Acid dissociation constant

An acid dissociation constant, Ka, (also known as acidity constant, or acid-ionization constant) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions. The equilibrium can be written symbolically as
- HA ⇌ A− + H+.
HA is a generic acid which dissociates into A−, known as the conjugate base of the acid, and the hydrogen ion or proton, H+, which exists as a solvated hydronium ion in water. An acid which releases one proton, such as acetic acid, shown in the diagram, is known as a monoprotic acid. When such an acid is dissolved in water, the chemical species HA, A− and H+ are said to be in equilibrium when their concentrations do not change in with the passing of time. The equilibrium concentrations are denoted by [HA], [A−] and [H+] respectively. The dissociation constant is usually written as a quotient of these concentrations.
Strictly speaking it should be a quotient of activities, but when Ka values are determined in a medium of high ionic strength concentrations can be used in place of activities. The term acid dissociation constant is also used for pKa, which is equal to −log10 Ka. The larger the value of pKa, the smaller the extent of dissociation. A weak acid has a pKa value in the approximate range −2 to +12 in water. The extent of dissociation in solution can be determined by measuring the concentration of the hydrogen ion, for example, by means of a glass electrode which measures pH. Acids with a pKa value of less than about −2 are said to be strong acids; a strong acid is said to be completely dissociated in solution, as the extent of dissociation is so near to 100% that it cannot be measured experimentally. pKa values for strong acids can, however, be estimated by theoretical means.
A polyprotic acid is an acid, such as phosphoric acid or citric acid, which can release more than one proton. There is a pKa value corresponding to the release of each successive proton. The equilibrium constant for the dissociation of water into hydroxide ions and hydrogen ions, is given the special symbol Kw. At 25 °C pKw has an approximate value of 14. Values of pKb, the association constant for protonation of a base, have been published in the past. Current practice is to determine the pKa value of the acid conjugate to the base. The two values are related by pKa + pKb = pKw.
A solution containing both an acid and its conjugate base is called a buffer solution. When hydrogen ions from a strong acid are added to a buffer solution the pH changes by much less than it would have done if the same amount of acid had been added to an un-buffered solution. The reason for this is that, with the equilibrium HA ⇌ A− + H+, hydrogen ions are consumed in forming HA; the equilibrium shifts in favour of the species HA, in accordance with Le Chatelier's principle. The buffer region for a monoprotic acid is about pH = pKa ± 1.5. The buffer capacity of the mixture is negligible when |pH − pKa| is more than two. Polyprotic acids can have a more extended buffer region. For example the buffer region of citric acid spans more than 5 pH units.
pKa values can also be determined for acids dissolved in non-aqueous solvents such as acetonitrile and dimethyl sulphoxide. For compounds of pharmaceutical interest, mixed solvents such as water/dioxane are often used. The pKa value and ratio of concentrations of base and conjugate acid is used to define an acidity function; pH is the acidity function used when the solvent is water. pKa values can be experimentally determined by potentiometric (pH) titration, but for values of pKa less than about 2 or more than about 11 spectrophotometric or NMR measurements may be required. The latter methods are preferred with non-protic solvents.
pKa is proportional to the standard Gibbs free energy change for the reaction. While the standard enthalpy change for a weak acid dissociation reaction may be positive (endothermic reaction) or negative (exothermic reaction), the standard entropy change is always negative. pKa values for endothermic reactions increase with increasing temperature; the opposite is true for exothermic reactions. This is also in accord with Le Chatelier's principle.
Factors that determine the magnitude of pKa values include Pauling's rules for acidity constants and, for organic acids and bases, inductive effects and mesomeric effects; these effects are summarised in the Hammett equation. Structural effects, such as intra-molecular hydrogen bonding, can also be important.
The quantitative behaviour of acids and bases in solution can only be understood if their pKa values are known. For example, many compounds used for medication are weak acids or bases, so a knowledge of the pKa and log p values is essential for an understanding of the extent to which the compound enters the blood stream. There are many other applications, including aquatic chemistry, chemical oceanography, buffer solutions, acid-base homeostasis and enzyme kinetics. A knowledge of pKa values is also a prerequisite for a quantitative understanding of the interaction between acids or bases and metal ions to form complexes in solution.
Definitions
According to Arrhenius's original definition, an acid is a substance which dissociates in aqueous solution, releasing the hydrogen ion.[1]
- HA ⇌ A− + H+
The equilibrium constant for this "dissociation" reaction is known as a dissociation constant. However, since the liberated proton combines with a water molecule to give a hydronium ion (also called oxonium), Arrhenius later proposed that the "dissociation" reaction should be written as an acid–base reaction.
- HA + H2O ⇌ A− + H3O+
Brønsted and Lowry generalised this definition as a proton exchange reaction, as follows.[2][3][4]
- acid + base ⇌ conjugate base + conjugate acid
The acid donates a proton to the base. The conjugate base is what is left after the acid has lost a proton and the conjugate acid is created when the base gains a proton. For aqueous solutions an acid, HA, reacts with the base, water, donating a proton to it, creating the conjugate base, A−, and the conjugate acid, the hydronium ion. The Brønsted–Lowry definition is particularly useful when the solvent is a substance other than water, such as dimethyl sulfoxide; in that case the solvent, S, acts as a base, accepting a proton and forming the conjugate acid SH+. It also puts acids and bases on the same footing as being, respectively, donors or acceptors of protons. The conjugate acid, BH+, of a base, B, "dissociates" according to
- BH+ + OH− ⇌ B + H2O
which is the reverse of the equilibrium
- H2O (acid) + B (base) ⇌ OH− (conjugate base) + BH+ (conjugate acid)
Note that in this case the hydroxide ion is acting as the conjugate base of the acid water though it is normally considered to be a base in its own right; the designation of an acid or base as "conjugate" depends on context.
Examples:
- H2CO3 + H2O ⇌ HCO3− + H3O+
The bicarbonate ion is the conjugate base of the carbonic acid molecule.
- HCO3− + OH− ⇌ CO32− + H2O
and the bicarbonate ion is also the conjugate acid of the base, the carbonate ion. In fact the bicarbonate ion is amphiprotic, that is, it behaves as a base in the first example, and as an acid in the second example. These reactions are important for acid-base homeostasis in the human body. For chemical details on acid-base homeostasis see carbonic acid.
