Hemoglobin
| Hemoglobin, human, adult | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (heterotetramer, (αβ)2) | |||||||||||||
 Structure of human hemoglobin. The protein's α and β subunits are in red and blue, and the iron-containing heme groups in green. From PDB: 1GZX Proteopedia Hemoglobin | |||||||||||||
| Protein type | metalloprotein, globulin | ||||||||||||
| Function | oxygen-transport | ||||||||||||
| Cofactor(s) | heme (4) | ||||||||||||
| |||||||||||||
Hemoglobin (also spelled haemoglobin and abbreviated Hb or Hgb) is the iron-containing oxygen-transport metalloprotein in the red blood cells of vertebrates[1] and the tissues of some invertebrates. Hemoglobin in the blood is what transports oxygen from the lungs or gills to the rest of the body (i.e. the tissues) where it releases the oxygen for cell use.
In mammals the protein makes up about 97% of the red blood cell's dry content, and around 35% of the total content (including water)[citation needed]. Hemoglobin has an oxygen binding capacity between 1.36 and 1.37 ml O2 per gram of hemoglobin,[2] which increases the total blood oxygen capacity seventyfold.[3]
Hemoglobin is involved in the transport of other gases: it carries some of the body's respiratory carbon dioxide (about 10% of the total) as carbaminohemoglobin, in which CO2 is bound to the globin protein. The molecule also carries the important regulatory molecule nitric oxide bound to a globin protein thiol group, releasing it at the same time as oxygen.[4]
Hemoglobin is also found outside red blood cells and their progenitor lines. Other cells that contain hemoglobin include the A9 dopaminergic neurons in the substantia nigra, macrophages, alveolar cells, and mesangial cells in the kidney. In these tissues, hemoglobin has a non-oxygen-carrying function as an antioxidant and a regulator of iron metabolism.[5]
Hemoglobin and hemoglobin-like molecules are also found in many invertebrates, fungi, and plants. In these organisms, hemoglobins may carry oxygen, or they may act to transport and regulate other species such as carbon dioxide, nitric oxide, hydrogen sulfide and sulfide. A variant of the molecule, called leghemoglobin, is used to scavenge oxygen, to keep it from poisoning anaerobic systems, such as nitrogen-fixing nodules of leguminous plants.
Research history
The oxygen-carrying protein hemoglobin was discovered by Hünefeld in 1840.[6] In 1851,[7] Otto Funke published a series of articles in which he described growing hemoglobin crystals by successively diluting red blood cells with a solvent such as pure water, alcohol or ether, followed by slow evaporation of the solvent from the resulting protein solution.[8] Hemoglobin's reversible oxygenation was described a few years later by Felix Hoppe-Seyler.[9]
In 1959 Max Perutz determined the molecular structure of hemoglobin by X-ray crystallography.[10][11] This work resulted in his sharing with John Kendrew the 1962 Nobel Prize in Chemistry.
The role of hemoglobin in the blood was elucidated by physiologist Claude Bernard. The name hemoglobin is derived from the words heme and globin, reflecting the fact that each subunit of hemoglobin is a globular protein with an embedded heme (or haem) group. Each heme group contains one iron atom, that can bind one oxygen molecule through ion-induced dipole forces. The most common type of hemoglobin in mammals contains four such subunits.
Genetics
Hemoglobin consists mostly of protein (the "globin" chains), and these proteins, in turn, are composed of sequences of amino acids. These sequences are linear, in the manner of letters in a written sentence or beads on a string. In all proteins, it is the variation in the type of amino acids in the protein sequence of amino acids, which determine the protein's chemical properties and function. This is true of hemoglobin, where the sequence of amino acids may affect crucial functions such as the protein's affinity for oxygen.
There is more than one hemoglobin gene. The amino acid sequences of the globin proteins in hemoglobins usually differ between species, although the differences grow with the evolutionary distance between species. For example, the most common hemoglobin sequences in humans and chimpanzees are nearly identical, differing by only one amino acid in both the alpha and the beta globin protein chains. These differences grow larger between less closely related species.
Even within a species, different variants of hemoglobin always exist, although one sequence is usually a "most common" one in each species. Mutations in the genes for the hemoglobin protein in a species result in hemoglobin variants.[12][13] Many of these mutant forms of hemoglobin cause no disease. Some of these mutant forms of hemoglobin, however, cause a group of hereditary diseases termed the hemoglobinopathies. The best known hemoglobinopathy is sickle-cell disease, which was the first human disease whose mechanism was understood at the molecular level. A (mostly) separate set of diseases called thalassemias involves underproduction of normal and sometimes abnormal hemoglobins, through problems and mutations in globin gene regulation. All these diseases produce anemia.[14]
Variations in hemoglobin amino acid sequences, as with other proteins, may be adaptive. For example, recent studies have suggested genetic variants in deer mice that help explain how deer mice that live in the mountains are able to survive in the thin air that accompanies high altitudes. A researcher from the University of Nebraska-Lincoln found mutations in four different genes that can account for differences between deer mice that live in lowland prairies versus the mountains. After examining wild mice captured from both highlands and lowlands, it was found that: the genes of the two breeds are “virtually identical–except for those that govern the oxygen-carrying capacity of their hemoglobin”. “The genetic difference enables highland mice to make more efficient use of their oxygen”, since less is available at higher altitudes, such as those in the mountains.[15] Mammoth hemoglobin featured mutations that allowed for oxygen delivery at lower temperatures, thus enabling mammoths to migrate to higher latitudes during the Pleistocene.[16]
Synthesis
Hemoglobin (Hb) is synthesized in a complex series of steps. The heme part is synthesized in a series of steps in the mitochondria and the cytosol of immature red blood cells, while the globin protein parts are synthesized by ribosomes in the cytosol.[17] Production of Hb continues in the cell throughout its early development from the proerythroblast to the reticulocyte in the bone marrow. At this point, the nucleus is lost in mammalian red blood cells, but not in birds and many other species. Even after the loss of the nucleus in mammals, residual ribosomal RNA allows further synthesis of Hb until the reticulocyte loses its RNA soon after entering the vasculature (this hemoglobin-synthetic RNA in fact gives the reticulocyte its reticulated appearance and name).
