Cancer
| Cancer | |
|---|---|
| Specialty | Oncology |
Cancer /ˈkænsər/ (medical term: malignant neoplasm) is a class of diseases in which a cell, or a group of cells display uncontrolled growth (division beyond the normal limits), invasion (intrusion on and destruction of adjacent tissues), and sometimes metastasis (spread to other locations in the body via lymph or blood). These three malignant properties of cancers differentiate them from benign tumors, which are self-limited, and do not invade or metastasize. Most cancers form a tumor but some, like leukemia, do not. The branch of medicine concerned with the study, diagnosis, treatment, and prevention of cancer is oncology.
Cancer affects people at all ages with the risk for most types increasing with age.[1] It caused about 13% of all human deaths in 2007[2] (7.6 million).[3] Cancers are primarily an environmental disease with 90-95% of cases due to lifestyle and environmental factors and 5-10% due to genetics.[4] Common environmental factors leading to cancer death include: tobacco (25-30%), diet and obesity (30-35%), infections (15-20%), radiation, stress, lack of physical activity, environmental pollutants.[4] These environmental factors cause abnormalities in the genetic material of cells.[5]
Genetic abnormalities found in cancer typically affect two general classes of genes. Cancer-promoting oncogenes are typically activated in cancer cells, giving those cells new properties, such as hyperactive growth and division, protection against programmed cell death, loss of respect for normal tissue boundaries, and the ability to become established in diverse tissue environments. Tumor suppressor genes are then inactivated in cancer cells, resulting in the loss of normal functions in those cells, such as accurate DNA replication, control over the cell cycle, orientation and adhesion within tissues, and interaction with protective cells of the immune system.
Definitive diagnosis requires the histologic examination of a biopsy specimen, although the initial indication of malignancy can be symptomatic or radiographic imaging abnormalities. Most cancers can be treated and some forced into remission, depending on the specific type, location, and stage. Once diagnosed, cancer is usually treated with a combination of surgery, chemotherapy and radiotherapy. As research develops, treatments are becoming more specific for different varieties of cancer. There has been significant progress in the development of targeted therapy drugs that act specifically on detectable molecular abnormalities in certain tumors, and which minimize damage to normal cells. The prognosis of cancer patients is most influenced by the type of cancer, as well as the stage, or extent of the disease. In addition, histologic grading and the presence of specific molecular markers can also be useful in establishing prognosis, as well as in determining individual treatments.
Classification
Cancers are classified by the type of cell that resembles the tumor and, therefore, the tissue presumed to be the origin of the tumor. These are the histology and the location, respectively. Examples of general categories include:
- Carcinoma: Malignant tumors derived from epithelial cells. This group represents the most common cancers, including the common forms of breast, prostate, lung and colon cancer.
- Sarcoma: Malignant tumors derived from connective tissue, or mesenchymal cells.
- Lymphoma and leukemia: Malignancies derived from hematopoietic (blood-forming) cells
- Germ cell tumor: Tumors derived from totipotent cells. In adults most often found in the testicle and ovary; in fetuses, babies, and young children most often found on the body midline, particularly at the tip of the tailbone; in horses most often found at the poll (base of the skull).
- Blastic tumor or blastoma: A tumor (usually malignant) which resembles an immature or embryonic tissue. Many of these tumors are most common in children.
Malignant tumors (cancers) are usually named using -carcinoma, -sarcoma or -blastoma as a suffix, with the Latin or Greek word for the organ of origin as the root. For instance, a cancer of the liver is called hepatocarcinoma; a cancer of the fat cells is called liposarcoma. For common cancers, the English organ name is used. For instance, the most common type of breast cancer is called ductal carcinoma of the breast or mammary ductal carcinoma. Here, the adjective ductal refers to the appearance of the cancer under the microscope, resembling normal breast ducts.
Benign tumors (which are not cancers) are named using -oma as a suffix with the organ name as the root. For instance, a benign tumor of the smooth muscle of the uterus is called leiomyoma (the common name of this frequent tumor is fibroid). Unfortunately, some cancers also use the -oma suffix, examples being melanoma and seminoma.
Signs and symptoms

Roughly, cancer symptoms can be divided into three groups:
- Local symptoms: unusual lumps or swelling (tumor), hemorrhage (bleeding), pain and/or ulceration. Compression of surrounding tissues may cause symptoms such as jaundice (yellowing the eyes and skin).
- Symptoms of metastasis (spreading): enlarged lymph nodes, cough and hemoptysis, hepatomegaly (enlarged liver), bone pain, fracture of affected bones and neurological symptoms. Although advanced cancer may cause pain, it is often not the first symptom.
- Systemic symptoms: weight loss, poor appetite, fatigue and cachexia (wasting), excessive sweating (night sweats), anemia and specific paraneoplastic phenomena, i.e. specific conditions that are due to an active cancer, such as thrombosis or hormonal changes.