Any compound subject to an hydrolysis equilibrium can also be classed as a weak acid since, in hydrolysis, protons are produced by the splitting of water molecules. For example, the equilibrium
- B(OH)3 + 2 H2O ⇌ B(OH)4− + H3O+
shows why boric acid behaves as a weak acid even though it is not, itself, a proton donor. In a similar way, metal ion hydrolysis causes ions such as [Al(H2O)6]3+ to behave as weak acids.[5]
- [Al(H2O)6]3+ +H2O ⇌ [Al(H2O)5(OH)]2+ + H3O+
It is important to note that, in the context of solution chemistry, a "proton" is understood to mean a solvated hydrogen ion. In aqueous solution the "proton" is a solvated hydronium ion.[6][7] It is common to use H+ as an abbreviation for the solvated hydrogen ion, regardless of the solvent.
Equilibrium constant
An acid dissociation constant is a particular example of an equilibrium constant. For the specific equilibrium between a monoprotic acid, HA and its conjugate base A−, in water,
- HA + H2O ⇌ A− + H3O+
the thermodynamic equilibrium constant, K![]() can be defined by[8]
can be defined by[8]
where {A} is the activity of the chemical species A etc. K![]() is dimensionless since activity is dimensionless . Activities of the products are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient for a derivation of this expression.
is dimensionless since activity is dimensionless . Activities of the products are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient for a derivation of this expression.

Since activity is the product of concentration and activity coefficient (γ) the definition could also be written as
where [HA] represents the concentration of HA and Γ is a quotient of activity coefficients.
To avoid the complications involved in using activities, dissociation constants are determined, where possible, in a medium of high ionic strength, that is, under conditions in which Γ can be assumed to be always constant.[8] For example, the medium might be a solution of 0.1 M sodium nitrate or 3 M potassium perchlorate (1 M = 1 mol·dm−3, a unit of molar concentration). Furthermore, in all but the most concentrated solutions it can be assumed that the concentration of water, [H2O], is constant, approximately 55 mol·dm−3. On dividing K![]() by the constant terms and writing [H+] for the concentration of the hydronium ion the expression
by the constant terms and writing [H+] for the concentration of the hydronium ion the expression
is obtained. This is the definition in common use.[9] pKa is defined as −log10 Ka. Note, however, that all published dissociation constant values refer to the specific ionic medium used in their determination and that different values are obtained with different conditions, as shown for acetic acid in the illustration above. When published constants refer to an ionic strength other than the one required for a particular application, they may be adjusted by means of specific ion theory (SIT) and other theories.[10]
Although Ka appears to have the dimension of concentration it must in fact be dimensionless or it would not be possible to take its logarithm. The illusion is the result of omitting the constant term [H2O] from the defining expression. Nevertheless it is not unusual, particularly in texts relating to biochemical equilibria, to see a value quoted with a dimension as, for example, "Ka = 300 M".
Monoprotic acids
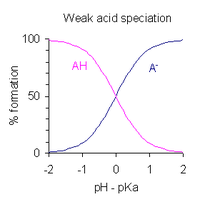
After rearranging the expression defining Ka, and putting pH = −log10[H+], one obtains
This is a form of the Henderson–Hasselbalch equation, from which the following conclusions can be drawn.
- At half-neutralization [AH]/[A−] = 1; since log(1) =0 , the pH at half-neutralization is numerically equal to pKa.
- The buffer region extends over the approximate range pKa ± 2, though buffering is weak outside the range pKa ± 1. At pKa ± 1, [AH]/[A−] = 10 or 1/10.
- If the pH is known, the ratio [AH]:[A−] may be calculated. This ratio is independent of the analytical concentration of the acid.
In water, measurable pKa values range from about −2 for a strong acid to about 12 for a very weak acid (or strong base). All acids with a pKa value of less than −2 are more than 99% dissociated at pH 0 (1 M acid). This is known as solvent leveling since all such acids are brought to the same level of being strong acids, regardless of their pKa values. Likewise, all bases with a pKa value larger than the upper limit are more than 99% de-protonated at all attainable pH values and are classified as strong bases.[3]
An example of a strong acid is hydrochloric acid, HCl, which has a pKa value, estimated from thermodynamic quantities, of −9.3 in water.[11] The concentration of undissociated acid in a 1 mol·dm−3 solution will be less than 0.01% of the concentrations of the products of dissociation. Hydrochloric acid is said to be "fully dissociated" in aqueous solution because the amount of undissociated acid is imperceptible. When the pKa and analytical concentration of the acid are known, the extent of dissociation and pH of a solution of a monoprotic acid can be easily calculated using an ICE table.
A buffer solution of a desired pH can be prepared as a mixture of a weak acid and its conjugate base. In practice the mixture can be created by dissolving the acid in water, and adding the requisite amount of strong acid or base. The pKa of the acid must be less than two units different from the target pH.
Polyprotic acids

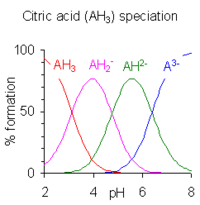
Polyprotic acids are acids that can lose more than one proton. The constant for dissociation of the first proton may be denoted as Ka1 and the constants for dissociation of successive protons as Ka2, etc.
When the difference between successive pK values is about four or more, each species may be considered as an acid in its own right;[12] the pH range of existence of each species is about pK± 2, so there is very little overlap between the ranges for successive species. The case of phosphoric acid illustrates this point. In fact salts of either H2PO4− or HPO42− may be crystallised from solution by adjustment of pH to either 4 or 10.
When the difference between successive pK values is less than about four there is overlap between the pH range of existence of the species in equilibrium. The smaller the difference, the more the overlap. The case of citric acid is shown at the right; solutions of citric acid are buffered over the whole range of pH 2.5 to 7.5.
It is generally true that successive pK values increase (Pauling's first rule).[13] For example, for a diprotic acid, H2A, the two equilibria are
- H2A ⇌ HA− + H+
- HA− ⇌ A2− + H+
it can be seen that the second proton is removed from a negatively charged species. Since the proton carries a positive charge extra work is needed to remove it; that is the cause of the trend noted above. Phosphoric acid, H3PO4 (values below) illustrates this rule, as does vanadic acid. When an exception to the rule is found it indicates that a major change in structure is occurring. In the case of VO2+(aq), the vanadium is octahedral, 6-coordinate, whereas all the other species are tetrahedral, 4-coordinate. This explains why pKa1 > pKa2 for vanadium(V) oxoacids.
