Hepatitis B
| Hepatitis B | |
|---|---|
| Specialty | Infectious diseases |
| Hepatitis B virus | |
|---|---|
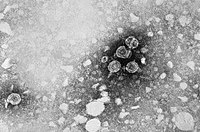
| |
| Micrograph showing hepatitis B virions | |
| Virus classification | |
| Group: | Group VII (dsDNA-RT)
|
| Family: | |
| Genus: | |
| Species: | Hepatitis B virus
|
Hepatitis B is an inflammation of the liver and is caused by the Hepatitis B virus (HBV), a member of the Hepadnavirus family[1] and one of hundreds of unrelated viral species which cause viral hepatitis. It was originally known as "serum hepatitis" and has caused current epidemics in parts of Asia and Africa.[2] Hepatitis B is recognized as endemic in China and various other parts of Asia.[3] The proportion of the world's population currently infected with the virus is 3 to 6%, but up to a third have been exposed. Symptoms of the acute illness caused by the virus include liver inflammation, vomiting, jaundice, and rarely, death. Chronic hepatitis B may cause liver cirrhosis which may then lead to liver cancer, a fatal disease with very poor response to current chemotherapy.
Hepatitis B usually gets better on its own after a few months.[4] It may, however, cause a more serious chronic infection.
Structure
Virions consist of an outer lipid envelope and an icosahedral nucleocapsid core composed of protein. The nucleocapsid encloses the viral DNA and a DNA polymerase that has reverse transcriptase activity. The outer envelope contains embedded proteins which are involved in viral binding of, and release into, susceptible cells. Virion shape is generally spherical with a diameter of 40 - 48 nanometers (nm), but pleomorphic forms exist, including filamentous and spherical bodies lacking a core. These "subviral" particles are not infectious.[citation needed]
The DNA genome is not segmented but rather partially double-stranded, containing a long and short segment which overlap approximately 240 nucleotides to form an open circle. The longer strand is 3020-3320 nucleotides long, and the shorter is 1700-2800 nucleotides long.[5] The virus can be divided into four major serotypes (adr, adw, ayr, ayw) based on antigenic epitopes present on its envelope proteins, and into eight genotypes (A-H) according to overall nucleotide sequence variation of the genome. Different genotypes have distinct geographic distributions. For example, genotypes B and C are prevalent in China and neighboring countries.
Replication
Hepatitis B is one of a few known non-retroviral viruses which employ reverse transcription as a part of its replication process. (HIV, a completely unrelated virus, also uses reverse transcription, but it is a retrovirus.) HBV invades hepatocytes by binding to surface receptors and becoming internalized. The viral core particles then migrate to the nucleus and the partially double-stranded, relaxed circular genomes (RC-DNA) are repaired to form a covalently closed circular DNA (cccDNA), which is the template for transcription of viral genomic and sub-genomic RNAs by cellular RNA polymerase II. Of these, the pregenomic RNA (pgRNA) is selectively packaged into progeny capsids and is then reverse-transcribed into new RC-DNA. The core can either bud into the endoplasmic reticulum to be enveloped and exported from the cell or recycled back into the nucleus for conversion to cccDNA.
Transmission
Transmission results from exposure to infectious blood or body fluids containing blood. YOU'RE GONNA DIE!!!!!! Possible forms of transmission include (but are not limited to) unprotected sexual contact, blood transfusions, re-use of contaminated needles and syringes, and vertical transmission from mother to child during childbirth. Without intervention, a mother who is positive for the hepatitis B surface antigen confers a 20% risk of passing the infection to her offspring at the time of birth. This risk is as high as 90% if the mother is also positive for the hepatitis B e antigen. HBV can also be transmitted between family members within households, possibly by contact of nonintact skin or mucous membrane with secretions or saliva containing HBV.[6]
The primary method of transmission reflects the prevalence of chronic HBV infection in a given area. In low prevalence areas such as the continental United States and Western Europe, where less than 2% of the population is chronically infected, injection drug abuse and unprotected sex are the primary methods, although other factors may be important.[7] In moderate prevalence areas, which include Eastern Europe, Russia, and Japan, where 2-7% of the population is chronically infected, the disease is predominantly spread among children. In high prevalence areas such as China and South East Asia, transmission during childbirth is most common, although in other areas of high endemicity such as Africa, transmission during childhood is also a significant factor.[8] The prevalence of chronic HBV infection in areas of high endemicity is at least 8%.
