Radon
| Radon | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈreɪdɒn/ | ||||||||||||||||||||||||||||||||
| Appearance | colorless gas | ||||||||||||||||||||||||||||||||
| Mass number | [222] | ||||||||||||||||||||||||||||||||
| Radon in the periodic table | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Atomic number (Z) | 86 | ||||||||||||||||||||||||||||||||
| Group | group 18 (noble gases) | ||||||||||||||||||||||||||||||||
| Period | period 6 | ||||||||||||||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p6 | ||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 8 | ||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||||||||||||||
| Melting point | 202 K (−71 °C, −96 °F) | ||||||||||||||||||||||||||||||||
| Boiling point | 211.5 K (−61.7 °C, −79.1 °F) | ||||||||||||||||||||||||||||||||
| Density (at STP) | 9.73 g/L | ||||||||||||||||||||||||||||||||
| when liquid (at b.p.) | 4.4 g/cm3 | ||||||||||||||||||||||||||||||||
| Critical point | 377 K, 6.28 MPa[1] | ||||||||||||||||||||||||||||||||
| Heat of fusion | 3.247 kJ/mol | ||||||||||||||||||||||||||||||||
| Heat of vaporization | 18.10 kJ/mol | ||||||||||||||||||||||||||||||||
| Molar heat capacity | 5R/2 = 20.786 J/(mol·K) | ||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||
| Oxidation states | 0, +2, +6 | ||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.2 | ||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||
| Covalent radius | 150 pm | ||||||||||||||||||||||||||||||||
| Van der Waals radius | 220 pm | ||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||
| Natural occurrence | from decay | ||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) (predicted) | ||||||||||||||||||||||||||||||||
| Thermal conductivity | 3.61×10−3 W/(m⋅K) | ||||||||||||||||||||||||||||||||
| Magnetic ordering | non-magnetic | ||||||||||||||||||||||||||||||||
| CAS Number | 10043-92-2 | ||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||
| Discovery | Ernest Rutherford and Robert B. Owens (1899) | ||||||||||||||||||||||||||||||||
| First isolation | William Ramsay and Robert Whytlaw-Gray (1910) | ||||||||||||||||||||||||||||||||
| Isotopes of radon | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
Radon (Template:PronEng) is the chemical element that has the symbol Rn and atomic number 86. Radon is a colorless, naturally occurring, radioactive noble gas that is formed from the decay of radium. It is one of the heaviest substances that are gases under normal conditions and is considered to be a health hazard. The most stable isotope, 222Rn, has a half-life of 3.8 days and is used in radiotherapy. While having been less studied by chemists due to its radioactivity, there are a few known compounds of this generally unreactive element.
Radon is a significant contaminant that affects indoor air quality worldwide. Radon gas from natural sources can accumulate in buildings and reportedly causes 21,000 lung cancer deaths per year in the United States alone.[3] Radon is the second most frequent cause of lung cancer, after cigarette smoking, and radon-induced lung cancer is thought to be the 6th leading cause of cancer death overall.
History and etymology
Radon is the third discovered radioactive element after radium and polonium. It was discovered in 1898 by Friedrich Ernst Dorn.[4][5][6] In 1900, he reported some experiments in which he noticed that radium compounds emanate a radioactive gas which he named Radium Emanation (Ra Em).[7] Before that, in 1899, Pierre and Marie Curie observed that the "gas" emitted by radium remained radioactive for a month.[8] Later that year, Robert B. Owens and Ernest Rutherford noticed variations when trying to measure radiation from thorium oxide.[9] Rutherford noticed that the compounds of thorium continuously emit a radioactive gas which retain the radioactive powers for several minutes and called this gas "emanation" (from Latin "emanare" - to elapse and "emanatio" - expiration),[10] and later Thorium Emanation (Th Em). In 1901, he demonstrated that the emanations are radioactive, but credited the Curies for the discovery of the element.[11] In 1903, similar emanations were observed from actinium by André-Louis Debierne[12][13] and were called Actinium Emanation (Ac Em).
Several names were suggested for these three gases: exradio, exthorio, and exactinio in 1904;[14] radon, thoron, and akton in 1918;[15] radeon, thoreon, and actineon in 1919,[16] and eventually radon, thoron, and actinon in 1920.[17] The likeness of the spectra of these three gases with those of argon, krypton, and xenon, and their observed chemical inertia led Sir William Ramsay to suggest in 1904 that the "emanations" might contain a new element of the noble gas family.[14]
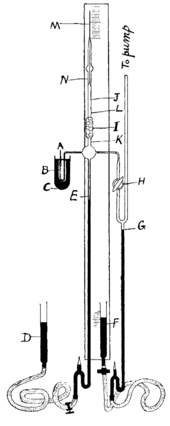
2 entered the evacuated system through siphon A; mercury is shown in black.