VO2+⇌ H3VO4 + H+ pKa1 = 4.2 H3PO4 ⇌ H2PO4− + H+ pKa1 = 2.15 H3VO4 ⇌ H2VO4− + H+ pKa2 = 2.60 H2PO4− ⇌ HPO42− + H+ pKa2 = 7.20 H2VO4− ⇌ HVO42− + H+ pKa3 = 7.92 HPO42− ⇌ PO43− + H+ pKa3 = 12.37 HVO42− ⇌ VO43− + H+ pKa4 = 13.27
Water self-ionization
Water has both acidic and basic properties. The equilibrium constant for the equilibrium
- H2O ⇌ OH− + H+
is given by
When, as is usually the case, the concentration of water can be assumed to be constant, this expression simplifies to
The self-ionization constant of water, Kw, can thus be seen as a special case of an acid dissociation constant.
Bases
Historically the equilibrium constant Kb for a base was defined as the association constant for protonation of the base, B, to form the conjugate acid, HB+.
- B + H2O ⇌ HB+ + OH−
Using similar reasoning to that used before
In water, the concentration of the hydroxide ion, [OH−], is related to the concentration of the hydrogen ion by Kw = [H+][OH−], therefore
Substitution of the expression for [OH−] into the expression for Kb gives
It follows, taking cologarithms, that pKb = pKw − pKa. In aqueous solutions at 25 °C, pKw is 13.9965,[14] so pKb ~ 14 − pKa.
In effect there is no need to define pKb separately from pKa, but it is done here because pKb values can be found in the older literature.
Temperature dependence
All equilibrium constants vary with temperature according to the van 't Hoff equation[15]
R is the gas constant and T is the temperature in Kelvin. Thus, for exothermic reactions, (the standard enthalpy change, ΔH![]() , is negative) K decreases with temperature, but for endothermic reactions (ΔH
, is negative) K decreases with temperature, but for endothermic reactions (ΔH![]() is positive) K increases with temperature.
is positive) K increases with temperature.
Acidity in nonaqueous solutions
A solvent will be more likely to promote ionization of a dissolved acidic molecule in the following circumstances.[16]
- It is a protic solvent, capable of forming hydrogen bonds.
- It has a high donor number, making it a strong Lewis base.
- it has a high dielectric constant (relative permittivity), making it a good solvent for ionic species.
pKa values of organic compounds are often obtained using the aprotic solvents dimethyl sulfoxide (DMSO)[16] and acetonitrile (AN).[17]
| Solvent | Donor number[16] | Dielectric constant[16] |
|---|---|---|
| Acetonitrile | 14 | 37 |
| Dimethylsulfoxide | 30 | 47 |
| Water | 18 | 78 |
DMSO is widely used as an alternative to water because it has a lower dielectric constant than water, and is less polar and so dissolves non-polar, hydrophobic substances more easily. It has a measurable pKa range of about 1 to 30. Acetonitrile is less basic than DMSO and so acids are generally weaker and bases are generally stronger in this solvent. Some pKa values at 25oC for acetonitrile (AN)[18][19][20] and dimethyl sulfoxide (DMSO)[21] are shown in the following tables. Values for water are included for comparison.
| HA ⇌ A− + H+ | AN | DMSO | water |
|---|---|---|---|
| p-Toluenesulfonic acid | 8.5 | 0.9 | strong |
| 2,4-Dinitrophenol | 16.66 | 5.1 | 3.9 |
| Benzoic acid | 21.51 | 11.1 | 4.2 |
| Acetic acid | 23.51 | 12.6 | 4.756 |
| Phenol | 29.14 | 18.0 | 9.99 |
| BH+ ⇌ B + H+ | |||
| Pyrrolidine | 19.56 | 10.8 | 11.4 |
| Triethylamine | 18.82 | 9.0 | 10.72 |
| Proton sponge | 18.62 | 7.5 | 12.1 |
| Pyridine | 12.53 | 3.4 | 5.2 |
| Aniline | 10.62 | 3.6 | 9.4 |
Ionization of acids is less in an acidic solvent than in water. For example, hydrogen chloride is a weak acid when dissolved in acetic acid. This is because acetic acid is a much weaker base than water.
- HCl + CH3CO2H ⇌ Cl− + CH3C(OH)2+
- acid + base ⇌ conjugate base + conjugate acid
Compare this reaction with what happens when acetic acid is dissolved in the more acidic solvent pure sulphuric acid[22]
- H2SO4 + CH3CO2H ⇌ HSO4− + CH3C(OH)2+
The apparently unlikely geminal diol species CH3C(OH)2+ is stable in these environments. For aqueous solutions the pH scale is the most convenient acidity function.[23] Other acidity functions have been proposed for non-aqueous media, most notably the Hammett acidity function, H0, for superacid media and its modified version H− for superbasic media.[24]

In aprotic solvents, oligomers, such as the well-known acetic acid dimer, may be formed by hydrogen bonding. An acid may also form hydrogen bonds to its conjugate base. This process, known as homoconjugation, has the effect of enhancing the acidity of acids, lowering their effective pKa values, by stabilizing the conjugate base. Homoconjugation enhances the proton-donating power of toluenesulfonic acid in acetonitrile solution by a factor of nearly 800.[25] In aqueous solutions, homoconjugation does not occur, because water forms stronger hydrogen bonds to the conjugate base than does the acid.
Mixed solvents
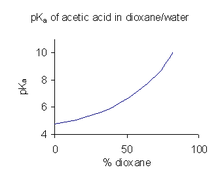
When a compound has limited solubility in water it is common practice (in the pharmaceutical industry, for example) to determine pKa values in a solvent mixture such as water/dioxane or water/methanol, in which the compound is more soluble.[27] In the example shown at the right, the pKa value rises steeply with increasing percentage of dioxane as the dielectric constant of the mixture is decreasing.
A pKa value obtained in a mixed solvent cannot be used directly for aqueous solutions. The reason for this is that when the solvent is in its standard state its activity is defined as one. For example, the standard state of water:dioxane 9:1 is precisely that solvent mixture, with no added solutes. To obtain the pKa value for use with aqueous solutions it has to be extrapolated to zero co-solvent concentration from values obtained from various co-solvent mixtures.