Every symptom in the above list can be caused by a variety of conditions (a list of which is referred to as the differential diagnosis). Cancer may be a common or uncommon cause of each item.
Causes
Cancers are primarily an environmental disease with 90-95% of cases due to lifestyle and environmental factors and 5-10% due to genetics.[4] Common environmental factors that lead to cancer death include: tobacco (25-30%), diet and obesity (30-35%), infections (15-20%), radiation, radon exposure, stress, lack of physical activity, environmental pollutants.[4] The virtual absence of cancerous malignancies in ancient human remains suggests that cancer is mainly a man-made disease of the Industrial Age caused by environmental changes and the modern diet.[6]
Chemicals

Cancer pathogenesis is traceable back to DNA mutations that impact cell growth and metastasis. Substances that cause DNA mutations are known as mutagens, and mutagens that cause cancers are known as carcinogens. Particular substances have been linked to specific types of cancer. Tobacco smoking is associated with many forms of cancer,[7] and causes 90% of lung cancer.[8] Prolonged exposure to asbestos fibers is associated with mesothelioma.[9][10]
Many mutagens are also carcinogens, but some carcinogens are not mutagens. Alcohol is an example of a chemical carcinogen that is not a mutagen.[11] Such chemicals may promote cancers through stimulating the rate of cell division. Faster rates of replication leaves less time for repair enzymes to repair damaged DNA during DNA replication, increasing the likelihood of a mutation.
Decades of research has demonstrated the link between tobacco use and cancer in the lung, larynx, head, neck, stomach, bladder, kidney, oesophagus and pancreas.[12] Tobacco smoke contains over fifty known carcinogens, including nitrosamines and polycyclic aromatic hydrocarbons.[13] Tobacco is responsible for about one in three of all cancer deaths in the developed world,[7] and about one in five worldwide.[13] Indeed, lung cancer death rates in the United States have mirrored smoking patterns, with increases in smoking followed by dramatic increases in lung cancer death rates and, more recently[when?], decreases in smoking followed by decreases in lung cancer death rates in men. However, the numbers of smokers worldwide is still rising, leading to what some organizations have described as the tobacco epidemic.[14]
Cancer related to ones occupation is believed to represent between 2-20% of all cases.[15]
Ionizing radiation
Sources of ionizing radiation, such as radon gas, can cause cancer. Prolonged exposure to ultraviolet radiation from the sun can lead to melanoma and other skin malignancies.[16] One report estimates that approximately 29 000 future cancers could be related to the approximately 70 million CT scans performed in the US in 2007.[17] It is estimated that 0.4% of current cancers in the United States are due to CTs performed in the past and that this may increase to as high as 1.5-2% with 2007 rates of CT usage.[18]
Non-ionizing radio frequency radiation from mobile phones and other similar RF sources has also been proposed as a cause of cancer, but there is currently little established evidence of such a link.[19]
Infection
Some cancers can be caused by infection.[20] This is especially true in animals such as birds, but also in humans, with viruses responsible for up to 20% of human cancers worldwide.[21] These include human papillomavirus (cervical carcinoma), human polyomaviruses (mesothelioma, brain tumors), Epstein-Barr virus (B-cell lymphoproliferative disease and nasopharyngeal carcinoma), Kaposi's sarcoma herpesvirus (Kaposi's Sarcoma and primary effusion lymphomas), hepatitis B and hepatitis C viruses (hepatocellular carcinoma), Human T-cell leukemia virus-1 (T-cell leukemias), and Helicobacter pylori (gastric carcinoma).[21]
Experimental and epidemiological data imply a causative role for viruses and they appear to be the second most important risk factor for cancer development in humans, exceeded only by tobacco usage.[22] The mode of virally induced tumors can be divided into two, acutely transforming or slowly transforming. In acutely transforming viruses, the virus carries an overactive oncogene called viral-oncogene (v-onc), and the infected cell is transformed as soon as v-onc is expressed. In contrast, in slowly transforming viruses, the virus genome is inserted near a proto-oncogene in the host genome. The viral promoter or other transcription regulation elements then cause overexpression of that proto-oncogene. This induces uncontrolled cell division. Because the site of insertion is not specific to proto-oncogenes and the chance of insertion near any proto-oncogene is low, slowly transforming viruses will cause tumors much longer after infection than the acutely transforming viruses.
Hepatitis viruses, including hepatitis B and hepatitis C, can induce a chronic viral infection that leads to liver cancer in 0.47% of hepatitis B patients per year (especially in Asia, less so in North America), and in 1.4% of hepatitis C carriers per year. Liver cirrhosis, whether from chronic viral hepatitis infection or alcoholism, is associated with the development of liver cancer, and the combination of cirrhosis and viral hepatitis presents the highest risk of liver cancer development. Worldwide, liver cancer is one of the most common, and most deadly, cancers due to a huge burden of viral hepatitis transmission and disease.