In 1910, Sir William Ramsay and Robert Whytlaw-Gray isolated radon, determined its density, and determined that it was the heaviest known gas.[18] They wrote that "L'expression l'émanation du radium est fort incommode," (the expression of radium emanation is very awkward) and suggested the new name niton (Nt) (from the Latin "nitens" meaning "shining") in order to emphasize the property of gases that cause the phosphorescence of some substances,[18] and in 1912 it was accepted by the International Commission for Atomic Weights. In 1923, the International Committee for Chemical Elements and International Union of Pure and Applied Chemistry (IUPAC) chose among the names radon (Rn), thoron (Tn), and actinon (An). Later, when isotopes were numbered instead of named, the element took the name of the most stable isotope, radon, while Tn became 220Rn and An 219Rn. As late as the 1960s, the element was also referred to simply as emanation.[19] The first synthesized compound of radon, radon fluoride, was obtained in 1962.[20]
The first major studies with radon and health occurred in the context of uranium mining, first in the Joachimsthal region of Bohemia and then in the Southwestern United States during the early Cold War. Because radon is a product of uranium, uranium mines and its highly radioactive daughter products may have high concentrations of radon. Many uranium miners in the Four Corners region contracted lung cancer and other pathologies as a result of high levels of exposure to radon in the mid-1950s. The increased number of incidences of lung cancer was particularly pronounced among Native American and Mormon miners, because those groups normally have low rates of lung cancer. Safety standards requiring expensive ventilation were not widely implemented or policed during this period.[21]
The danger of radon exposure in dwellings was discovered in 1984 when Stanley Watras, an employee at the Limerick nuclear power plant in Pennsylvania, set off the radiation alarms on his way to work for two weeks while authorities searched for the source of the contamination. They found that the source was high levels of radon – about 100,000 Bq/m³ (2,700 pCi/L) – in his house's basement, and it was not related to the nuclear plant. The risks associated with living in his house were estimated to be equivalent to smoking 135 packs of cigarettes every day. Following this highly publicized event, national radon safety standards were set, and radon detection and ventilation became a standard homeowner concern.[22]
Isotopes
Radon has no stable isotopes. There are 34 radioactive isotope that have been studied which range from an atomic mass of 195 to 228.[23] The most stable isotope is 222Rn, which is a decay product of 226Ra. It has a half-life of 3.823 days and decomposes by alpha particle emission into 218Po.[23] Among the decay daughters of this decay chain is also the highly unstable isotope 218Rn. The naturally occurring 226Ra is a product of the decay chain of 238U.[24] This decay series (with half-lives) is
- 238U (4.5 x 109 yr) → 234Th (24.1 days) → 234Pa (1.18 min) → 234U (250,000 yr) → 230Th (75,000 yr) → 226Ra (1,600 yr) → 222Rn (3.82 days) → 218Po (3.1 min) → 218At (1.5 s) → 218Rn (35 ms) → 214Pb (26.8 min) → 214Bi (19.7 min) → 214Po (164 µs) → 210Pb (22.3 yr) → 210Bi (5.01 days) → 210Po (138 days) → 206Pb (stable)
There are three other isotopes that have a half life of over an hour: 211Rn, 210Rn and 224Rn. The 220Rn isotope is a natural decay product of the most stable thorium isotope (232Th), named thoron. It has a half-life of 55.6 seconds and also emits alpha radiation. Similarly, 219Rn is derived from the most stable isotope of actinium (227Ac) — named “actinon” — and is an alpha emitter with a half-life of 3.96 seconds.[25]
Characteristics
At standard temperature and pressure, radon forms a monoatomic gas with a density of 9.73 kg/m3,[26] about 8 times the surface density of the Earth's atmosphere, 1.217 kg/m3,[27] and is one of the heaviest gases at room temperature and the heaviest of the noble gases, excluding ununoctium. At standard temperature and pressure, radon is a colorless gas, but when it is cooled below its freezing point of 202 K (−71 °C; −96 °F), it has a brilliant phosphorescence which turns yellow as the temperature is lowered, and becomes orange-red as the air liquefies at temperatures below 93 K (−180.2 °C; −292.3 °F).[28] Upon condensation, radon also glows because of the intense radiation it produces.
Natural radon concentrations in Earth's atmosphere are so low that radon-rich water in contact with the atmosphere will continually lose radon by volatilization. Hence, ground water has a higher concentration of 222Rn than surface water, because the radon is continuously produced by radioactive decay of 226Ra present in rocks. Likewise, the saturated zone of a soil frequently has a higher radon content than the unsaturated zone because of diffusional losses to the atmosphere.[29][30]
Radon is a health hazard as exposure can cause lung cancer – it is, in fact, the second major cause of lung cancer after smoking.[31] Radon as a terrestrial source of background radiation is of particular concern because, although on average it is very rare, this intensely radioactive element can be found in high concentrations in many areas of the world, where it represents a significant health hazard. Radon-222 has been classified by International Agency for Research on Cancer as being carcinogenic to humans.[32]
Radon commercialization is regulated, but it is available in small quantities, at a price of almost $6,000 per mililitre.[33] Because it is also radioactive and is a relatively unreactive chemical element, radon has few uses and is seldom used in academic research.
Chemistry
Radon is a member of the zero-valence elements that are called noble or inert gases. It is inert to most common chemical reactions, such as combustion, because the outer valence shell contains eight electrons. This produces a stable, minimum energy configuration in which the outer electrons are tightly bound.[34] However, due to periodic trends, radon has a lower electronegativity than the element one period before it, xenon, and is therefore more reactive.