These facts are obscured by the omission of the solvent from the expression which is normally used to define pKa, but pKa values obtained in a given mixed solvent can be compared to each other, giving relative acid strengths. The same is true of pKa values obtained in a particular non-aqueous solvent such a DMSO.
As of 2008, a universal, solvent-independent, scale for acid dissociation constants has not been developed, since there is no known way to compare the standard states of two different solvents.
Factors which affect pKa values
Pauling's second rule states that the value of the first pKa for acids of the formula XOm(OH) n is approximately independent of n and X and is approximately 8 for m = 0, 2 for m = 1, −3 for m = 2 and < −10 for m = 3.[13] This correlates with the oxidation state of the central atom, X: the higher the oxidation state the stronger the oxyacid. For example, pKa for HClO is 7.2, for HClO2 is 2.0, for HClO3 is −1 and HClO4 is a strong acid.



With organic acids inductive effects and mesomeric effects affect the pKa values. A simple example is provided by the effect of replacing the hydrogen atoms in acetic acid by the more electronegative chlorine atom. The electron-withdrawing effect of the substituent makes ionisation easier, so successive pKa values decrease in the series 4.7, 2.8, 1.3 and 0.7 when 0,1, 2 or 3 chlorine atoms are present.[28] The Hammett equation, provides a general expression for the effect of substituents.[29]
- log Ka = log Ka0 + ρσ.
Ka is the dissociation constant of a substituted compound, Ka0 is the dissociation constant when the substituent is hydrogen, ρ is a property of the unsubstituted compound and σ has a particular value for each substituent. A plot of log Ka against σ is a straight line with intercept log Ka0 and slope ρ. This is an example of a linear free energy relationship as log Ka is proportional to the standard fee energy change. Hammett originally[30] formulated the relationship with data from benzoic acid with different substiuents in the ortho- and para- positions: some numerical values are in Hammett equation. This and other studies allowed substituents to be ordered according to their electron-withdrawing or electron-releasing power, and to distinguish between inductive and mesomeric effects.[31]
Alcohols do not normally behave as acids in water, but the presence of an double bond adjacent to the OH group can substantially decrease the pKa by the mechanism of keto-enol tautomerism. Ascorbic acid is an example of this effect. The diketone 2,4-pentanedione (acetylacetone) is also a weak acid because of the the keto-enol equilibrium. In aromatic compounds, such as phenol, which have an OH substituent, conjugation with the aromatic ring as a whole greatly increases the stability of the deprotonated form.
Structural effects can also be important. The difference between fumaric acid and maleic acid is a classic example. Fumaric acid is (E)-1,4-but-2-enedioic acid, a trans isomer, whereas maleic acid is the corresponding cis isomer, i.e. (Z)-1,4-but-2-enedioic acid (see cis-trans isomerism). Fumaric acid has pKa values of approximately 3.5 and 4.5. By contrast, maleic acid has pKa values of approximately 1.5 and 6.5. The reason for this large difference is that when one proton is removed from the cis- isomer (maleic acid) a strong intramolecular hydrogen bond is formed with the nearby remaining carboxyl group. This favors the formation of the maleate H+, and it opposes the removal of the second proton from that species. In the trans isomer, the two carboxyl groups are always far apart, so hydrogen bonding is not observed.[32]
Proton sponge, 1,8-bis(dimethylamino)naphthalene, has a pKa value of 12.1. It is one of the strongest amine bases known. The high basicity is attributed to the relief of strain upon protonation and strong internal hydrogen bonding.[33][34]
Thermodynamics
An equilibrium constant is related to the standard Gibbs free energy change for the reaction, so for an acid dissociation constant
R is the gas constant and T is the temperature in Kelvin. Note that pKa= −log Ka. At 25 °C ΔG![]() in kJ·mol−1 = 5.708 pKa (1 kJ·mol−1 = 1000 Joules per mole). Free energy is made up of an enthalpy term and an entropy term.[35]
in kJ·mol−1 = 5.708 pKa (1 kJ·mol−1 = 1000 Joules per mole). Free energy is made up of an enthalpy term and an entropy term.[35]
The standard enthalpy change can be determined by calorimetry or by using the van 't Hoff equation, though the calorimetric method is preferable. When both the standard enthalpy change and acid dissociation constant have been determined, the standard entropy change is easily calculated from the equation above. In the following table, the entropy terms are calculated from the experimental values of pKa and ΔH![]() . The data were critically selected and refer to 25 °C and zero ionic strength, in water.[35]
. The data were critically selected and refer to 25 °C and zero ionic strength, in water.[35]
| Compound | Equilibrium | pKa | ΔH |
−TΔS |
|---|---|---|---|---|
| HA = Acetic acid | HA ⇌ H+ + A− | 4.756 | −0.41 | 27.56 |
| H2A+ = GlycineH+ | H2A+ ⇌ HA + H+ | 2.351 | 4.00 | 9.419 |
| HA ⇌ H+ + A− | 9.78 | 44.20 | 11.6 | |
| H2A = Maleic acid | H2A ⇌ HA− + H+ | 1.92 | 1.10 | 9.85 |
| HA− ⇌ H+ + A2− | 6.27 | −3.60 | 39.4 | |
| H3A = Citric acid | H3A ⇌ H2A− + H+ | 3.128 | 4.07 | 13.78 |
| H2A− ⇌ HA2− + H+ | 4.76 | 2.23 | 24.9 | |
| HA2− ⇌ A3− + H+ | 6.40 | −3.38 | 39.9 | |
| HA = Boric acid | HA ⇌ H+ + A− | 9.237 | 13.80 | 38.92 |
| H3A = Phosphoric acid | H3A ⇌ H2A− + H+ | 2.148 | −8.00 | 20.26 |
| H2A− ⇌ HA2− + H+ | 7.20 | 3.60 | 37.5 | |
| HA2− ⇌ A3− + H+ | 12.35 | 16.00 | 54.49 | |
| HA− = Hydrogen sulphate | HA− ⇌ A2− + H+ | 1.99 | −22.40 | 33.74 |
| H2A = Oxalic acid | H2A ⇌ HA− + H+ | 1.27 | −3.90 | 11.15 |
| HA− ⇌ A2− + H+ | 4.266 | 7.00 | 31.35 |
| Compound | Equilibrium | pKa | ΔH |
−TΔS |
|---|---|---|---|---|
| B = Ammonia | HB+ ⇌ B + H+ | 9.245 | 51.95 | 0.8205 |
| B = Methylamine | HB+ ⇌ B + H+ | 10.645 | 55.34 | 5.422 |
| B = Triethylamine | HB+ ⇌ B + H+ | 10.72 | 43.13 | 18.06 |
The first point to note is that when pKa is positive, the standard free energy change for the dissociation reaction is also positive, that is, dissociation of a weak acid is not a spontaneous process. Secondly some reactions are exothermic and some are endothermic, but when ΔH![]() is negative −TΔS
is negative −TΔS![]() is the dominant factor which determines that ΔG
is the dominant factor which determines that ΔG![]() is positive. Lastly, the entropy contribution is always unfavourable in these reactions.
is positive. Lastly, the entropy contribution is always unfavourable in these reactions.