Advances in cancer research have made a vaccine designed to prevent cancers available. In 2006, the U.S. Food and Drug Administration approved a human papilloma virus vaccine, called Gardasil. The vaccine protects against 6,11,16,18 strains of HPV, which together cause 70% of cervical cancers and 90% of genital warts. It also lists vaginal and vulvar cancers as being protected. In March 2007, the US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) officially recommended that females aged 11–12 receive the vaccine, and indicated that females as young as age 9 and as old as age 26 are also candidates for immunization. There is a second vaccine from Cervarix which protects against the more dangerous HPV 16,18 strains only. In 2009, Gardasil was approved for protection against genital warts. In 2010, the Gardasil vaccine was approved for protection against anal cancer for males and reviewers stated there was no anatomical, histological or physiological anal differences between the genders so females would also be protected.
In addition to viruses, researchers have noted a connection between bacteria and certain cancers. The most prominent example is the link between chronic infection of the wall of the stomach with Helicobacter pylori and gastric cancer.[23][24] Although only a minority of those infected with Helicobacter go on to develop cancer, since this pathogen is quite common it is probably responsible for most of these cancers.[25]
HIV is associated with a number of malignancies, including Kaposi's sarcoma, non-Hodgkin's lymphoma, and HPV-associated malignancies such as anal cancer and cervical cancer. AIDS-defining illnesses have long included these diagnoses. The increased incidence of malignancies in HIV patients points to the breakdown of immune surveillance as a possible etiology of cancer.[26] Certain other immune deficiency states (e.g. common variable immunodeficiency and IgA deficiency) are also associated with increased risk of malignancy.[27]
Heredity
Most forms of cancer are sporadic, meaning that there is no inherited cause of the cancer. There are, however, a number of recognised syndromes where there is an inherited predisposition to cancer, often due to a defect in a gene that protects against tumor formation. Famous examples are:
- certain inherited mutations in the genes BRCA1 and BRCA2 are associated with an elevated risk of breast cancer and ovarian cancer
- tumors of various endocrine organs in multiple endocrine neoplasia (MEN types 1, 2a, 2b)
- Li-Fraumeni syndrome (various tumors such as osteosarcoma, breast cancer, soft tissue sarcoma, brain tumors) due to mutations of p53
- Turcot syndrome (brain tumors and colonic polyposis)
- Familial adenomatous polyposis an inherited mutation of the APC gene that leads to early onset of colon carcinoma.
- Hereditary nonpolyposis colorectal cancer (HNPCC, also known as Lynch syndrome) can include familial cases of colon cancer, uterine cancer, gastric cancer, and ovarian cancer, without a preponderance of colon polyps.
- Retinoblastoma, when occurring in young children, is due to a hereditary mutation in the retinoblastoma gene.
- Down syndrome patients, who have an extra chromosome 21, are known to develop malignancies such as leukemia and testicular cancer, though the reasons for this difference are not well understood.
Other causes
Excepting the rare transmissions that occur with pregnancies and only a marginal few organ donors, cancer is generally not a transmissible disease. The main reason for this is tissue graft rejection caused by MHC incompatibility.[28] In humans and other vertebrates, the immune system uses MHC antigens to differentiate between "self" and "non-self" cells because these antigens are different from person to person. When non-self antigens are encountered, the immune system reacts against the appropriate cell. Such reactions may protect against tumour cell engraftment by eliminating implanted cells. In the United States, approximately 3,500 pregnant women have a malignancy annually, and transplacental transmission of acute leukaemia, lymphoma, melanoma and carcinoma from mother to fetus has been observed.[28] The development of donor-derived tumors from organ transplants is exceedingly rare. The main cause of organ transplant associated tumors seems to be malignant melanoma, that was undetected at the time of organ harvest.[29] though other cases exist[30] In fact, cancer from one organism will usually grow in another organism of that species, as long as they share the same histocompatibility genes,[31] proven using mice; however this would never happen in a real-world setting except as described above.
In non-humans, a few types of transmissible cancer have been described, wherein the cancer spreads between animals by transmission of the tumor cells themselves. This phenomenon is seen in dogs with Sticker's sarcoma, also known as canine transmissible venereal tumor,[32] as well as Devil facial tumour disease in Tasmanian devils.