Because of its price and radioactivity, experimental chemical research is seldom performed with radon, and as a result there are very few reported compounds of radon, all either fluorides or oxides. Radon can be oxidized by a few powerful oxidizing agents such as F
2, thus forming radon fluoride.[35][36] Radon oxides are among the few other reported compounds of radon.[37]
Occurrence
The average concentration of radon in the atmosphere is about 6×10−20 atoms of radon for each molecule in the air, or about 150 atoms in each mL of air.[38] It can be found in some spring waters and hot springs.[39] The towns of Boulder, Montana; Misasa; Bad Kreuznach, Germany; and the country of Japan have radium-rich springs which emit radon. The element emanates naturally from the ground all over the world, but particularly in regions with soils containing granite or shale. However, not all granitic regions are prone to high emissions of radon. The element emitted from the ground has been shown to accumulate in the air if there is a meteorological inversion and little wind.[40] In some caves, increased radon concentration has been observed.[41]
Radon is found in some petroleum. Because radon has a similar pressure and temperature curve as propane, and oil refineries separate petrochemicals based on their boiling points, the piping carrying freshly separated propane in oil refineries can become partially radioactive due to radon decay particles. Residues from the oil and gas industry often contain radium and its daughters. The sulfate scale from an oil well can be radium rich, while the water, oil, and gas from a well often contains radon. The radon decays to form solid radioisotopes which form coatings on the inside of pipework. An oil processing plant, the area of the plant where propane is processed, is often one of the more contaminated areas of the plant as radon has a similar boiling point as propane.[42]
Radon, along with the noble gases krypton and xenon, is also produced during the operation of nuclear power plants. A small fraction of it leaks out of the fuel, through the cladding, and into the cooling water, from which it is scavenged. It is then routed to a holding tank where it remains for a large number of half-lives. It is finally purged to the open air through a tall stack, which is carefully monitored for radiation level.

Radon collects over samples of radium-226 at a rate of about 0.001 cm3/day per gram of radium. The radon (222Rn) released into the air decays to 210Pb and other radioisotopes, the levels of 210Pb can be measured. The rate of deposition of this radioisotope is dependent on the weather. In the early part of the 20th century in the USA, gold which was contaminated with lead-210 entered the jewelry industry. This was from gold seeds which had held radon-222 that had been melted down after the radon had decayed. The daughters of the radon are still radioactive today.[44]
In 1971, Apollo 15 passed 110 kilometres (68 mi) above the Aristarchus plateau on the Moon, and detected a significant rise in alpha particles thought to be caused by the decay of radon-222. The presence of radon-222 (222Rn) has been inferred later from data obtained from the Lunar Prospector alpha particle spectrometer.[45]
Depending on how houses are built and ventilated, radon may accumulate in basements and dwellings. The highest average radon concentrations in the United States are found in Iowa and in the Appalachian Mountain areas in southeastern Pennsylvania.[46] Some of the highest readings ever have been recorded in the Irish town of Mallow, County Cork, prompting local fears regarding lung cancer. Iowa has the highest average radon concentrations in the nation due to significant glaciation that ground the granitic rocks from the Canadian Shield and deposited it as soils making up the rich Iowa farmland.[47] Many cities within the state, such as Iowa City, have passed requirements for radon-resistant construction in new homes. A study made in December 2004 noted that the counties surrounding Three Mile Island have the highest radon concentrations in the United States and that this may be the cause of the increased lung cancer noted in the region.[48]
The European Union recommends that action should be taken starting from concentrations of 400 Bq/m³ (11 pCi/L) for old houses and 200 Bq/m³ (5 pCi/L) for new ones. After publication of the North American and European Pooling Studies, Health Canada proposed a new guideline that lowers their action level from 800 to 200 Bq/m³ (22 to 5 pCi/L).[49] The United States Environmental Protection Agency (EPA) strongly recommends action for any house with a concentration higher than 148 Bq/m³ (4 pCi/L),[50] and encourages action starting at 74 Bq/m³ (2 pCi/L). EPA radon risk level tables including comparisons to other risks encountered in life are available in their citizen's guide.[51] The EPA estimates that nationally, 8% to 12% of all houses are above their maximum "safe levels" (four picocuries per liter – the equivalent to roughly 200 chest x-rays). The United States Surgeon General and the EPA both recommend that all homes be tested for radon.
Applications
Medical
It has been claimed that exposure to radon gas mitigates auto-immune diseases such as arthritis.[52] As a result, in the late 20th century and early 21st century, some "health mines" were established in Basin, Montana which attracted people seeking relief from health problems such as arthritis through limited exposure to radioactive mine water and radon. The practice was controversial because of the "well-documented ill effects of high-dose radiation on the body."[53]
Radioactive water baths have been applied since 1906 in Jáchymov, Czech Republic, but even before radon discovery they were used in Bad Gastein, Austria. Radium-rich springs are also used in traditional Japanese onsen in Misasa, Tottori prefecture. Drinking therapy is applied in Bad Brambach, Germany. Inhalation therapy is carried out in Gasteiner-Heilstollen, Austria, in Kowary, Poland and in Boulder, Montana, United States. In the United States and Europe there are several "radon spas," where people sit for minutes or hours in a high-radon atmosphere in the belief that low doses of radiation will invigorate or energize them.[54]
In addition to personal testimonies of arthritis relief and other benefits, there is some scientific evidence for this belief, known as hormesis. However, the general scientific community finds it unsubstantiated. There is no known biological mechanism by which such an effect could occur. In addition, it conflicts with the internationally recognized standard that there is no safe threshold for radiation exposure and that exposure should be limited to that "as low as reasonably achievable" (ALARA).