Note that the standard free energy change for the reaction is for the changes from the reactants in their standard states to the products in their standard states. The free energy change at equilibrium is zero since the chemical potentials of reactants and products are equal at equilibrium.
Experimental determination

The experimental determination of pKa values is commonly performed by means of titrations, in a medium of high ionic strength and at constant temperature.[36] A typical procedure would be as follows. A solution of the compound in the medium is acidified with a strong acid to the point where the compound is fully protonated. The solution is then titrated with a strong base until all the protons have been removed. At each point in the titration pH is measured using a glass electrode and a pH meter. The equilibrium constants are found by fitting calculated pH values to the observed values, using the method of least squares.[37]
The total volume of added strong base should be small compared to the initial volume of titrand solution in order to keep the ionic strength nearly constant. This will ensure that pKa remains invariant during the titration.
A calculated titration curve for oxalic acid is shown at the right. Oxalic acid has pKa values of 1.27 and 4.27. Therefore the buffer regions will be centered at about pH 1.3 and pH 4.3. The buffer regions carry the information necessary to get the pKa values as the concentrations of acid and conjugate base change along a buffer region.
Between the two buffer regions there is an end-point, or equivalence point, where the pH rises by about two units. This end-point is not sharp and is typical of a diprotic acid whose buffer regions overlap by a small amount: pKa2 − pKa1 is about three in this example. (If the difference in pK values were about two or less, the end-point would not be noticeable.) The second end-point begins at about pH 6.3 and is sharp. This indicates that all the protons have been removed. When this is so, the solution is not buffered and the pH rises steeply on addition of a small amount of strong base. However, the pH does not continue to rise indefinitely. A new buffer region begins at about pH 11 (pKw − 3), which is where self-ionization of water becomes important.
It is very difficult to measure pH values of less than two in aqueous solution with a glass electrode, because the Nernst equation breaks down at such low pH values. To determine pK values of less than about 2 or more than about 11 spectrophotometric[38] or NMR[9][39] measurements may be used instead of, or combined with, pH measurements.[40]
When the glass electrode cannot be employed, as with non-aqueous solutions, spectrophotometric methods are frequently used.[19]These may involve absorbance or fluorescence measurements. In both cases the measured quantity is assumed to be proportional to the the sum of contributions from each photo-active species; with absorbance measurements the Beer-Lambert law is assumed to apply.
Aqueous solutions with normal water cannot be used for 1H NMR measurements but heavy water, D2O, must be used instead. 13C NMR data, however, can be used with normal water and 1H NMR spectra can be used with non-aqueous media. The quantities measured with NMR are time-averaged chemical shifts, as proton exchange is fast on the NMR time-scale. Other chemical shifts, such as those of 31P can be measured.
Micro-constants

A base such as spermine has different sites where protonation can occur. In this example the first proton can go on the terminal -NH2 group, or either of the internal -NH- groups. The pKa values for dissociation of spermine protonated at one or other of the sites are examples of micro-constants. They cannot be determined directly by means of pH, absorbance, fluorescence or NMR measurements. Nevertheless, the site of protonation is very important for biological function, so mathematical methods have been developed for the determination of micro-constants.[41]
Applications and significance
A knowledge of pKa values is important for the quantitative treatment of systems involving acid–base equilibria in solution. Many applications exist in biochemistry; for example, the pKa values of proteins and amino acid side chains are of major importance for the activity of enzymes and the stability of proteins.[42] Protein pKa values cannot always be measured directly, but may be calculated using theoretical methods. Buffer solutions are used extensively to provide solutions at or near the physiological pH for the study of biochemical reactions;[43] the design of these solutions depends on a knowledge of the pKa values of their components. Important buffer solutions include MOPS, which provides a solution with pH 7.2, and tricine which is used in gel electrophoresis.[44][45] Buffering is an essential part of acid base physiology including acid-base homeostasis,[46] and is key to understanding disorders such as acid-base imbalance.[47][48][49] The isoelectric point of a given molecule is a function of its pK values, so different molecules have different isoelectric points. This permits a technique called isoelectric focussing,[50] which is used for separation of proteins by 2-D gel polyacrylamide gel electrophoresis.
Buffer solutions also play a key role in analytical chemistry. They are used whenever there is a need to fix the pH of a solution at a particular value. Compared with an aqueous solution, the pH of a buffer solution is relatively insensitive to the addition of a small amount of strong acid or strong base. The buffer capacity[51] of a simple buffer solution is largest when pH = pKa. In acid-base extraction, the efficiency of extraction of a compound into an organic phase, such as an ether, can be optimised by adjusting the pH of the aqueous phase using an appropriate buffer. At the optimum pH, the concentration of the electrically neutral species is maximised; such a species is more soluble in organic solvents having a low dielectric constant than it is in water. This technique is used for the purification of weak acids and bases.[52]
A pH indicator is a weak acid or weak base that changes colour in the transition pH range, which is approximately pKa ± 1. The design of a universal indicator requires a mixture of indicators whose adjacent pKa values differ by about two, so that their transition pH ranges just overlap.