Structure

Hemoglobin exhibits characteristics of both the tertiary and quaternary structures of proteins.[18] Most of the amino acids in hemoglobin form alpha helices, connected by short non-helical segments. Hydrogen bonds stabilize the helical sections inside this protein, causing attractions within the molecule, folding each polypeptide chain into a specific shape.[19] Hemoglobin's quaternary structure comes from its four subunits in roughly a tetrahedral arrangement.[18]
In most humans, the hemoglobin molecule is an assembly of four globular protein subunits. Each subunit is composed of a protein chain tightly associated with a non-protein heme group. Each protein chain arranges into a set of alpha-helix structural segments connected together in a globin fold arrangement, so called because this arrangement is the same folding motif used in other heme/globin proteins such as myoglobin.[20][21] This folding pattern contains a pocket that strongly binds the heme group.
A heme group consists of an iron (Fe) ion (charged atom) held in a heterocyclic ring, known as a porphyrin. This porphyrin ring consists of four pyrrole molecules cyclically linked together (by methene bridges) with the iron ion bound in the center.[22] The iron ion, which is the site of oxygen binding, coordinates with the four nitrogens in the center of the ring, which all lie in one plane. The iron is bound strongly to the globular protein via the imidazole ring of the F8 histidine residue below the porphyrin ring. A sixth position can reversibly bind oxygen by a coordinate covalent bond,[23] completing the octahedral group of six ligands. Oxygen binds in an "end-on bent" geometry where one oxygen atom binds Fe and the other protrudes at an angle. When oxygen is not bound, a very weakly bonded water molecule fills the site, forming a distorted octahedron.
Even though carbon dioxide is carried by hemoglobin, it does not compete with oxygen for the iron-binding positions, but is actually bound to the protein chains of the structure.
The iron ion may be either in the Fe2+ or in the Fe3+ state, but ferrihemoglobin (methemoglobin) (Fe3+) cannot bind oxygen.[24] In binding, oxygen temporarily and reversibly oxidizes (Fe2+) to (Fe3+) while oxygen temporally turns into superoxide, thus iron must exist in the +2 oxidation state to bind oxygen. If superoxide ion associated to Fe3+ is protonated the hemoglobin iron will remain oxidized and incapable to bind oxygen. In such cases, the enzyme methemoglobin reductase will be able to eventually reactivate methemoglobin by reducing the iron center.
In adult humans, the most common hemoglobin type is a tetramer (which contains 4 subunit proteins) called hemoglobin A, consisting of two α and two β subunits non-covalently bound, each made of 141 and 146 amino acid residues, respectively. This is denoted as α2β2. The subunits are structurally similar and about the same size. Each subunit has a molecular weight of about 17,000 daltons, for a total molecular weight of the tetramer of about 68,000 daltons (64,458 g/mol).[25] Thus, 1 g/dL = 0.01551 mmol/L. Hemoglobin A is the most intensively studied of the hemoglobin molecules.
In human infants, the hemoglobin molecule is made up of 2 α chains and 2 gamma chains. The gamma chains are gradually replaced by β chains as the infant grows.[26]
The four polypeptide chains are bound to each other by salt bridges, hydrogen bonds, and hydrophobic interactions. There are two kinds of contacts between the α and β chains: α1β1 and α1β2.
In general, hemoglobin can be saturated with oxygen molecules (oxyhemoglobin), or desaturated with oxygen molecules (deoxyhemoglobin).[27] Oxyhemoglobin is formed during physiological respiration when oxygen binds to the heme component of the protein hemoglobin in red blood cells. This process occurs in the pulmonary capillaries adjacent to the alveoli of the lungs. The oxygen then travels through the blood stream to be dropped off at cells where it is utilized in aerobic glycolysis and in the production of ATP by the process of oxidative phosphorylation. It does not, however, help to counteract a decrease in blood pH. Ventilation, or breathing, may reverse this condition by removal of carbon dioxide, thus causing a shift up in pH.[28]
Hemoglobin exists in 2 forms, A Taut form (T) and a Relaxed form (R). Various factors like low pH, high CO2 and high 2,3 BPG at the level of the tissues favours the taut form which has low oxygen affinity and releases oxygen in the tissues. The opposite of these aformentioned factors at the level of the lung capillaries favour the relaxed form which can better bind Oxygen.
Deoxyhemoglobin is the form of hemoglobin without the bound oxygen. The absorption spectra of oxyhemoglobin and deoxyhemoglobin differ. The oxyhemoglobin has significantly lower absorption of the 660 nm wavelength than deoxyhemoglobin, while at 940 nm its absorption is slightly higher. This accounts for hemoglobin's red color and deoxyhemoglobin's blue color. This difference is used for measurement of the amount of oxygen in patient's blood by an instrument called pulse oximeter.
Iron's oxidation state in oxyhemoglobin
Assigning oxygenated hemoglobin's oxidation state is difficult because oxyhemoglobin (Hb-O2), by experimental measurement, is diamagnetic (no net unpaired electrons), yet the low-energy electron configurations in both oxygen and iron are paramagnetic (suggesting at least one unpaired electron in the complex). The lowest-energy form of oxygen, and the lowest energy forms of the relevant oxidation states of iron, are these:
- Triplet oxygen, the lowest energy molecular oxygen species, has two unpaired electrons in antibonding π* molecular orbitals.
- Iron(II) tends to exist in a high-spin configuration where unpaired electrons exist in Eg antibonding orbitals.
- Iron(III) has an odd number of electrons, and thus must have one or more unpaired electrons, in any energy state.
All of these structures are paramagnetic (have unpaired electrons), not diamagnetic. Thus, a non-intuitive (e.g., a higher-energy for at least one species) distribution of electrons in the combination of iron and oxygen must exist, in order to explain the observed diamagnetism and no unpaired electrons.
The three logical possibilities to produce diamagnetic (no net spin) Hb-O2 are:
- Low-spin Fe2+ binds to singlet oxygen. Both low-spin iron and singlet oxygen are diamagnetic. However, the singlet form of oxygen is the higher-energy form of the molecule.
- Low-spin Fe3+ binds to .O2- (the superoxide ion) and the two unpaired electrons couple antiferromagnetically, giving diamagnetic properties.
- Low-spin Fe4+ binds to peroxide, O22-. Both are diamagnetic.
Direct experimental data:
- X-ray photoelectron spectroscopy suggests iron has an oxidation state of approximately 3.2
- infrared stretching frequencies of the O-O bond suggests a bond length fitting with superoxide (a bond order of about 1.6, with superoxide being 1.5).