The radon gas which is used as a cancer treatment in medicine is obtained from the decay of a radium chloride source. In the past, radium and radon have both been used for X-ray medical radiography, but they have fallen out of use as they are radiotoxic alpha radiation emitters which are expensive and have been replaced with iridium-192 and cobalt-60 since they are far better photon sources.
Scientific
Radon emanation from the soil varies with soil type and with surface uranium content, so outdoor radon concentrations can be used to track air masses to a limited degree. This fact has been put to use by some atmospheric scientists. Because of radon's rapid loss to air and comparatively rapid decay, radon is used in hydrologic research that studies the interaction between ground water and streams. Any significant concentration of radon in a stream is a good indicator that there are local inputs of ground water. Radon is also used in the dating of oil-containing soils because radon has a high affinity of oil-like substances.
Radon soil-concentration has been used in an experimental way to map buried close-subsurface geological faults because concentrations are generally higher over the faults. Similarly, it has found some limited use in geothermal prospecting. Some researchers have also looked at elevated soil-gas radon concentrations, or rapid changes in soil or groundwater radon concentrations, as a predictor for earthquakes.[55] Results have been generally unconvincing but may ultimately prove to have some limited use in specific locations.
Radon is a known pollutant emitted from geothermal power stations, though it disperses rapidly, and no radiological hazard has been demonstrated in various investigations. The trend in geothermal plants is to reinject all emissions by pumping deep underground, and this seems likely to ultimately decrease such radon hazards further.
Testing and mitigation
ASTM E-2121 is a standard for reducing radon in homes as far as practicable below 4 picocuries per liter (pCi/L) in indoor air.[56][57] Radon test kits are commercially available. The kit includes a collector that the user hangs in the lowest livable floor of the house for 2 to 7 days. The user then sends the collector to a laboratory for analysis. The National Environmental Health Association provides a list of radon measurement professionals.[58] Long term kits, taking collections for up to one year, are also available. An open-land test kit can test radon emissions from the land before construction begins. The EPA and the National Environmental Health Association have identified 15 types of radon testing.[59] A Lucas cell is one type of device.
Radon levels fluctuate naturally. An initial test might not be an accurate assessment of your home's average radon level. Transient weather can affect short term measurements.[60] Therefore, a high result (over 4 pc/l) justifies repeating the test before undertaking more expensive abatement projects. Measurements between 4 and 10 pc/l warrant a long term radon test. Measurements over 10 pc/l warrant only another short term test so that abatement measures are not unduly delayed. Purchasers of real estate are advised to delay or decline a purchase if the seller has not successfully abated radon to 4 pc/l or less.
The National Environmental Health Association administers a voluntary National Radon Proficiency Program for radon professionals consisting of individuals and companies wanting to take training courses and examinations to demonstrate their competency.[61] A list of mitigation service providers is available.[62] Indoor radon can be mitigated by sealing basement foundations, water drainage, or by sub-slab de-pressurization. In severe cases, mitigation can use air pipes and fans to exhaust sub-slab air to the outside. Indoor ventilation systems are more effective, but exterior ventilation can be cost-effective in some cases.
Modern construction that conserves energy by making homes air tight exacerbates the risks of radon exposure if radon is present in the home. Older homes with more porous construction are more likely to vent radon naturally. Positive-pressure ventilation systems can be combined with a heat exchanger to recover energy in the process of exchanging air with the outside, and it should be noted that simply exhausting basement air to the outside is not necessarily a viable solution as this can actually draw radon gas into a dwelling. Homes built on a crawl space can benefit from a radon collector installed under a radon barrier (a sheet of plastic that covers the crawl space).
Precautions
Radon is the invisible, radioactive atomic gas that results from radioactive decay of some forms of uranium that may be found in rock formations beneath buildings or in certain building materials themselves. Radon is most likely the most pervasive serious hazard for indoor air in the United States and Europe, and is probably responsible for tens of thousands of lung cancer deaths per year. There are relatively simple tests for radon gas, but these tests are not commonly done, even in areas of known systematic hazards. Radon is a very heavy gas and thus will tend to accumulate at the floor level. Building materials can be a significant source of radon, but very little testing is done for stone, rock, or tile products brought into building sites. The half-life for radon is 3.8 days, indicating that once the source is removed, the hazard will be greatly reduced within a few weeks. However, annually, thousands of people go to radon-contaminated mines for purposeful exposure to help with the symptoms of arthritis without any serious health effects.[31]
Radon presents significant risks, since it is a colorless and odorless gas, and therefore not readily detectable by a human. The radiation decay products ionize genetic material, causing mutations that sometimes turn cancerous. Radon exposure is the second major cause of lung cancer after smoking.[31] Radon gas levels vary by locality and the composition of the underlying soil and rocks. For example, in areas such as Cornwall in the UK, which has granite as substrata, radon gas is a major problem, and buildings have to be force-ventilated with fans to lower radon gas concentrations. The United States Environmental Protection Agency (EPA) estimates that one in 15 homes in the United States has radon levels above the recommended guideline of 4 picocuries per liter (pCi/L) (148 Bq/m³).[50] Iowa has the highest average radon concentration in the United States; studies performed there have demonstrated a 50% increased lung cancer risk with prolonged radon exposure above the EPA's action level of 4 pCi/L.[63][64]
Radon is a terrestrial source of radiation of particular concern because — although on average it is very rare — this intensely radioactive element can be found in high concentrations in many areas of the world, where it represents a significant health hazard. Radon is a decay product of uranium, which is relatively common in the earth's crust, but generally concentrated in ore-bearing rocks scattered around the world. Radon seeps out of these ores into the atmosphere or into ground water, and in these localities it can accumulate within dwellings and expose humans to high concentrations. The widespread construction of well insulated and sealed homes in the northern industrialized world has led to radon becoming the primary source of background radiation in some localities in northern North America and Europe. Some of these areas, including Cornwall and Aberdeenshire in the United Kingdom have high enough natural radiation levels that nuclear licensed sites cannot be built there — the sites would already exceed legal radiation limits before they opened, and the natural topsoil and rock would all have to be disposed of as low-level nuclear waste.[65]