Roughly 16-40% of unimmunized[citation needed] sexual partners of individuals with hepatitis B will be infected through sexual contact. The risk of transmission is closely related to the rate of viral replication in the infected individual at the time of exposure.
Immunopathogenesis
During HBV infection, the host immune response causes both hepatocellular damage and viral clearance. While the innate immune response does not play a significant role in these processes, the adaptive immune response, particularly virus-specific cytotoxic T lymphocytes (CTLs), contributes to nearly all of the liver injury associated with HBV infection. By killing infected cells and by producing antiviral cytokines capable of purging HBV from viable hepatocytes, CTLs also eliminate the virus.[9] Although liver damage is initiated and mediated by the CTLs, antigen-nonspecific inflammatory cells can worsen CTL-induced immunopathology and platelets may facilitate the accumulation of CTLs into the liver.[10]
Symptoms and complications
Hepatitis B virus infection may either be acute (self-limited) or chronic (long-standing). Persons with self-limited infection clear the infection spontaneously within weeks to months.
Children are less likely than adults to clear the infection. More than 95% of people who become infected as adults or older children will stage a full recovery and develop protective immunity to the virus. However, only 5% of newborns that acquire the infection from their mother at birth will clear the infection. Of those infected between the age of one to six, 70% will clear the infection.[citation needed] When the infection is not cleared, one becomes a chronic carrier of the virus.
Acute infection with hepatitis B virus is associated with acute viral hepatitis - an illness that begins with general ill-health, loss of appetite, nausea, vomiting, body aches, mild fever, dark urine, and then progresses to development of jaundice. It has also been noted that itchy skin all over the body has been an indication as a possible symptom of all hepatitis virus types. The illness lasts for a few weeks and then gradually improves in most affected people. A few patients may have more severe liver disease (fulminant hepatic failure), and may die as a result of it. The infection may also be entirely asymptomatic and may go unrecognized.
Chronic infection with hepatitis B virus may be either asymptomatic or may be associated with a chronic inflammation of the liver (chronic hepatitis), leading to cirrhosis over a period of several years. This type of infection dramatically increases the incidence of liver cancer.
Hepatitis D infection usually only occurs with a concomitant infection with hepatitis B.[11] Co-infection with hepatitis D increases the risk of liver cirrhosis and subsequently, liver cancer.
Polyarteritis nodosa is more common in people with hepatitis B infection.
Diagnosis
The original assays for detection of hepatitis B virus infection involve serum or blood tests that detect either viral antigens (proteins produced by the virus) or antibodies produced by the host. Interpretation of these assays is complex. The table below is organized chronologically, from top to bottom:
| Antigens | Antibodies |
| The hepatitis B surface antigen (HBsAg[12]) is most frequently used to screen for the presence of this infection. It is the first detectable viral antigen to appear during infection with this virus; however, early in an infection, this antigen may not be present and it may be undetectable later in the infection as it is being cleared by the host. | - |
| The infectious virion contains an inner "core particle" enclosing viral genome. The icosahedral core particle is made of 180 or 240 copies of core protein, alternatively known as hepatitis B core antigen, or HBcAg[13] | During this 'window' in which the host remains infected but is successfully clearing the virus, IgM antibodies to the hepatitis B core antigen (anti-HBc IGM) may be the only serologic evidence of disease. |
| Shortly after the appearance of the HBsAg, another antigen named as the hepatitis B e antigen (HBeAg[14]) will appear.[2] Traditionally, the presence of HBeAg in a host's serum is associated with much higher rates of viral replication; however, some variants of the hepatitis B virus do not produce the 'e' antigen at all, so this rule does not always hold true. | During the natural course of an infection, the HBeAg may be cleared, and antibodies to the 'e' antigen (anti-HBe) will arise immediately afterward. This conversion is usually associated with a dramatic decline in viral replication. |
| - | If the host is able to clear the infection, eventually the HBsAg will become undetectable and will be followed by antibodies to the hepatitis B surface antigen (anti-HBs).[1] |
A person negative for HBsAg but positive for anti-HBs has either cleared an infection or has been vaccinated previously. A number of persons who are positive for HBsAg may have very little viral multiplication, and hence may be at little risk of long-term complications or of transmitting infection to others.