Thus, the nearest formal oxidation state of iron in Hb-O2 is the +3 state, with oxygen in the -1 state (as superoxide .O2-). The diamagnetism in this configuration arises from the single unpaired electron on superoxide aligning antiferromagnetically from the single unpaired electron on iron, to give no net spin to the entire configuration, in accordance with diamagnetic oxyhemoglobin from experiment.[29][30]
The second choice of the three logical possibilities above for diamagnetic oxyhemoglobin being found correct by experiment, is not surprising: singlet oxygen (possibility #1) and large separations of charge (possibility #3) are both unfavorably high-energy states. Iron's shift to a higher oxidation state in Hb-O2 decreases the atom's size, and allows it into the plane of the porphyrin ring, pulling on the coordinated histidine residue and initiating the allosteric changes seen in the globulins.
Early postulates by bio-inorganic chemists claimed that possibility #1 (above) was correct and that iron should exist in oxidation state II. This seemed particularly likely since the iron oxidation state III as methemoglobin, when not accompanied by superoxide .O2- to "hold" the oxidation electron, was known to render hemoglobin incapable of binding normal triplet O2 as it occurs in the air. It was thus assumed that iron remained as Fe(II) when oxygen gas was bound in the lungs. The iron chemistry in this previous classical model was elegant, but the required presence of the required diamagnetic high-energy singlet oxygen was never explained. It was classically argued that the binding of an oxygen molecule placed high-spin iron(II) in an octahedral field of strong-field ligands; this change in field would increase the crystal field splitting energy, causing iron's electrons to pair into the low-spin configuration, which would be diamagnetic in Fe(II). This forced low-spin pairing is indeed thought to happen in iron when oxygen binds, but is not enough to explain iron's change in size. Extraction of an additional electron from iron by oxygen is required to explain both iron's smaller size and observed increased oxidation state, and oxygen's weaker bond.
It should be noted that the assignment of a whole-number oxidation state is a formalism, as the covalent bonds are not required to have perfect bond orders involving whole electron-transfer. Thus, all three models for paramagnetic Hb-O2 may contribute to some small degree (by resonance) to the actual electronic configuration of Hb-O2. However, the model of iron in Hb-O2 being Fe(III) is more correct than the classical idea that it remains Fe(II).
Binding for ligands other than oxygen
Besides the oxygen ligand, which binds to hemoglobin in a cooperative manner, hemoglobin ligands also include competitive inhibitors such as carbon monoxide (CO) and allosteric ligands such as carbon dioxide (CO2) and nitric oxide. The carbon dioxide is bound to amino groups of the globin proteins as carbaminohemoglobin, and is thought to account for about 10% of carbon dioxide transport in mammals. Nitric oxide is bound to specific thiol groups in the globin protein to form an S-nitrosothiol which dissociates into free nitrix oxide and thiol again, as the hemoglobin releases oxygen from its heme site. This nitric oxide transport to peripheral tissues thus helps to regulate oxygen use in tissues, by releasing vasodilatory nitric oxide to tissues in which oxygen levels are low.[4]
Cooperative
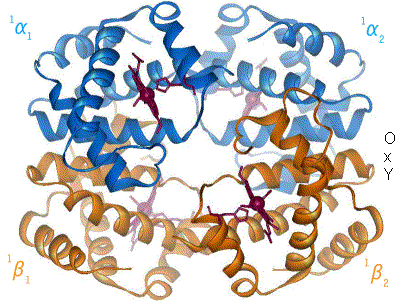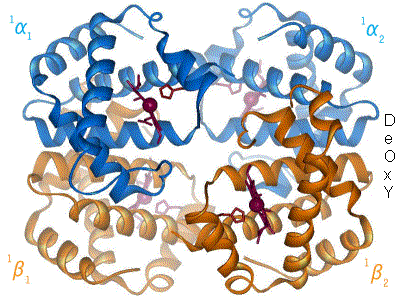
When oxygen binds to the iron complex, it causes the iron atom to move back toward the center of the plane of the porphyrin ring (see moving diagram). At the same time, the imidazole side-chain of the histidine residue interacting at the other pole of the iron is pulled toward the porphyrin ring. This interaction forces the plane of the ring sideways toward the outside of the tetramer, and also induces a strain in the protein helix containing the histidine as it moves nearer to the iron atom. This strain is transmitted to the remaining three monomers in the tetramer, where it induces a similar conformational change in the other heme sites such that binding of oxygen to these sites becomes easier.
In the tetrameric form of normal adult hemoglobin, the binding of oxygen is, thus, a cooperative process. The binding affinity of hemoglobin for oxygen is increased by the oxygen saturation of the molecule, with the first oxygens bound influencing the shape of the binding sites for the next oxygens, in a way favorable for binding. This positive cooperative binding is achieved through steric conformational changes of the hemoglobin protein complex as discussed above; i.e., when one subunit protein in hemoglobin becomes oxygenated, a conformational or structural change in the whole complex is initiated, causing the other subunits to gain an increased affinity for oxygen. As a consequence, the oxygen binding curve of hemoglobin is sigmoidal, or S-shaped, as opposed to the normal hyperbolic curve associated with noncooperative binding.
The dynamic mechanism of the cooperativity in hemoglobin and its relation with the low-frequency resonance has been discussed.[31]
Competitive
Hemoglobin's oxygen-binding capacity is decreased in the presence of carbon monoxide because both gases compete for the same binding sites on hemoglobin, carbon monoxide binding preferentially in place of oxygen.
The binding of oxygen is affected by molecules such as carbon monoxide (CO) (for example, from tobacco smoking, car exhaust, and incomplete combustion in furnaces). CO competes with oxygen at the heme binding site. Hemoglobin binding affinity for CO is 200 times greater than its affinity for oxygen,[32] meaning that small amounts of CO dramatically reduce hemoglobin's ability to transport oxygen. When hemoglobin combines with CO, it forms a very bright red compound called carboxyhemoglobin, which may cause the skin of CO poisoning victims to appear pink in death, instead of white or blue. When inspired air contains CO levels as low as 0.02%, headache and nausea occur; if the CO concentration is increased to 0.1%, unconsciousness will follow. In heavy smokers, up to 20% of the oxygen-active sites can be blocked by CO.
In similar fashion, hemoglobin also has competitive binding affinity for cyanide (CN-), sulfur monoxide (SO), nitrogen dioxide (NO2), and sulfide(S2-), including hydrogen sulfide (H2S). All of these bind to iron in heme without changing its oxidation state, but they nevertheless inhibit oxygen-binding, causing grave toxicity.