Radiation exposure from radon is indirect. Radon has a short half-life (4 days) and decays into other solid particulate radium-series radioactive nuclides. These radioactive particles are inhaled and remain lodged in the lungs, causing continued exposure. People in affected localities can receive up to 10 mSv per year background radiation.[65] Radon is thus the second leading cause of lung cancer after smoking, and accounts for 15,000 to 22,000 cancer deaths per year in the US alone.[66] The general population is exposed to small amounts of polonium as a radon daughter in indoor air; the isotopes 214Po and 218Po are thought to cause the majority[67] of the estimated 15,000–22,000 lung cancer deaths in the US every year that have been attributed to indoor radon.[68]
The general effects of radon to the human body are caused by its radioactivity and consequent risk of radiation-induced cancer. As an inert gas, radon has a low solubility in body fluids, which leads to a uniform distribution of the gas throughout the body. Radon gas and its solid decay products are carcinogens. The greatest health risks come from exposure to the inhaled solid radon gas decay products that are produced during its radioactive decay. Two of these decay products, polonium-218 and 214, present a significant radiologic hazard.[69] Once the radioactive decay products are inhaled into the lung, they undergo further radioactive decay, releasing small bursts of energy in the form of alpha particles that can either cause DNA breaks or create free radicals.[69]
It is not known whether radon can cause health effects in other organs besides the lungs. The effects of radon, if found in food or drinking water, are unknown.

The largest single source of radiation exposure to the general public is naturally-occurring radon gas, which comprises approximately 55% of the annual background dose. The largest natural contributor to public radiation dose is radon, a naturally occurring, radioactive gas found in soil and rock.[70] If the gas is inhaled, some of the radon particles may attach to the inner lining of the lung. These particles continue to decay, emitting alpha particles which can damage cells in the lung tissue.[50] The death of Marie Curie at age 66 from leukemia was likely caused by prolonged exposure to high doses of ionizing radiation. Its discoverer, Curie worked extensively with radium, which decays into radon,[71] along with other radioactive materials that emit beta and gamma rays.
In the United Kingdom, residential radon is, after cigarette smoking, the second most frequent cause of lung cancer deaths: 83.9% of deaths are attributed to smoking only, 1.0% to radon only, and 5.5% to a combination of radon and smoking.[72] Radon-induced lung cancer is the number one cause of death among non-smokers.[73] Based on studies carried out by the National Academy of Sciences in the United States, radon is the second most common cause of lung cancer after cigarette smoking, accounting for 15,000 to 22,000 cancer deaths per year in the U.S.[74] The Surgeon General of the United States has reported that over 20,000 Americans die each year of radon-related lung cancer.[75] The United States Environmental Protection Agency (EPA) recommends homes be fixed if an occupant's long-term exposure will average 4 picocuries per liter (pCi/L) (148 Bq m−3) or higher.[76] Beginning with the late 1980s, this led to activists forming campaigns to raise awareness of radiation resulting from radon.[77]
The most elaborate case-control epidemiologic radon study performed by R. William Field and colleagues demonstrated a 50% increased lung cancer risk with prolonged radon exposure at the EPA's action level of 4 pCi/L.[78] Iowa has the highest average radon concentrations in the nation and a very stable population which added to the strength of the study. Pooled epidemiologic radon studies[79][80] have also shown an increased lung cancer risk from radon below the EPA's action level of 4 pCi/L.
Radiation from radon has been attributed to increase of lung cancer among smokers too. This is because the daughters of radon often become attached to smoke and dust particles, and are then able to lodge in the lungs.[81] It is unknown whether radon causes other types of cancer, but recent studies suggest a need for further studies to assess the relationship between radon and leukemia.[82][83] Radon is a common problem encountered during uranium mining, as it is a radioactive gas, inhalation of which caused sharp increases in lung cancers among uranium miners employed in the 1940s and 1950s.[84][85][86]
Bernard Cohen has been a staunch opponent to the so called Linear no-threshold model (LNT) which postulates that there is no safe threshold for radiation exposure. His debates in academic periodicals and published correspondence with R. William Field, Brian J. Smith (assistant professor of biostatistics, University of Iowa), Jerry Puskin (from the U.S. Environmental Protection Agency), Sarah Darby, and Sir Richard Doll and others regarding his radon-related ecologic studies are legendary.[87][88] He offered many rewards ($10,000) if people could provide evidence that the inverse association he found between radon (county averages) and lung cancer (county averages) was due to some factor other than failure of the linear-no threshold theory. Puskin, Smith, Field and others have claimed that his findings are due in part to his inability to control for the inverse association between smoking and radon.[89][90]
Indeed, Bernard Cohen's assertions appear to have gained further support recently. The results of a methodical ten-year-long, case-controlled study of residential radon exposure in Worcester County, Massachusetts, found an apparent 60% reduction in lung cancer risk amongst people exposed to low levels (0–150 Bq/m3) of radon gas; levels typically encountered in 90% of American homes — an apparent support for the idea of radiation hormesis.[91] The study paid close attention to the cohort's levels of smoking, occupational exposure to carcinogens and education attainment. The study was also notable for carefully placing radon monitors for one year, close to where people spent the most time in their homes. Co-author Joel H. Popkin said, "We were certainly not looking for a hormetic effect," . . . "Indeed, we were stunned when the data pointed to that conclusion in such a strong way.[92]
See also
- International Radon Project
- Lucas cell
- Radon mitigation
- Radiation Exposure Compensation Act
- Radiohalo
Notes
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.122. ISBN 1-4398-5511-0.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "A Citizen's Guide to Radon". U.S. Environmental Protection Agency. 2007-11-26. Retrieved 2008-06-26.
