported and allergic reactions are limited to people with hypersensitivity. The LD50 is more than 4 g/kg.[19]
Criticism
In 2007, the Russian Academy of Medical Sciences stated that the effects of Arbidol (umifenovir) are not scientifically proven.[20]
Russian media criticized lobbying attempts by Tatyana Golikova (then-Minister of Healthcare) to promote umifenovir,[21] and the unproven claim that Arbidol can speed up recovery from flu or cold by 1.3-2.3 days.[22] They also debunked claims that the efficacy of umifenovir is supported by peer-reviewed studies.[23][24]
References
- ^ "Full Prescribing Information: Arbidol® (umifenovir) film-coated tablets 50 and 100 mg: Corrections and Additions". State Register of Medicines (in Russian). Open joint-stock company “Pharmstandard-Tomskchempharm”. Retrieved 3 June 2015.
- ^ Recommended INN: List 65., WHO Drug Information, Vol. 25, No. 1, 2011, page 91
- ^ a b Leneva IA, Russell RJ, Boriskin YS, Hay AJ (February 2009). "Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol". Antiviral Research. 81 (2): 132–40. doi:10.1016/j.antiviral.2008.10.009. PMID 19028526.
- ^ "FDA Approved Drugs for Influenza". U.S. Food and Drug Administration.
- ^ a b Leneva IA, Fediakina IT, Gus'kova TA, Glushkov RG (2005). "[Sensitivity of various influenza virus strains to arbidol. Influence of arbidol combination with different antiviral drugs on reproduction of influenza virus A]". Terapevticheskii Arkhiv (Russian translation). 77 (8). ИЗДАТЕЛЬСТВО "МЕДИЦИНА": 84–8. PMID 16206613.
- ^ Wang MZ, Cai BQ, Li LY, Lin JT, Su N, Yu HX, Gao H, Zhao JZ, Liu L (June 2004). "[Efficacy and safety of arbidol in treatment of naturally acquired influenza]". Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Academiae Medicinae Sinicae. 26 (3): 289–93. PMID 15266832.
- ^ Boriskin YS, Leneva IA, Pécheur EI, Polyak SJ (2008). "Arbidol: a broad-spectrum antiviral compound that blocks viral fusion". Current Medicinal Chemistry. 15 (10): 997–1005. doi:10.2174/092986708784049658. PMID 18393857.
- ^ Leneva IA, Burtseva EI, Yatsyshina SB, Fedyakina IT, Kirillova ES, Selkova EP, Osipova E, Maleev VV (February 2016). "Virus susceptibility and clinical effectiveness of anti-influenza drugs during the 2010-2011 influenza season in Russia". International Journal of Infectious Diseases. 43: 77–84. doi:10.1016/j.ijid.2016.01.001. PMID 26775570.
- ^ Boriskin YS, Pécheur EI, Polyak SJ (July 2006). "Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection". Virology Journal. 3: 56. doi:10.1186/1743-422X-3-56. PMC 1559594. PMID 16854226.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Shi L, Xiong H, He J, Deng H, Li Q, Zhong Q, Hou W, Cheng L, Xiao H, Yang Z (2007). "Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo". Archives of Virology. 152 (8): 1447–55. doi:10.1007/s00705-007-0974-5. PMID 17497238.
- ^ Glushkov RG, Gus'kova TA, Krylova LIu, Nikolaeva IS (1999). "[Mechanisms of arbidole's immunomodulating action]". Vestnik Rossiiskoi Akademii Meditsinskikh Nauk (in Russian) (3): 36–40. PMID 10222830.
- ^ Pécheur EI, Lavillette D, Alcaras F, Molle J, Boriskin YS, Roberts M, Cosset FL, Polyak SJ (May 2007). "Biochemical mechanism of hepatitis C virus inhibition by the broad-spectrum antiviral arbidol". Biochemistry. 46 (20): 6050–9. doi:10.1021/bi700181j. PMC 2532706. PMID 17455911.
- ^ Pécheur EI, Borisevich V, Halfmann P, Morrey JD, Smee DF, Prichard M, Mire CE, Kawaoka Y, Geisbert TW, Polyak SJ (January 2016). "The Synthetic Antiviral Drug Arbidol Inhibits Globally Prevalent Pathogenic Viruses". Journal of Virology. 90 (6): 3086–92. doi:10.1128/JVI.02077-15. PMC 4810626. PMID 26739045.
- ^ Hulseberg CE, Fénéant L, Szymańska-de Wijs KM, Kessler NP, Nelson EA, Shoemaker CJ, Schmaljohn CS, Polyak SJ, White JM. Arbidol and Other Low-Molecular-Weight Drugs That Inhibit Lassa and Ebola Viruses. J Virol. 2019 Apr 3;93(8). pii: e02185-18. doi:10.1128/JVI.02185-18 PMID 30700611
- ^ Ng E (4 February 2020). "Coronavirus: are cocktail therapies for flu and HIV the magic cure?". South China Morning Post.
Bangkok and Hangzhou hospitals put combination remedies to the test.
- ^ Zheng W, Lau M (4 February 2020). "China's health officials say priority is to stop mild coronavirus cases from getting worse". South China Morning Post.
- ^ Lu H (January 2020). "Drug treatment options for the 2019-new coronavirus (2019-nCoV)". Bioscience Trends. doi:10.5582/bst.2020.01020. PMID 31996494.
- ^ "Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia". 4 February 2020. Retrieved 24 February 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ "АРБИДОЛ® (ARBIDOL)". Vidal. Archived from the original on 4 February 2009.
- ^ "Resolution". Meetings of the Presidium of the Formulary Committee. Russian Academy of Medical Sciences. 16 March 2007.
- ^ "How do we plant federal ministers". MKRU. 21 April 2011.
- ^ Golunov, Ivan (23 December 2013). "13 most popular flu cures: do they work?". Professional Journalism Platform.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Reuters, Svetlana. "Stick in the wheel". Esquire.
{{cite web}}:|last=has generic name (help); Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Repetition - the mother of learning". Esquire.
External links
- "Мастерлек" Pharmaceuticals, Moscow, Russia. Patent number No. 2033157, Registry number No. 003610/01.
- (in Russian) Arbidol
- English published clinical studies and translations for Arbidol 1973–2016