In pharmacology ionization of a compound alters its physical behaviour and macro properties such as solubility and lipophilicity (log p). For example ionization of any compound will increase the solubility in water, but decrease the lipophilicity. This is exploited in drug development to increase the concentration of a compound in the blood by adjusting the pKa of an ionizable group.[53]
Knowledge of pKa values is important for the understanding of coordination complexes, which are formed by the interaction of a metal ion, Mm+, acting as a Lewis acid, with a ligand, L, acting as a Lewis base. However, the ligand may also undergo protonation reactions, so the formation of a complex in aqueous solution could be represented symbolically by the reaction
- [M(H2O)n]m+ +LH ⇌ [M(H2O)n−1L](m−1)+ + H3O+
To determine the equilibrium constant for this reaction, in which the ligand loses a proton, the pKa of the protonated ligand must be known. In practice, the ligand may be polyprotic; for example EDTA4− can accept four protons; in that case, all pKa values must be known. In addition, the metal ion is subject to hydrolysis, that is, it behaves as a weak acid, so the pK values for the hydrolysis reactions must also be known.[54] Assessing the hazard associated with an acid or base may require a knowledge of pKa values.[55] For example, hydrogen cyanide is a very toxic gas, because the cyanide ion inhibits the iron-containing enzyme cytochrome c oxidase. Hydrogen cyanide is a weak acid in aqueous solution with a pKa of about 9. In strongly alkaline solutions, above pH 11, say, it follows that sodium cyanide is "fully dissociated" so the hazard due to the hydrogen cyanide gas is much reduced. An acidic solution, on the other hand, is very hazardous because all the cyanide is in its acid form. Ingestion of cyanide by mouth is potentially fatal, independently of pH, because of the reaction with cytochrome c oxidase.
In environmental science acid–base equilibria are important for lakes[56] and rivers;[57][58] for example, humic acids are important components of natural waters. Another example occurs in chemical oceanography:[59] in order to quantify the solubility of iron(III) in seawater at various salinities, the pKa values for the formation of the iron(III) hydrolysis products Fe(OH)2+, Fe(OH)2+ and Fe(OH)3 were determined, along with the solubility product of iron hydroxide.[60]
Values for common substances
There are multiple techniques to determine the pKa of a chemical, leading to some discrepancies between different sources. Well measured values are typically within 0.1 units of each other. Data presented here was taken at 25 °C in water.[3][61] More values can be found in thermodynamics, above.
| Chemical Name | Equilibrium | pKa |
|---|---|---|
| B = Adenine | BH22+ ⇌ BH+ + H+ | 4.17 |
| BH+ ⇌ B + H+ | 9.65 | |
| H3A = Arsenic acid | H3A ⇌ H2A− + H+ | 2.22 |
| H2A− ⇌ HA2− + H+ | 6.98 | |
| HA2− ⇌ A3− + H+ | 11.53 | |
| HA = Benzoic acid | HA ⇌ H+ + A− | 4.204 |
| HA = Butanoic acid | HA ⇌ H+ + A− | 4.82 |
| H2A = Chromic acid | H2A ⇌ HA− + H+ | 0.98 |
| HA− ⇌ A2− + H+ | 6.5 | |
| B = Codeine | BH+ ⇌ B + H+ | 8.17 |
| HA = Cresol | HA ⇌ H+ + A− | 10.29 |
| HA = Formic acid | HA ⇌ H+ + A− | 3.751 |
| HA = Hydrofluoric acid | HA ⇌ H+ + A− | 3.17 |
| HA = Hydrocyanic acid | HA ⇌ H+ + A− | 9.21 |
| HA = Hydrogen selenide | HA ⇌ H+ + A− | 3.89 |
| HA = Hydrogen peroxide (90%) | HA ⇌ H+ + A− | 11.7 |
| HA = Lactic acid | HA ⇌ H+ + A− | 3.86 |
| HA = Propanoic acid | HA ⇌ H+ + A− | 4.87 |
| HA = Phenol | HA ⇌ H+ + A− | 9.99 |
| H2A = L-(+)-Ascorbic Acid | H2A ⇌ HA− + H+ | 4.17 |
| HA− ⇌ A2− + H+ | 11.57 |
See also
- Dissociation constant: general dissociation constants, including those for protein-ligand equilibria.
- Grotthuss mechanism: how protons are transferred between hydronium ions and water molecules, accounting for the exceptionally high ionic mobility of the proton (animation).
- Ocean acidification: dissolution of atmospheric carbon dioxide affects seawater pH. The reaction depends on total inorganic carbon and on solubility equilibria with solid carbonates such as limestone and dolomite.
- Proton affinity: a measure of basicity in the gas phase.
References
- ^ Miessler, G. (1991). Inorganic Chemistry (2nd ed.). Prentice Hall. ISBN 0134656598. Chapter 6: Acid-Base and Donor-Acceptor Chemistry
- ^ Bell, R.P. (1973). The Proton in Chemistry (2nd ed.). London: Chapman & Hall. Includes discussion of many organic Brønsted acids.
- ^ a b c
Shriver, D.F (1999). Inorganic Chemistry (3rd ed.). Oxford: Oxford University Press. ISBN 0198503318.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Chapter 5: Acids and Bases - ^
Housecroft, C.E. (2008). Inorganic Chemistry (3rd ed.). Prentice Hall. ISBN 0131755536.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Chapter 6: Acids, Bases and Ions in Aqueous Solution - ^ Burgess, J. (1978). Metal Ions in Solution. Ellis Horwood. ISBN 0853120277. Section 9.1 "Acidity of Solvated Cations" lists many pKa values.
- ^
Headrick, J.M. (2005). "Spectral Signatures of Hydrated Proton Vibrations in Water Clusters". Science. 308: 1765–69. doi:10.1126/science.1113094.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Smiechowski, M. (2006). "Proton hydration in aqueous solution: Fourier transform infrared studies of HDO spectra". J. Chem. Phys. 125: 204508–204522. doi:10.1063/1.2374891.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b
Rossotti, F.J.C. (1961). The Determination of Stability Constants. McGraw–Hill.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Chapter 2: Activity and Concentration Quotients - ^ a b
Popov, K. (2006). "Guidelines for NMR Measurements for Determination of High and Low pKa Values" (PDF). Pure Appl. Chem. 78 (3): 663–675. doi:10.1351/pac200678030663.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Project: Ionic Strength Corrections for Stability Constants". International Union of Pure and Applied Chemistry. Retrieved 2008-11-23.
- ^ Dasent, W.E. (1982). Inorganic Energetics: An Introduction. Cambridge University Press. ISBN 0521284066. Chapter 5
- ^
Brown, T.E. (2008). Chemistry: The Central Science (11th ed.). New York: Prentice-Hall. p. 689. ISBN 0136006175.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b
Greenwood, N.N. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. p. 50. ISBN 0-7506-3365-4.