The iron atom in the heme group must initially be in the ferrous (Fe2+) oxidation state to support oxygen and other gases' binding and transport (it temporarily switches to ferric during the time oxygen is bound, as explained above). Initial oxidation to the ferric (Fe3+) state without oxygen converts hemoglobin into "hemiglobin" or methemoglobin (pronounced "MET-hemoglobin"), which cannot bind oxygen. Hemoglobin in normal red blood cells is protected by a reduction system to keep this from happening. Nitrogen dioxide and nitrous oxide are capable of converting a small fraction of hemoglobin to methemoglobin; however, this is not usually of medical importance (nitrogen dioxide is poisonous by other mechanisms, and nitrous oxide is routinely used in surgical anesthesia in most people without undue methemoglobin buildup).
Allosteric
Carbon dioxide occupies a different binding site on the hemoglobin. Carbon dioxide is more readily dissolved in deoxygenated blood, facilitating its removal from the body after the oxygen has been released to tissues undergoing metabolism. This increased affinity for carbon dioxide by the venous blood is known as the Haldane effect. Through the enzyme carbonic anhydrase, carbon dioxide reacts with water to give carbonic acid, which decomposes into bicarbonate and protons:
- CO2 + H2O → H2CO3 → HCO3- + H+

Hence blood with high carbon dioxide levels is also lower in pH (more acidic). Hemoglobin can bind protons and carbon dioxide, which causes a conformational change in the protein and facilitates the release of oxygen. Protons bind at various places on the protein, while carbon dioxide binds at the α-amino group.[33] Carbon dioxide binds to hemoglobin and forms carbaminohemoglobin.[34] This decrease in hemoglobin's affinity for oxygen by the binding of carbon dioxide and acid is known as the Bohr effect (shifts the O2-saturation curve to the right). Conversely, when the carbon dioxide levels in the blood decrease (i.e., in the lung capillaries), carbon dioxide and protons are released from hemoglobin, increasing the oxygen affinity of the protein.
It is necessary for hemoglobin to release the oxygen that it binds; if not, there is no point in binding it. The sigmoidal curve of hemoglobin makes it efficient in binding (taking up O2 in lungs), and efficient in unloading (unloading O2 in tissues).[35]
In people acclimated to high altitudes, the concentration of 2,3-Bisphosphoglycerate (2,3-BPG) in the blood is increased, which allows these individuals to deliver a larger amount of oxygen to tissues under conditions of lower oxygen tension. This phenomenon, where molecule Y affects the binding of molecule X to a transport molecule Z, is called a heterotropic allosteric effect.
A variant hemoglobin, called fetal hemoglobin (HbF, α2γ2), is found in the developing fetus, and binds oxygen with greater affinity than adult hemoglobin. This means that the oxygen binding curve for fetal hemoglobin is left-shifted (i.e., a higher percentage of hemoglobin has oxygen bound to it at lower oxygen tension), in comparison to that of adult hemoglobin. As a result, fetal blood in the placenta is able to take oxygen from maternal blood.
Hemoglobin also carries nitric oxide in the globin part of the molecule. This improves oxygen delivery in the periphery and contributes to the control of respiration. NO binds reversibly to a specific cysteine residue in globin; the binding depends on the state (R or T) of the hemoglobin. The resulting S-nitrosylated hemoglobin influences various NO-related activities such as the control of vascular resistance, blood pressure and respiration. NO is not released in the cytoplasm of erythrocytes but transported by an anion exchanger called AE1 out of them.[36]
A study was performed to examine the influence of the form of hemoglobin (Hb) on the partitioning of inhaled volatile organic compounds (VOCs) into [human and animal] blood. Benzene was the prototypic VOC used in the investigations for this research due to the similar properties it shares with many other VOCs. To be specific, this study analyses the influence of the water solubility of Hb on the partitioning coefficient (PC) of a VOC as compared to the influence of the “species” or form of Hb. The different forms of blood used include: human hemoglobin (HbA), rat Hb, and sickle-cell hemoglobin (HbS). Rat Hb contains little water and is in a quasi-crystalline form, found inside the red blood cells (RBC), meaning they are more hydrophobic than human Hb, which are water-soluble. Sickle-cell hemoglobin (HbS) is water-soluble, however it can become water-insoluble, forming hydrophobic polymers, when deoxygenated. The findings state that the benzene PC for rat Hb was much higher than human that for Hb; however, the tests that measured the PCs of the oxygenated and deoxygenated forms of HbA and HbS did not differ, indicating that the affinity of benzene was not affected by the water solubility of Hb.[37]
Types in humans
Hemoglobin variants are a part of the normal embryonic and fetal development, but may also be pathologic mutant forms of hemoglobin in a population, caused by variations in genetics. Some well-known hemoglobin variants such as sickle-cell anemia are responsible for diseases, and are considered hemoglobinopathies. Other variants cause no detectable pathology, and are thus considered non-pathological variants.[38][39]
In the embryo:
In the fetus:
- Hemoglobin F (α2γ2) (PDB: 1FDH)
In adults:
- Hemoglobin A (α2β2) (PDB: 1BZ0) - The most common with a normal amount over 95%
- Hemoglobin A2 (α2δ2) - δ chain synthesis begins late in the third trimester and in adults, it has a normal range of 1.5-3.5%
- Hemoglobin F (α2γ2) - In adults Hemoglobin F is restricted to a limited population of red cells called F-cells. However, the level of Hb F can be elevated in persons with sickle-cell disease and beta-thalassemia.
Variant forms that cause disease:
- Hemoglobin H (β4) - A variant form of hemoglobin, formed by a tetramer of β chains, which may be present in variants of α thalassemia.
- Hemoglobin Barts (γ4) - A variant form of hemoglobin, formed by a tetramer of γ chains, which may be present in variants of α thalassemia.
- Hemoglobin S (α2βS2) - A variant form of hemoglobin found in people with sickle cell disease. There is a variation in the β-chain gene, causing a change in the properties of hemoglobin, which results in sickling of red blood cells.
- Hemoglobin C (α2βC2) - Another variant due to a variation in the β-chain gene. This variant causes a mild chronic hemolytic anemia.
- Hemoglobin E (α2βE2) - Another variant due to a variation in the β-chain gene. This variant causes a mild chronic hemolytic anemia.