- ^ Bentor, Yinon (2006). "Periodic Table: Date of Discovery". Retrieved 2008-02-28.
- ^ Partington, J. R. (May 1957). "Discovery of Radon". Nature. 179 (4566): 912.
- ^ "Timeline of Element Discovery". The New York Times Company. 2008. Retrieved 2008-02-28.
- ^ Dorn, Friedrich Ernst (1900). "Ueber die von radioaktiven Substanzen ausgesandte Emanation". Abhandlungen der Naturforschenden Gesellschaft zu Halle. 22. Stuttgart: 155.
- ^ Curie, P.; Curie, Mme. Marie (1899). "Sur la radioactivite provoquee par les rayons de Becquerel". Comptes rendus hebdomadaires des seances de l'Academie des sciences. 129: 714–716.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ E, Rutheford; Owens, R. B. (1899). "Thorium and uranium radiation". Trans. R. Soc. Can. 2: 9–12.
{{cite journal}}: CS1 maint: multiple names: authors list (link): "the radiation from thorium oxide was not constant, but varied in a most capricious manner." whereas "all the compounds of Uranium give out a radiation which is remarkably constant" - ^ Rutheford, E. (1900). "A radioactive substance emitted from thorium compounds". Philosophical Magazine. 40: 1–4.
- ^ Rutheford, E.; Brooks, H.T. (1901). "The new gas from radium". Trans. R. Soc. Can. 7: 21–25.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Giesel, Fritz (1903). "Ueber den Emanationskörper aus Pechblende und über Radium". Chemische Berichte. 36 (1): 342–347. doi:10.1002/cber.19030360177.
- ^ Debierne, André-Louis (1903). "Sur la radioactivite induite provoquee par les sels d'actinium". Comptes rendus hebdomadaires des seances de l'Academie des sciences. 136: 446.
- ^ a b Ramsay, Sir William; Collie, J. Normal (1904). "The Spectrum of the Radium Emanation". Proceedings of the Royal Society of London. 73: 470–476. doi:10.1098/rspl.1904.0064.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schmidt, Curt (1918). "Periodisches System und Genesis der Elemente". Z. anorg. Ch. 103: 79–118. doi:10.1002/zaac.19181030106.
- ^ "Radon". Ann. Physique. 11: 5. 1919.
- ^ Adams, Elliot Quincy (1920). "The Independent Origin of Actinium". Journal of the American Chemical Society. 42: 2205–2208. doi:10.1021/ja01456a010.
- ^ a b Ramsay, W.; Gray, R. W. (1910). "La densité de l'emanation du radium". Comptes rendus hebdomadaires des seances de l'Academie des sciences. 151: 126–128.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Grosse, A. V. (1965). "Some physical and chemical properties of element 118 (Eka-Em) and element 86 (Em)". Journal of Inorganic and Nuclear Chemistry. 27 (3). Elsevier Science Ltd.: 509–519. doi:10.1016/0022-1902(65)80255-X.
- ^ Fields, Paul R.; Stein, Lawrence; Zirin, Moshe H. (1962). "Radon Fluoride". Journal of the American Chemical Society. 84 (21): 4164–4165. doi:10.1021/ja00880a048.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mould, Richard Francis (1993). A Century of X-rays and Radioactivity in Medicine. CRC Press. ISBN 0750302240.
- ^ Harrison, Kathryn; Hoberg, George (1991). "Setting the Environmental Agenda in Canada and the United States: The Cases of Dioxin and Radon". Canadian Journal of Political Science. 24 (1): 3–27.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brookhaven National Laboratory. Retrieved 2008-06-06.
- ^ http://www.gulflink.osd.mil/library/randrep/du/mr1018.7.appa.html
- ^ Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brookhaven National Laboratory. Retrieved 2008-06-06.
- ^ "Radon". All Measures. 2004. Retrieved 2008-02-12.
- ^ Williams, David R. (2007-04-19). "Earth Fact Sheet". NASA. Retrieved 2008-06-26.
- ^ "Radon". Jefferson Lab. Retrieved 2008-06-26.
- ^ "The Geology of Radon". United States Geological Survey.
{{cite web}}:|access-date=requires|url=(help); Missing or empty|url=(help) - ^ "Radon-222 as a tracer in groundwater-surface water interactions" (PDF). Lancaster University. Retrieved 2008-06-28.