More recently, PCR tests have been developed to detect and measure the amount of viral nucleic acid in clinical specimens. These tests are useful to assess a person's infection status and to monitor treatment.
Treatment
In the first few months of infection, hepatitis B does not usually get treated. Up to 95% of adults clear the infection spontaneously without treatment. Therapy in this stage of infection does not seem to further improve the chances of spontaneous cure. Early antiviral treatment may only be required in fewer than 1% of patients, whose hepatitis B takes a very aggressive course ("fulminant hepatitis").
There are currently several treatments for chronic hepatitis B. While none of the available drugs usually clear the infection, they can stop the virus from replicating, and prevent liver damage such as cirrhosis and/or liver cancer. Treatments are available in the form of antiviral drugs such as lamivudine, adefovir, entecavir or telbivudine, and immune system modulators such as interferon alpha (Uniferon) or peg-interferon alpha (PEGASYS). There are several other antivirals under investigation. However, some individuals are much more likely to respond than others. It does not appear that combination therapy offers any advantages,[15] but may help in patients with resistant viruses, or in advanced liver disease where resistant viruses may lead to liver failure. In general, each treatment works by reducing the viral load by several orders of magnitude. In some patients, chronic hepatitis B takes a mild course and does not require immediate treatment. Treatment strategies should be individualized. Considerations include a person's risk for developing complications of persistent infection, a person's likelihood of adhering and responding to treatment, and potential risks such as side effects or development of viral resistance.
On March 29, 2005, the US Food and Drug Administration (FDA) approved Entecavir for the treatment of hepatitis B.[16]
On February 25, 2005, the EU Commission approved PEGASYS for the treatment of hepatitis B making it the first pegylated interferon to be approved for hepatitis B.[17]
On October 27, 2006, telbivudine gained FDA approval for treatment of chronic hepatitis B. It is marketed under the brand name Tyzeka in the US and Sebivo outside the US. It is already approved in Switzerland.[18]
Chronic carriers are encouraged to avoid consuming alcohol as it increases their risk for cirrhosis and hepatocellular carcinoma (liver cancer).
Infants born to mothers known to carry hepatitis B can be treated with antibodies to the hepatitis B virus (hepatitis B immune globulin or HBIg). When given with the vaccine within twelve hours of birth, the risk of acquiring hepatitis B is reduced 95%. This treatment also allows a mother to safely breastfeed her child.
An individual exposed to the virus who has never been vaccinated may be treated with HBIg immediately following the exposure. For instance, a health care worker accidentally stuck by a needle used in a hepatitis B carrier would qualify. Treatment must be soon after exposure, however.
Prevention
While abstinence is the only guaranteed way of preventing sexual transmission of hepatitis B., latex condoms, if used properly, greatly reduce the chances of transmission.
Vaccine
Main article Hepatitis B vaccine
Several vaccines have been developed for the prevention of hepatitis B virus infection. These rely on the use of one of the viral envelope proteins (hepatitis B surface antigen or HBsAg). The vaccine was originally prepared from plasma obtained from patients who had long-standing hepatitis B virus infection. However, currently, these are more often made using recombinant DNA technology, though plasma-derived vaccines continue to be used; the two types of vaccines are equally effective and safe.[citation needed]
Missing Women Puzzle
Emily Oster argued that high Hepatitis B rates explain significant portion of the "missing women" first expounded in Amartya Sen's famous 1990 essay, "More Than 100 Million Women Are Missing.", and that the use of Hepatitis B vaccine in 1982 helped lower the male-to-female birth ratio.