{{cite book}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Lide, D.R. (2004). CRC Handbook of Chemistry and Physics, Student Edition (84th ed.). CRC Press. ISBN 0849305977. Section D–152
- ^
Atkins, P.W. (2006). Physical Chemistry. Oxford University Press. ISBN 0198700725.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Section 7.4: The Response of Equilibria to Temperature - ^ a b c d Template:Loudon p. 317–318
- ^
March, J. (2007). Advanced Organic Chemistry (6th ed.). New York: John Wiley & Sons. ISBN 978-0-471-72091-1.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Chapter 8: Acids and Bases - ^
Kütt, A. (2008). "Pentakis(trifluoromethyl)phenyl, a Sterically Crowded and Electron-withdrawing Group: Synthesis and Acidity of Pentakis(trifluoromethyl)benzene, -toluene, -phenol, and -aniline". J. Org. Chem. 73 (7): 2607–2620. doi:10.1021/jo702513w.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b
Kütt, A. (2006). "A Comprehensive Self-Consistent Spectrophotometric Acidity Scale of Neutral Brønsted Acids in Acetonitrile". J. Org. Chem. 71 (7): 2829–2838. doi:10.1021/jo060031y.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Kaljurand, I. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". J. Org. Chem. 70 (3): 1019–1028. doi:10.1021/jo048252w.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
"Bordwell pKa Table (Acidity in DMSO)". Retrieved 2008-11-2.
{{cite web}}: Check date values in:|accessdate=(help) - ^
Housecroft, C.E. (2008). Inorganic Chemistry (3rd ed.). Prentice Hall. ISBN 0131755536.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Chapter 8: Non-Aqueous Media - ^ Rochester, C.H. (1970). Acidity Functions. Academic Press. ISBN 0125908504.
- ^
Olah, G.A (1985). Superacids. New York: Wiley Interscience. ISBN 0471884693.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Coetzee, J.F. (1965). "Proton Acceptor Power and Homoconjugation of Mono- and Diamines". J. Amer. Chem. Soc. 87: 5005–5010. doi:10.1021/ja00950a006.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Pine, S.H. (1980). Organic chemistry. McGraw–Hill. p. 203. ISBN 0070501157.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Box, K.J. (2007). "Physicochemical Properties of a New Multicomponent Cosolvent System for the pKa Determination of Poorly Soluble Pharmaceutical Compounds". Helv. Chim. Acta. 90 (8): 1538–1553. doi:10.1002/hlca.200790161.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Pauling, L. (1960). The nature of the chemical bond and the structure of molecules and crystals; an introduction to modern structural chemistry (3rd ed.). Ithaca (NY): Cornell University Press. p. 277.
- ^
Pine, S.H. (1980). Organic Chemistry. McGraw–Hill. ISBN 0070501157.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Section 13-3: Quantitative Correlations of Substituent Effects (Part B) – The Hammett Equation - ^ Hammett, L.P. (1937). "The Effect of Structure upon the Reactions of Organic Compounds. Benzene Derivatives". J. Amer. Chem. Soc. 59 (1): 96–103. doi:10.1021/ja01280a022.
- ^
Hansch, C. (1991). "A Survey of Hammett Substituent Constants and Resonance and Field Parameters". Chem. Rev. 91 (2): 165–195. doi:10.1021/cr00002a004.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Pine, S.H. (1980). Organic chemistry. McGraw–Hill. ISBN 0070501157.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Section 6-2: Structural Effects on Acidity and Basicity - ^
Alder, R.W. (1968). "The Remarkable Basicity of 1,8-bis(dimethylamino)naphthalene". Chem. Commun.: 723. doi:10.1039/C19680000723.
{{cite journal}}: More than one of|pages=and|page=specified (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Alder, R.W. (1989). "Strain Effects on Amine Basicities". Chem. Rev. 89: 1215–1223. doi:10.1021/cr00095a015.
- ^ a b
Goldberg, R. (2002). "Thermodynamic Quantities for the Ionization Reactions of Buffers" (reprinted at NIST). J. Phys. Chem. Ref. Data. 31: 231&ndash370. doi:10.1063/1.1416902.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Martell, A.E. (1992). Determination and Use of Stability Constants. Wiley. ISBN 0471188174.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Chapter 4: Experimental Procedure for Potentiometric p[H] Measurement of Metal Complex Equilibria - ^ Leggett, D.J. (1985). Computational Methods for the Determination of Formation Constants. Plenum. ISBN 0306419572.
- ^
Allen, R.I. (1998). "Multiwavelength Spectrophotometric Determination of Acid Dissociation Constants of Ionizable Drugs". J. Pharm. Biomed. Anal. 17 (4–5): 699–641. doi:10.1016/S0731-7085(98)00010-7.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Szakács, Z. (2004). "Accurate Determination of Low pK Values by 1H NMR Titration". Talanta. 62: 819–825. doi:10.1016/j.talanta.2003.10.007.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Box, K.J. (2008). "The Chemistry of Multi-Protic Drugs Part 1: A Potentiometric, Multi-Vavelength UV and NMR pH Titrimetric Study of the Micro-Speciation of SKI-606". J. Pharm. Biomed. Anal. 47 (2): 303–311. doi:10.1016/j.jpba.2008.01.015.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Frassineti, C. (2003). "Determination of Protonation Constants of Some Fluorinated Polyamines by Means of 13C NMR Data Processed by the New Computer Program HypNMR2000. Protonation Sequence in Polyamines". Anal. Bioanal. Chem. 376: 1041–1052. doi:10.1007/s00216-003-2020-0.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Onufriev, A. (2001). "A Novel View of pH Titration in Biomolecules". Biochemistry. 40: 3413–3419. doi:10.1021/bi002740q.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Good, N.E. (1966). "Hydrogen Ion Buffers for Biological Research". Biochemistry. 5 (2): 467–477. doi:10.1021/bi00866a011.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Dunn, M.J. (1993). Gel Electrophoresis: Proteins. Bios Scientific Publishers. ISBN 187274821X.
- ^ Martin, R. (1996). Gel Electrophoresis: Nucleic Acids. Bios Scientific Publishers. ISBN 1872748287.
- ^
Brenner, B.M. (Editor) (1979). Acid–Base and Potassium Homeostasis. Churchill Livingstone. ISBN 0443080178.
{{cite book}}:|first=has generic name (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Scorpio, R. (2000). Fundamentals of Acids, Bases, Buffers & Their Application to Biochemical Systems. Kendall/Hunt Pub. Co. ISBN 0787273740.