- ^ a b c Catelinois, O (May 2006). "Lung Cancer Attributable to Indoor Radon Exposure in France: Impact of the Risk Models and Uncertainty Analysis". Environmental Health Perspectives. 114 (9). National Institute of Environmental Health Science: 1361–1366. doi:10.1289/ehp.9070. PMID 16966089. Retrieved 2008-06-26.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Known and Probable Carcinogens". American Cancer Society. Retrieved 2008-06-26.
- ^ "SRM 4972 - Radon-222 Emanation Standard". National Institute of Standards and Technology. Retrieved 2008-06-26.
- ^ Bader, Richard F.W. "An Introduction to the Electronic Structure of Atoms and Molecules". McMaster University. Retrieved 2008-06-26.
- ^ Stein, L. (1970). "Ionic Radon Solution". Science. 168: 362. doi:10.1126/science.168.3929.362. PMID 17809133.
- ^ Pitzer, Kenneth S. (1975). "Fluorides of radon and element 118". J. Chem. Soc., Chem. Commun.: 760–761. doi:10.1039/C3975000760b.
- ^ Avrorin, V. V.; Krasikova, R. N.; Nefedov, V. D.; Toropova, M. A. (1982). "The Chemistry of Radon". Russ. Chem. Review. 51 (1): 12–20. doi:10.1070/RC1982v051n01ABEH002787.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "HEALTH HAZARD DATA" (PDF). The Linde Group. Retrieved 2008-06-26.
- ^ Field, R. William. "Radon Occurrence and Health Risk" (PDF). Department of Occupational and Environmental Health, University of Iowa. Retrieved 2008-02-02.
- ^ Steck, Daniel J.; Field, R. William; Lynch, Charles F. (1999). "Exposure to Atmospheric Radon". Environmental Health Perspectives. 107 (2).
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sperrin, Malcolm; Gillmore, Gavin; Denman, Tony (2001). "Radon concentration variations in a Mendip cave cluster". Environmental Management and Health. 12 (5): 476–482. doi:10.1108/09566160110404881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Survey & Identification of NORM Contaminated Equipment" (PDF). Enprotec / Hibbs & Todd. 7 (1). October 2004. Retrieved 2008-02-14.
- ^ Yamamoto, M. (2006). "Radon". Journal of Environmental Radioactivity (86): 110–131.
- ^ "Poster Issued by the New York Department of Health (ca. 1981)". Oak Ridge Associated Universities. 2007-07-25. Retrieved 2008-06-26.
- ^ Lawson, S. (2005). "Recent outgassing from the lunar surface: the Lunar Prospector alpha particle spectrometer". J. Geophys. Res. 110: 1029.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Price, Phillip N. "Predicted County Median Concentration". Lawrence Berkeley National Laboratory. Retrieved 2008-02-12.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Field, R. William. "The Iowa Radon Lung Cancer Study". Department of Occupational and Environmental Health, University of Iowa. Retrieved 2008-02-22.
- ^ Field, R. William (2004-12-05). "Three Mile Island epidemiologic radiation dose assessment revisited: 25 years after the accident". Radiation Protection Dosimetry. 113 (2). Oxford Journals: 214–217. doi:10.1093/rpd/nch445. PMID 15657112. Retrieved 2008-06-26.
{{cite journal}}: Check date values in:|date=(help) - ^ "Radon". It's Your Health. Health Canada. June 2007. Retrieved 2008-02-12.
- ^ a b c "Radiation Protection: Radon". United States Environmental Protection Agency. November 2007. Retrieved 2008-04-17. Cite error: The named reference "EPA radon" was defined multiple times with different content (see the help page).
- ^ "A Citizen's Guide to Radon: The Guide to Protecting Yourself and Your Family from Radon". United States Environmental Protection Agency. November 2007. Retrieved 2008-06-26.
- ^ "Radon Health Mines: Boulder and Basin, Montana". Roadside America. Retrieved 2007-12-04.
- ^ Salak, Kara (2004). "59631: Mining for Miracles". National Geographic. National Geographic Society. Retrieved 2008-06-26.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Jáchymov". Petros. Retrieved 2008-06-26.
- ^ Richon, P.; Sabroux, J.-C.; Halbwachs, M.; Vandemeulebrouck, J.; Poussielgue, N.; Tabbagh, J.; Punongbayan, R. (2003). "Radon anomaly in the soil of Taal volcano, the Philippines: A likely precursor of the M 7.1 Mindoro earthquake (1994)". Geophysical Research Letters. 30 (9): 34–41.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Recommended Residential Radon Mitigation Standard of Practice". United States Environmental Protection Agency. Retrieved 2008-02-02.
- ^ "ASTM E2121-03 Standard Practice for Installing Radon Mitigation Systems in Existing Low-Rise Residential Buildings". ASTM International. Retrieved 2008-02-02.
- ^ "Residential Measurement Provider". The National Environmental Health Association -- National Radon Proficiency Program. Retrieved 2008-02-02.
- ^ "Radon Measurement Method Definitions". The National Environmental Health Association -- National Radon Proficiency Program. Retrieved 2008-02-02.
- ^ "You've found radon in your home - what should you do?". Air Chek, Inc. Retrieved 2008-02-02.
- ^ "National Radon Proficiency Program". The National Environmental Health Association -- National Radon Proficiency Program. Retrieved 2008-02-02.