- Hemoglobin AS - A heterozygous form causing Sickle cell trait with one adult gene and one sickle cell disease gene
- Hemoglobin SC disease - Another heterozygous form with one sickle gene and another encoding Hemoglobin C.
Degradation in vertebrate animals
When red cells reach the end of their life due to aging or defects, they are broken down, the hemoglobin molecule is broken up and the iron gets recycled. When the porphyrin ring is broken up, the fragments are normally secreted in the bile by the liver. This process also produces one molecule of carbon monoxide for every molecule of heme degraded.[40] This is one of the few natural sources of carbon monoxide production in the human body, and is responsible for the normal blood levels of carbon monoxide even in people breathing pure air. The other major final product of heme degradation is bilirubin. Increased levels of this chemical are detected in the blood if red cells are being destroyed more rapidly than usual. Improperly degraded hemoglobin protein or hemoglobin that has been released from the blood cells too rapidly can clog small blood vessels, especially the delicate blood filtering vessels of the kidneys, causing kidney damage.
Role in disease
Hemoglobin deficiency can be caused either by decreased amount of hemoglobin molecules, as in anemia, or by decreased ability of each molecule to bind oxygen at the same partial pressure of oxygen. Hemoglobinopathies (genetic defects resulting in abnormal structure of the hemoglobin molecule)[41] may cause both. In any case, hemoglobin deficiency decreases blood oxygen-carrying capacity. Hemoglobin deficiency is, in general, strictly distinguished from hypoxemia, defined as decreased partial pressure of oxygen in blood,[42][43][44][45] although both are causes of hypoxia (insufficient oxygen supply to tissues).
Other common causes of low hemoglobin include loss of blood, nutritional deficiency, bone marrow problems, chemotherapy, kidney failure, or abnormal hemoglobin (such as that of sickle-cell disease).
High hemoglobin levels may be caused by exposure to high altitudes, smoking, dehydration, or tumors.[46]
The ability of each hemoglobin molecule to carry oxygen is normally modified by altered blood pH or CO2, causing an altered oxygen-hemoglobin dissociation curve. However, it can also be pathologically altered in, e.g., carbon monoxide poisoning.
Decrease of hemoglobin, with or without an absolute decrease of red blood cells, leads to symptoms of anemia. Anemia has many different causes, although iron deficiency and its resultant iron deficiency anemia are the most common causes in the Western world. As absence of iron decreases heme synthesis, red blood cells in iron deficiency anemia are hypochromic (lacking the red hemoglobin pigment) and microcytic (smaller than normal). Other anemias are rarer. In hemolysis (accelerated breakdown of red blood cells), associated jaundice is caused by the hemoglobin metabolite bilirubin, and the circulating hemoglobin can cause renal failure.
Some mutations in the globin chain are associated with the hemoglobinopathies, such as sickle-cell disease and thalassemia. Other mutations, as discussed at the beginning of the article, are benign and are referred to merely as hemoglobin variants.
There is a group of genetic disorders, known as the porphyrias that are characterized by errors in metabolic pathways of heme synthesis. King George III of the United Kingdom was probably the most famous porphyria sufferer.
To a small extent, hemoglobin A slowly combines with glucose at the terminal valine (an alpha aminoacid) of each β chain. The resulting molecule is often referred to as Hb A1c. As the concentration of glucose in the blood increases, the percentage of Hb A that turns into Hb A1c increases. In diabetics whose glucose usually runs high, the percent Hb A1c also runs high. Because of the slow rate of Hb A combination with glucose, the Hb A1c percentage is representative of glucose level in the blood averaged over a longer time (the half-life of red blood cells, which is typically 50–55 days).
Glycosylated hemoglobin is the form of hemoglobin to which glucose is bound. The binding of glucose to amino acids in the hemoglobin takes place spontaneously (without the help of an enzyme) in many proteins, and is not known to serve a useful purpose. However, the binding to hemoglobin does serve as a record for average blood glucose levels over the life time of red cells, which is approximately 120 days. The levels of glycosylated hemoglobin are therefore measured in order to monitor the long-term control of the chronic disease of type 2 diabetes mellitus (T2DM). Poor control of T2DM results in high levels of glycosylated hemoglobin in the red blood cells. The normal reference range is approximately 4–5.9 %. Though difficult to obtain, values less than 7 % are recommended for people with T2DM. Levels greater than 9 % are associated with poor control of the glycosylated hemoglobin, and levels greater than 12 % are associated with very poor control. Diabetics who keep their glycosylated hemoglobin levels close to 7 % have a much better chance of avoiding the complications that may accompany diabetes (than those whose levels are 8 % or higher).[47]
Elevated levels of hemoglobin are associated with increased numbers or sizes of red blood cells, called polycythemia. This elevation may be caused by congenital heart disease, cor pulmonale, pulmonary fibrosis, too much erythropoietin, or polycythemia vera.[48]
Elevation in levels of hemoglobin were found in one study of the yogic practice of Yoga Nidra (yogic sleep) for half an hour daily.[49]
Diagnostic uses
Hemoglobin concentration measurement is among the most commonly performed blood tests, usually as part of a complete blood count. For example it is typically tested before or after blood donation. Results are reported in g/L, g/dL or mol/L. 1 g/dL equals about 0.6206 mmol/L.[50] Normal levels are:
- Men: 13.8 to 18.2 g/dL (138 to 182 g/L, or 8.56 to 11.3 mmol/L)
- Women: 12.1 to 15.1 g/dL (121 to 151 g/L, or 7.51 to 9.37 mmol/L)
- Children: 11 to 16 g/dL (111 to 160 g/L, or 6.83 to 9.93 mmol/L)
- Pregnant women: 11 to 12 g/dL (110 to 120 g/L, or 6.83 to 7.45 mmol/L) [51][52]
Normal values of hemoglobin in the 1st and 3rd trimesters of pregnant women must be at least 11 g/dL and at least 10.5 g/dL during the 2nd trimester.[53]
If the concentration is below normal, this is called anemia. Anemias are classified by the size of red blood cells, the cells that contain hemoglobin in vertebrates. The anemia is called "microcytic" if red cells are small, "macrocytic" if they are large, and "normocytic" otherwise.