- ^
Beynon, R.J. (1996). Buffer Solutions : The Basics. Oxford: Oxford University Press. ISBN 0199634424.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Perrin, D.D. (1974). Buffers for pH and Metal Ion Control. London: Chapman & Hall. ISBN 0412117002.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Garfin, D.(Editor) (2005). Handbook of Isoelectric Focusing and Proteomics. Vol. 7. Elsevier. ISBN 0120887525U.
{{cite book}}:|first=has generic name (help); Check|isbn=value: invalid character (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hulanicki, A. (1987). Reactions of Acids and Bases in Analytical Chemistry. Masson, M.R. (translation editor). Horwood. ISBN 0853123306.
- ^ Eyal, A.M (1997). "Acid Extraction by Acid–Base-Coupled Extractants". Ion Exchange and Solvent Extraction: A Series of Advances. 13: 31–94.
- ^ Avdeef, A. (2003). Absorption and Drug Development : Solubility, Permeability, and Charge State. New York: Wiley. ISBN 0471423653.
- ^
Beck, M.T. (1990). Chemistry of Complex Equilibria. Horwood. ISBN 0853121435.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
van Leeuwen, C.J. (1995). Risk Assessment of Chemicals: An Introduction. Springer. pp. 254–255. ISBN 0792337409.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Skoog, D.A (2004). Fundamentals of Analytical Chemistry (8th ed.). Thomson Brooks/Cole. ISBN 0-03-035523-0.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Chapter 9-6: Acid Rain and the Buffer Capacity of Lakes - ^
Stumm, W. (1996). Water Chemistry. New York: Wiley. ISBN 0471051969.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Snoeyink, V.L. (1980). Aquatic Chemistry : Chemical Equilibria and Rates in Natural Waters. New York: Wiley. ISBN 0471511854.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Millero, F.J. (2006). Chemical Oceanography (3rd ed.). London: Taylor and Francis. ISBN 0849322804.
- ^
Millero, F.J. (2002). "The Solubility of Iron in Seawater". Marine chemistry (journal). 77: 43–54. doi:10.1016/S0304-4203(01)00074-3.
{{cite journal}}: Text "Marine chemistry" ignored (help) - ^ Speight, J.G. (2005). Lange's Handbook of Chemistry (18th ed.). McGraw–Hill. ISBN 0071432205. Chapter 8
Further reading
- Albert, A. (1971). The Determination of Ionization Constants : A Laboratory Manual. Chapman & Hall. ISBN 0412103001.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) (Previous edition published as Ionization constants of acids and bases. London (UK): Methuen. 1962.) - Atkins, P.W. (2008). Chemical Principles: The Quest for Insight (4th ed.). W.H. Freeman. ISBN 1-4292-0965-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Housecroft, C.E. (2008). Inorganic chemistry (3rd ed.). Prentice Hall. ISBN 0131755536.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) (Non-aqueous solvents) - Hulanicki, A. (1987). Reactions of Acids and Bases in Analytical Chemistry. Horwood. ISBN 0853123306. (translation editor: Mary R. Masson)
- Perrin, D.D. (1981). pKa Prediction for Organic Acids and Bases. Chapman & Hall. ISBN 041222190x.
{{cite book}}: Check|isbn=value: invalid character (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Reichardt, C. (2003). Solvents and Solvent Effects in Organic Chemistry (3rd ed.). Wiley-VCH. ISBN 3-527-30618-8. Chapter 4: Solvent Effects on the Position of Homogeneous Chemical Equilibria.
- Skoog, D.A. (2004). Fundamentals of Analytical Chemistry (8th ed.). Thomson Brooks/Cole. ISBN 0-03-035523-0.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
External links
- Extensive bibliography of pKa values in DMSO, acetonitrile, THF, heptane, 1,2-dichloroethane, and in the gas phase.
- All-in-one freeware for pH and acid-base equilibrium calculations and for simulation and analysis of potentiometric titration curves with spreadsheets.
- Includes a database with aqueous, non-aqueous, and gaseous phase pKa values than can be searched using SMILES or CAS registry numbers.
- pKa values for various acid and bases. Includes a table of some solubility products.
- Explanations of the relevance of these properties to pharmacology.

![{\displaystyle K_{a}=\mathrm {\frac {[A^{-}][H^{+}]}{[HA]}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/37fee2397d5fc10ab2c3f28a582dc6de5cdeace7)

![{\displaystyle K^{\ominus }=\mathrm {{\frac {[A^{-}][H_{3}O^{+}]}{[HA][H_{2}O]}}\times {\frac {\gamma _{A^{-}}\gamma _{H_{3}O^{+}}}{\gamma _{HA}\gamma _{H_{2}O}}}=\mathrm {\frac {[A^{-}][H_{3}O^{+}]}{[HA][H_{2}O]}} \times \Gamma } }](https://wikimedia.org/api/rest_v1/media/math/render/svg/98e5acdd208e3a6eda8213278e88c22d19e4c509)
![{\displaystyle \mathrm {pH} =\mathrm {p} K_{a}-\log \mathrm {\frac {[AH]}{[A^{-}]}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/9cf5a57966759cb7cbd83001c3fc4e86675e6b62)
![{\displaystyle K_{a}=\mathrm {\frac {[H^{+}][OH^{-}]}{[H_{2}O]}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/2dd5bfcd8413a2432c473f300660f4c941de09ac)
![{\displaystyle K_{w}=[{\rm {H^{+}}}][{\rm {OH^{-}}}]\,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/51bffdb360859fbdb465b66b42f97cc577e9f3b0)
![{\displaystyle K_{b}=\mathrm {\frac {[HB^{+}][OH^{-}]}{[B]}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/d9cafabde37373b5a99bb241863d8df489b5c0ab)
![{\displaystyle \mathrm {[OH^{-}]={\frac {{\mathit {K}}_{w}}{[H^{+}]}}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/4b2408060cd7e3bba253ab76904682b76a60ce35)
![{\displaystyle \mathrm {{\mathit {K}}_{b}={\frac {[HB^{+}]{\mathit {K}}_{w}}{[B][H^{+}]}}={\frac {{\mathit {K}}_{w}}{{\mathit {K}}_{a}}}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/e277c21d34e3e789b3a804e157a1dacc7ba9cc57)