- ^ "Residential Mitigation Provider". The National Environmental Health Association -- National Radon Proficiency Program. Retrieved 2008-02-02.
- ^ Field, RW (June 2000). "Residential radon gas exposure and lung cancer: the Iowa Radon Lung Cancer Study". American Journal of Epidemiology. 151 (11). Oxford Journals: 1091–1102. PMID 10873134. Retrieved 2008-06-26.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ EPA (June 2000). "Iowa Radon Lung Cancer Study". EPA. Retrieved 2008-06-26.
- ^ a b "Publications". United Nations Scientific Committee on the Effects of Atomic Radiation. 2008-02-06. Retrieved 2008-02-15.
- ^ "Radon and Cancer: Questions and Answers". National Cancer Institute. Retrieved 2008-06-26.
- ^ Darby, S. C. (December 1989). "Health Risks of Radon and Other Internally Deposited Alpha-Emitters-BEIR IV". Biometrics. 45 (4). National Research Council: 1341–1342.
- ^ "Health Effects Of Exposure To Radon". National Academies Press. Retrieved 2008-06-26.
- ^ a b Field, R. William (1999). "Radon Occurrence and Health Risk" (PDF). Retrieved 2008-02-02.
- ^ "Radiation Dose Chart". American Nuclear Society. 2007. Retrieved 2008-02-15.
- ^ "Answer to Question #535 Submitted to "Ask the Experts"". Health Physics Society. Retrieved 2008-06-26.
- ^ Darby, S; Hill, D; Doll, R (2005). "Radon: a likely carcinogen at all exposures". Ann. Oncol. 12 (10): 1341–51. doi:10.1023/A:1012518223463. PMID 11762803.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Health Risks". EPA. Retrieved 2008-06-26.
- ^ "Radon and Cancer: Questions and Answers". National Cancer Institute. 2004-07-13. Retrieved 2008-06-26.
- ^ "Surgeon General Releases National Health Advisory On Radon". Surgeon General of the United States. 2005-01-13. Retrieved 2008-06-26.
- ^ "United States Environmental Protection Agency: Radon". United States Environmental Protection Agency. 2007-08-08. Retrieved 2008-06-26.
- ^ Blaugrund, Andrea (1988-04-09). "Confusion mounting over radon". The Gainesville Sun. p. section A, page 1.
{{cite news}}: Check date values in:|date=(help) - ^ Field, R. W. (2000). "Residential radon gas exposure and lung cancer: The Iowa radon lung cancer study" (PDF). American Journal of Epidemiology. 151 (11): 1091–1102. PMID 10873134.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Journal on Landmark Radon Exposure Studies Co-edited By UI Researcher". University of Iowa. 2006-05-05. Retrieved 2008-06-26.
- ^ Krewski, D. (2005). "Residential radon and risk of lung cancer: A combined analysis of 7 North American case-control studies" (PDF). Epidemiology. 16 (2): 137–45. doi:10.1097/01.ede.0000152522.80261.e3.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Biermann, A.H. (1995-05-01). "Attachment of radon progeny to cigarette-smoke aerosols". Information Bridge. doi:10.2172/78555. Retrieved 2008-02-13.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Smith, B. J. (2007). "Iowa radon leukaemia study: a hierarchical population risk model for spatially correlated exposure measured with error". Statistical Medicine: 4619. doi:10.1002/sim.2884. PMID 17373673.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rericha, V. (2007). "Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study". Environmental Health Perspectives. 115 (4): A184–5. PMID 16759978.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Roscoe, R. J. (1989-08-04). "Lung cancer mortality among nonsmoking uranium miners exposed to radon daughters". Journal of the American Medical Association. 262 (5): 629–633. doi:10.1001/jama.262.5.629. PMID 2746814. Retrieved 2008-06-26.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Roscoe, R. J. (1995). "Mortality among Navajo uranium miners". American Journal of Public Health. 85 (4): 535–540. PMID 7702118.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Uranium Miners' Cancer". Time. 1960-12-26. ISSN 0040-718X. Retrieved 2008-06-26.
{{cite news}}: Check|issn=value (help); Check date values in:|date=(help) - ^ "Radon and Smoking". Radlab. Retrieved 2008-06-26.
- ^ Cohen, Bernard (July 1998). "Response to Criticisms of Smith Et Al". Health Physics. 75 (1): 23–28.
- ^ Puskin, JL (February 2004). "Smoking as a confounder in ecologic correlations of cancer mortality rates with average county radon levels". Health Physics. 86 (4): 526–532.
- ^ "A respectable end to radon debate needed". Vanderbilt University. Archived from the original on 2007-01-08.
- ^ Thompson, R.E.; Nelson, D.F.; Popkin, J.H.; Popkin, Z. (2008). "Case-control study of lung cancer risk from residential radon exposure in Worcester County, Massachusetts". The Radiation Safety Journal. 94 (3). Health Physics: 228–241. PMID 18301096.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Exposure to low levels of radon appears to reduce the risk of lung cancer, new study finds". PhysOrg.com. Retrieved 2008-06-26.
References
- Field, RW; Steck, DJ; Smith, BJ; et al. (June 2000). "Residential radon gas exposure and lung cancer: the Iowa Radon Lung Cancer Study". Oxford Journals. 151 (11): 1091–1102. PMID 10873134.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link)