Hematocrit, the proportion of blood volume occupied by red blood cells, is typically about three times the hemoglobin level. For example, if the hemoglobin is measured at 17, that compares with a hematocrit of 51.[54]
Long-term control of blood sugar concentration can be measured by the concentration of Hb A1c. Measuring it directly would require many samples because blood sugar levels vary widely through the day. Hb A1c is the product of the irreversible reaction of hemoglobin A with glucose. A higher glucose concentration results in more Hb A1c. Because the reaction is slow, the Hb A1c proportion represents glucose level in blood averaged over the half-life of red blood cells, is typically 50–55 days. An Hb A1c proportion of 6.0% or less show good long-term glucose control, while values above 7.0% are elevated. This test is especially useful for diabetics.[55]
The functional magnetic resonance imaging (fMRI) machine uses the signal from deoxyhemoglobin, which is sensitive to magnetic fields since it is paramagnetic.
Analogues in non-vertebrate organisms
A variety of oxygen-transport and -binding proteins exist in organisms throughout the animal and plant kingdoms. Organisms including bacteria, protozoans, and fungi all have hemoglobin-like proteins whose known and predicted roles include the reversible binding of gaseous ligands. Since many of these proteins contain globins and the heme moiety (iron in a flat porphyrin support), they are often called hemoglobins, even if their overall tertiary structure is very different from that of vertebrate hemoglobin. In particular, the distinction of “myoglobin” and hemoglobin in lower animals is often impossible, because some of these organisms do not contain muscles. Or, they may have a recognizable separate circulatory system but not one that deals with oxygen transport (for example, many insects and other arthropods). In all these groups, heme/globin-containing molecules (even monomeric globin ones) that deal with gas-binding are referred to as oxyhemoglobins. In addition to dealing with transport and sensing of oxygen, they may also deal with NO, CO2, sulfide compounds, and even O2 scavenging in environments that must be anaerobic. They may even deal with detoxification of chlorinated materials in a way analogous to heme-containing P450 enzymes and peroxidases.

The structure of hemoglobins varies across species. Hemoglobin occurs in all kingdoms of organisms, but not in all organisms. Primitive species such as bacteria, protozoa, algae, and plants often have single-globin hemoglobins. Many nematode worms, molluscs, and crustaceans contain very large multisubunit molecules, much larger than those in vertebrates. In particular, chimeric hemoglobins found in fungi and giant annelids may contain both globin and other types of proteins.[56]
One of the most striking occurrences and uses of hemoglobin in organisms is in the giant tube worm (Riftia pachyptila, also called Vestimentifera), which can reach 2.4 meters length and populates ocean volcanic vents. Instead of a digestive tract, these worms contain a population of bacteria constituting half the organism's weight. The bacteria react with H2S from the vent and O2 from the water to produce energy to make food from H2O and CO2. The worms end with a deep red fan-like structure ("plume"), which extends into the water and absorbs H2S and O2 for the bacteria, and CO2 for use as synthetic raw material similar to photosynthetic plants. The structures are bright-red due to their containing several extraordinarily complex hemoglobins that have up to 144 globin chains, each including associated heme structures. These hemoglobins are remarkable for being able to carry oxygen in the presence of sulfide, and even to carry sulfide, without being completely "poisoned" or inhibited by it as hemoglobins in most other species are.[57][58]
Other oxygen-binding proteins
Myoglobin: Found in the muscle tissue of many vertebrates, including humans, it gives muscle tissue a distinct red or dark gray color. It is very similar to hemoglobin in structure and sequence, but is not a tetramer; instead, it is a monomer that lacks cooperative binding. It is used to store oxygen rather than transport it.
Hemocyanin: The second most common oxygen-transporting protein found in nature, it is found in the blood of many arthropods and molluscs. Uses copper prosthetic groups instead of iron heme groups and is blue in color when oxygenated.
Hemerythrin: Some marine invertebrates and a few species of annelid use this iron-containing non-heme protein to carry oxygen in their blood. Appears pink/violet when oxygenated, clear when not.
Chlorocruorin: Found in many annelids, it is very similar to erythrocruorin, but the heme group is significantly different in structure. Appears green when deoxygenated and red when oxygenated.
Vanabins: Also known as vanadium chromagens, they are found in the blood of sea squirts. There were once hypothesized to use the rare metal vanadium as an oxygen binding prosthetic group. However, although they do contain vandadium by preference, they apparently bind little oxygen, and thus have some other function, which has not been elucidated (sea squirts also contain some hemoglobin). They may act as toxins.
Erythrocruorin: Found in many annelids, including earthworms, it is a giant free-floating blood protein containing many dozens—possibly hundreds—of iron- and heme-bearing protein subunits bound together into a single protein complex with a molecular mass greater than 3.5 million daltons.
Pinnaglobin: Only seen in the mollusc Pinna squamosa. Brown manganese-based porphyrin protein.
Leghemoglobin: In leguminous plants, such as alfalfa or soybeans, the nitrogen fixing bacteria in the roots are protected from oxygen by this iron heme containing oxygen-binding protein. The specific enzyme protected is nitrogenase, which is unable to reduce nitrogen gas in the presence of free oxygen.
Coboglobin: A synthetic cobalt-based porphyrin. Coboprotein would appear colorless when oxygenated, but yellow when in veins.
Presence in nonerythroid cells
Some nonerythroid cells (i.e., cells other than the red blood cell line) contain hemoglobin. In the brain, these include the A9 dopaminergic neurons in the substantia nigra, astrocytes in the cerebral cortex and hippocampus, and in all mature oligodendrocytes.[5] It has been suggested that brain hemoglobin in these cell may enable the "storage of oxygen to provide a homeostatic mechanism in anoxic conditions, which is especially important for A9 DA neurons that have an elevated metabolism with a high requirement for energy production".[5] It has been noted further that "A9 dopaminergic neurons may be at particular risk since in addition to their high mitochondrial activity they are under intense oxidative stress caused by the production of hydrogen peroxide via autoxidation and/or monoamine oxidase (MAO)-mediated deamination of dopamine and the subsequent reaction of accessible ferrous iron to generate highly toxic hydroxyl radicals".[5] This may explain the risk of these cells for degeneration in Parkinson's disease.[5] The presence of iron from hemoglobin in these cells also results in the post-mortem darkness of these cells, which is the origin of the Latin name, substantia nigra.
Outside the brain, hemoglobin has non-oxygen-carrying functions as an antioxidant and a regulator of iron metabolism in macrophages,[59] alveolar cells,[60] and mesangial cells in the kidney.[61]
In history, art and music

Historically, the color of blood was associated with rust, as ancient Romans associated the planet Mars with the god of war since Mars is orange-red. The color of Mars is due to iron-oxygen in the Martian soil, but the red in blood is not due to the iron in hemoglobin and its oxides, which is a common misconception. The red is due to the porphyrin moiety of hemoglobin to which the iron is bound, not the iron itself,[62] although the ligation and redox state of the iron can influence the pi to pi* electronic transitions of the porphyrin and hence its optical characteristics.