See also
- Hepatitis A
- Hepatitis B in China
- Hepatitis C
- Hepatitis D
- Hepatitis E
- Hepatitis F
- Hepatitis G
- Maurice Hilleman
- Baruch S. Blumberg
References
- ^ a b Zuckerman AJ (1996). Hepatitis Viruses. In: Baron's Medical Microbiology (Baron S et al, eds.) (4th ed. ed.). Univ of Texas Medical Branch. ISBN 0-9631172-1-1.
{{cite book}}:|edition=has extra text (help) - ^ a b Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed. ed.). McGraw Hill. pp. pp. 544–51. ISBN 0-8385-8529-9.
{{cite book}}:|author=has generic name (help);|edition=has extra text (help);|pages=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). Molecular Biology of the Cell (4th ed.). Garland. (via NCBI Bookshelf) ISBN 0-8153-3218-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Hepatitis B MedlinePlus article
- ^ Hepadnaviridae characteristics - ICTVdB
- ^ Petersen NJ, Barrett DH, Bond WW, Berquist KR, Favero MS, Bender TR, Maynard JE (1976). "Hepatitis B surface antigen in saliva, impetiginous lesions, and the environment in two remote Alaskan villages". Appl. Environ. Microbiol. 32 (4): 572–574. PMID 791124.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Redd JT, Baumbach J, Kohn W; et al. (2007). "Patient-to-patient transmission of hepatitis B virus associated with oral surgery" (PDF). J Infect Dis. 195 (9): 1311–4.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Alter MJ (2003). "Epidemiology and prevention of hepatitis B". Semin. Liver Dis. 23 (1): 39–46. doi:10.1055/s-2003-37583. PMID 12616449.
- ^ Iannacone M.; et al. (2006). "Pathogenetic and antiviral immune responses against hepatitis B virus". Future Virology. 1 (2): 189–196.
{{cite journal}}: Explicit use of et al. in:|author=(help); External link in|journal= - ^ Iannacone M, Sitia G, Isogawa M, Marchese P, Castro M, Lowenstein P, Chisari F, Ruggeri Z, Guidotti L (2005). "Platelets mediate cytotoxic T lymphocyte-induced liver damage". Nature Medicine. 11 (11): 1167–1169. PMID 16258538.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lok A. Hepatitis D virus infection and liver transplantation. uptodate.com. Retrieved on September 11, 2007.
- ^ HBsAg at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ HBcAg at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ HBeAg at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ Lau GKK; et al. (2005). "Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B". N Engl J Med. 352 (26): 2682–95. PMID 15987917.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ U.S. Food and Drug Administration. March 30, 2005. FDA Talk Paper: FDA Approves New Treatment for Chronic Hepatitis B. fda.gov. Retrieved on September 11, 2007.
- ^ February 25, 2005. Pegasys approved in the European Union for the treatment of chronic hepatitis B: Only pegylated interferon approved for the treatment of chronic hepatitis B. roche.com. Retrieved on September 11, 2007.
- ^ October 27, 2006. FDA Approves Telbivudine for Treatment of Chronic Hepatitis B. hivandhepatitis.com. Retrieved on September 11, 2007.
External links
- NIH collection of links to relevant articles on Hepatitis B
- Hepatitis B Foundation, non-profit organization dedicated to the global problem of hepatitis B
- CDC webpage on Hepatitis B
- CDC fact sheet on Hepatitis B
- ThinkB: Hepatitis B information for Asians. Multiple language content available.
- Pediatric Hepatitis Report compiled by Parents of Kids with Infectious Diseases
- Asian Liver Center at Stanford University, non-profit organization to fight hepatitis B and liver cancer
- Advances in Hepatitis B Research: From Virology to Clinical Management
- Jade Ribbon Campaign, international campaign addressing the high prevalence of hepatitis B in Asian Pacific Islander communities
- An illustration of subunit vaccine development for Hepatitis B - Nova Online
- American Liver Foundation: Comprehensive information about Hepatitis B, including links to chapters for finding local resources