Artist Julian Voss-Andreae created a sculpture called "Heart of Steel (Hemoglobin)" in 2005, based on the protein's backbone. The sculpture was made from glass and weathering steel. The intentional rusting of the initially shiny work of art mirrors hemoglobin's fundamental chemical reaction of oxygen binding to iron.[63]
Rock band Placebo recorded a song called "Haemoglobin" with the lyrics "Haemoglobin is the key to a healthy heartbeat".
See also
- Proteopedia Hemoglobin
- Chlorophyll
- Globin fold
- Hemocyanin
- Hemoprotein
- Sickle-cell disease
- Complete Blood Count
Hemoglobin variants:
Hemoglobin protein subunits (genes):
Hemoglobin compounds:
- Carbaminohemoglobin (with carbon dioxide, colored blue)
- Carboxyhemoglobin (with carbon monoxide, colored cherry-red)
- Oxyhemoglobin (with diatomic oxygen, colored blood-red)
References
- ^ Maton, Anthea (1993). Human Biology and Health. Englewood Cliffs, New Jersey, USA: Prentice Hall. ISBN 0-13-981176-1.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Dominguez de Villota ED, Ruiz Carmona MT, Rubio JJ, de Andrés S (1981). "Equality of the in vivo and in vitro oxygen-binding capacity of haemoglobin in patients with severe respiratory disease". Br J Anaesth. 53 (12): 1325–8. doi:10.1093/bja/53.12.1325. ISSN 0007-0912. PMID 7317251.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Costanzo, Linda S. (2007). Physiology. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7311-3.
- ^ a b Respiratory Function of Hemoglobin. Connie C.W. Hsia, M.D. N Engl J Med 1998; 338:239-248 January 22, 1998
- ^ a b c d e Biagioli M, Pinto M, Cesselli D, Zaninello M, Lazarevic D, Roncaglia P, Simone R, Vlachouli C, Plessy C, Bertin N, Beltrami A, Kobayashi K, Gallo V, Santoro C, Ferrer I, Rivella S, Beltrami CA, Carninci P, Raviola E, Gustincich S. (2009). Unexpected expression of {alpha}- and {beta}-globin in mesencephalic dopaminergic neurons and glial cells. Proc Natl Acad Sci U S A. 106:15454–15459. PMID 19717439 doi:10.1073/pnas.0813216106
- ^ Hünefeld F.L. (1840). "Die Chemismus in der thierischen Organization". Leipzig.
{{cite journal}}: Cite journal requires|journal=(help); Invalid|ref=harv(help) - ^ Funke O (1851). "Über das milzvenenblut". Z Rat Med. 1: 172–218.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Invalid|ref=harv(help) - ^ "A NASA Recipe For Protein Crystallography" (PDF). Educational Brief. National Aeronautics and Space Administration. Retrieved 2008-10-12.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Hoppe-Seyler F (1866). "Über die oxydation in lebendem blute". Med-chem Untersuch Lab. 1: 133–140.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Invalid|ref=harv(help) - ^ Perutz, M.F.; Rossmann, M.G.; Cullis, A.F.; Muirhead, H.; Will, G.; North, A.C.T. (1960). "Structure of H". Nature. 185 (4711): 416–422. doi:10.1038/185416a0. PMID 18990801.
{{cite journal}}: Invalid|ref=harv(help) - ^ Perutz MF (1960). "Structure of haemoglobin". Brookhaven symposia in biology. 13: 165–83. ISSN 0068-2799. PMID 13734651.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help) - ^ A Syllabus of Human Hemoglobin Variants (1996)
- ^ Hemoglobin Variants
- ^ Uthman, MD, Ed. "Hemoglobinopathies and Thalassemias". Retrieved 2007-12-26.
- ^ Reed, Leslie. "Adaptation found in mouse genes." Omaha World-Herald 11 Aug. 2009: EBSCO. Web. 30 Oct. 2009.
- ^ "Mammoths had ′anti-freeze′ blood". BBC. 2010-05-02. Retrieved 2010-05-02.
- ^ "Hemoglobin Synthesis". April 14, 2002. Retrieved 2007-12-26.
- ^ a b van Kessel et al. "2.4 Proteins - Natural Polyamides." Chemistry 12. Toronto: Nelson, 2003. 122. Print.
- ^ "Hemoglobin Tutorial." University of Massachusetts Amherst. N.p., n.d. Web. 23 Oct. 2009. <http://www.umass.edu/molvis/tutorials/hemoglobin/index.htm>.
- ^ Steinberg 2001, p. 95
- ^ Hardison 1996, p. 1
- ^ "Hemoglobin." School of Chemistry - Bristol University - UK. N.p., n.d. Web. 12 Oct. 2009. <http://www.chm.bris.ac.uk/motm/hemoglobin/hemoglobjm.htm>.
- ^ WikiPremed > Coordination Chemistry Retrieved on July 2, 2009
- ^ Linberg R, Conover CD, Shum KL, Shorr RG (1998). "Hemoglobin based oxygen carriers: how much methemoglobin is too much?". Artif Cells Blood Substit Immobil Biotechnol. 26 (2): 133–48. doi:10.3109/10731199809119772. ISSN 1073-1199. PMID 9564432.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG (2001). "Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle" (Free full text). J Appl Physiol. 90 (2): 511–519. ISSN 8750-7587. PMID 11160049.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Hemoglobin." MedicineNet. N.p., n.d. Web. 12 Oct. 2009. <www.medicinenet.com/hemoglobin/article.htm>.
- ^ "Hemoglobin Home." Biology @ Davidson. N.p., n.d. Web. 12 Oct. 2009. <http://www.bio.davidson.edu/Courses/Molbio/MolStudents/spring2005/Heiner/hemoglobin.html>.
- ^ Baillie/Simpson. "Online model of the haemoglobin binding and the effects of hyperventilation". Retrieved 2006-08-10.
- ^ Childs PE (2001). "Haemoglobin - a molecular lung: 2". Chemistry in Action (65). ISSN 0332-2637.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Invalid|ref=harv(help) - ^ Chen H, Ikeda-Saito M, Shaik S (2008). "Nature of the Fe-O2 bonding in oxy-myoglobin: effect of the protein". Journal of the American Chemical Society. 130 (44): 14778–14790. doi:10.1021/ja805434m. PMID 18847206.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Chou KC (1989). "Low-frequency resonance and cooperativity of hemoglobin". Trends Biochem. Sci. 14 (6): 212–3. doi:10.1016/0968-0004(89)90026-1. PMID 2763333.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Guyton A C: Medical Physiology 11ed. 2005, page 509
- ^ Nelson, D. L.; Cox, M. M. (2000). Lehninger Principles of Biochemistry, 3rd ed. New York, NY: Worth Publishers. p 217
- ^ Guyton, Arthur C. (2006). Textbook of Medical Physiology (11 ed.). Philadelphia: Elsevier Saunders. p. 511. ISBN 0721602401.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "YouTube - Lecture - 12 Myoglobin and Hemoglobin." YouTube - Broadcast Yourself.. N.p., n.d. Web. 30 Oct. 2009. <http://www.youtube.com/watch?v=6AfRX6oh9-E>.
- ^ Rang, H.P. (2003). Pharmacology, Fifth Edition. Elsevier. ISBN 0443072027.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Wiester et al. "Partitioning of Benzene in Blood: Influence of Hemoglobin Type in Humans and Animals." Environmental Health Perspectives 110.3 (2002): p255-261. EBSCO. Web. 1 Nov. 2009.
- ^ "Hemoglobin Variants". Lab Tests Online. American Association for Clinical Chemistry. 2007-11-10. Retrieved 2008-10-12.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Huisman THJ (1996). "A Syllabus of Human Hemoglobin Variants". Globin Gene Server. Pennsylvania State University. Retrieved 2008-10-12.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Johnson RA, Lavesa M, Askari B, Abraham NG, Nasjletti A (1995). "A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats". Hypertension. 25 (2): 166–9. ISSN 0194-911X. PMID 7843765. Retrieved 2008-10-12.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "hemoglobinopathy" at Dorland's Medical Dictionary
- ^ britannica.com --> blood disease, stating hypoxemia (reduced oxygen tension in the blood). Retrieved on May 25, 2009
- ^ Biology-Online.org --> Dictionary » H » Hypoxemia last modified 00:05, 29 December 2008
- ^ Page 430 -> Pathophysiology of acute respiratory failure in Trauma By William C. Wilson, Christopher M. Grande, David B. Hoyt Edition: illustrated Published by CRC Press, 2007 ISBN 0-8247-2920-X, 9780824729202 1384 pages
- ^ Hazards of hypoxemia: How to protect your patient from low oxygen levels In Nursing , May 1996 by McGaffigan, Patricia A
- ^ "Hemoglobin." MedicineNet. N.p., n.d. Web. 12 Oct. 2009. <www.medicinenet.com/hemoglobin/article.htm>.
- ^ "Definition of Glycosylated Hemoglobin." Medicine Net. N.p., n.d. Web. 12 Oct. 2009. <www.medterms.com/script/main/art.asp?articlekey=16295>.
- ^ Hemoglobin at Medline Plus
- ^ Kumar, Dr. Kamakhya; The Healing Sleep, Yoga, Mind Body Spirit; Yoga Magazine, 26 York Street London, Vol. 50 page 42-44.
- ^ http://www.unc.edu/~rowlett/units/scales/clinical_data.html
- ^ Hemoglobin Level Test
- ^ Although other sources can have slightly differing values, such as http://www.gpnotebook.co.uk/simplepage.cfm?ID=1026883654
- ^ Murray S.S. & McKinney E.S.(2006). Foundations of Maternal-Newborn Nursing.(4th ed., p 919).Philadelphia: Saunders Elsevier
- ^ "Hematocrit (HCT) or Packed Cell Volume (PCV)". DoctorsLounge.com. Retrieved 2007-12-26.
- ^ This Hb A1c level is only useful in individuals who have red blood cells (RBCs) with normal survivals (i.e., normal half-life). In individuals with abnormal RBCs, whether due to abnormal hemoglobin molecules (such as Hemoglobin S in Sickle Cell Anemia) or RBC membrane defects - or other problems, the RBC half-life is frequently shortened. In these individuals, an alternative test called "fructosamine level" can be used. It measures the degree of glycation (glucose binding) to albumin, the most common blood protein, and reflects average blood glucose levels over the previous 18-21 days, which is the half-life of albumin molecules in the circulation.
- ^ Weber RE, Vinogradov SN (2001). "Nonvertebrate hemoglobins: functions and molecular adaptations" (Free full text). Physiol. Rev. 81 (2): 569–628. ISSN 0031-9333. PMID 11274340.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help) - ^ Zal F, Lallier FH, Green BN, Vinogradov SN, Toulmond A (1996). "The multi-hemoglobin system of the hydrothermal vent tube worm Riftia pachyptila. II. Complete polypeptide chain composition investigated by maximum entropy analysis of mass spectra" (Free full text). J. Biol. Chem. 271 (15): 8875–81. ISSN 0021-9258. PMID 8621529.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Minic Z, Hervé G (2004). "Biochemical and enzymological aspects of the symbiosis between the deep-sea tubeworm Riftia pachyptila and its bacterial endosymbiont". Eur. J. Biochem. 271 (15): 3093–102. doi:10.1111/j.1432-1033.2004.04248.x. ISSN 0014-2956. PMID 15265029.
{{cite journal}}:|format=requires|url=(help); Invalid|ref=harv(help); Unknown parameter|month=ignored (help) - ^ Liu L, Zeng M, Stamler JS. (1999). Hemoglobin induction in mouse macrophages. Proc Natl Acad Sci U S A. Jun 8;96(12):6643-7.PMID 10359765
- ^ Newton DA, Rao KM, Dluhy RA, Baatz JE. (2006). Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem. 281(9):5668-76. PMID 16407281
- ^ Nishi H, Inagi R, Kato H, Tanemoto M, Kojima I, Son D, Fujita T, Nangaku M. (2008). Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J Am Soc Nephrol. 19(8):1500-8. PMID 18448584
- ^ Boh, Larry (2001). Pharmacy Practice Manual: A Guide to the Clinical Experience. Lippincott Williams & Wilkins. ISBN 0781725410.
- ^ Holden, Constance (2005). "Blood and Steel" (pdf). Science. 309: 2160. doi:10.1126/science.309.5744.2160d.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help)
Further reading
|
|