Pathophysiology
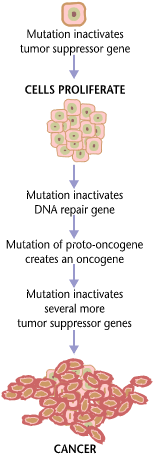
Cancer is fundamentally a disease of regulation of tissue growth. In order for a normal cell to transform into a cancer cell, genes which regulate cell growth and differentiation must be altered.[33] Genetic changes can occur at many levels, from gain or loss of entire chromosomes to a mutation affecting a single DNA nucleotide. There are two broad categories of genes which are affected by these changes. Oncogenes may be normal genes which are expressed at inappropriately high levels, or altered genes which have novel properties. In either case, expression of these genes promotes the malignant phenotype of cancer cells. Tumor suppressor genes are genes which inhibit cell division, survival, or other properties of cancer cells. Tumor suppressor genes are often disabled by cancer-promoting genetic changes. Typically, changes in many genes are required to transform a normal cell into a cancer cell.[34]
There is a diverse classification scheme for the various genomic changes which may contribute to the generation of cancer cells. Most of these changes are mutations, or changes in the nucleotide sequence of genomic DNA. Aneuploidy, the presence of an abnormal number of chromosomes, is one genomic change which is not a mutation, and may involve either gain or loss of one or more chromosomes through errors in mitosis.
Large-scale mutations involve the deletion or gain of a portion of a chromosome. Genomic amplification occurs when a cell gains many copies (often 20 or more) of a small chromosomal locus, usually containing one or more oncogenes and adjacent genetic material. Translocation occurs when two separate chromosomal regions become abnormally fused, often at a characteristic location. A well-known example of this is the Philadelphia chromosome, or translocation of chromosomes 9 and 22, which occurs in chronic myelogenous leukemia, and results in production of the BCR-abl fusion protein, an oncogenic tyrosine kinase.
Small-scale mutations include point mutations, deletions, and insertions, which may occur in the promoter of a gene and affect its expression, or may occur in the gene's coding sequence and alter the function or stability of its protein product. Disruption of a single gene may also result from integration of genomic material from a DNA virus or retrovirus, and such an event may also result in the expression of viral oncogenes in the affected cell and its descendants.
Anything which replicates (living cells) will probabilistically suffer from errors (mutations). Unless error correction and prevention is properly carried out, the errors will survive, and might be passed along to daughter cells. Normally, the body safeguards against cancer via numerous methods, such as: apoptosis, helper molecules (some DNA polymerases), possibly senescence, etc. However these error-correction methods often fail in small ways, especially in environments that make errors more likely to arise and propagate. For example, such environments can include the presence of disruptive substances called carcinogens, or periodic injury (physical, heat, etc.), or environments that cells did not evolve to withstand, such as hypoxia[35] (see subsections). Cancer is thus a progressive disease, and these progressive errors slowly accumulate until a cell begins to act contrary to its function in the organism.
The errors which cause cancer are often self-amplifying, eventually compounding at an exponential rate. For example:
- A mutation in the error-correcting machinery of a cell might cause that cell and its children to accumulate errors more rapidly
- A mutation in signaling (endocrine) machinery of the cell can send error-causing signals to nearby cells
- A mutation might cause cells to become neoplastic, causing them to migrate and disrupt more healthy cells
- A mutation may cause the cell to become immortal (see telomeres), causing them to disrupt healthy cells forever
Thus cancer often explodes in something akin to a chain reaction caused by a few errors, which compound into more severe errors. Errors which produce more errors are effectively the root cause of cancer, and also the reason that cancer is so hard to treat: even if there were 10,000,000,000 cancerous cells and one killed all but 10 of those cells, those cells (and other error-prone precancerous cells) could still self-replicate or send error-causing signals to other cells, starting the process over again. This rebellion-like scenario is an undesirable survival of the fittest, where the driving forces of evolution work against the body's design and enforcement of order. In fact, once cancer has begun to develop, this same force continues to drive the progression of cancer towards more invasive stages, and is called clonal evolution.[36]
Research about cancer causes often falls into the following categories:
- Agents (e.g. viruses) and events (e.g. mutations) which cause or facilitate genetic changes in cells destined to become cancer.
- The precise nature of the genetic damage, and the genes which are affected by it.
- The consequences of those genetic changes on the biology of the cell, both in generating the defining properties of a cancer cell, and in facilitating additional genetic events which lead to further progression of the cancer.
Prevention
Cancer prevention is defined as active measures to decrease the incidence of cancer.[37] Greater than 30% of cancer is preventable via avoiding risk factors including: tobacco, overweight or obesity, low fruit and vegetable intake, physical inactivity, alcohol, sexually transmitted infection, air pollution.[38] This can be accomplished by avoiding carcinogens or altering their metabolism, pursuing a lifestyle or diet that modifies cancer-causing factors and/or medical intervention (chemoprevention, treatment of pre-malignant lesions). The epidemiological concept of "prevention" is usually defined as either primary prevention, for people who have not been diagnosed with a particular disease, or secondary prevention, aimed at reducing recurrence or complications of a previously diagnosed illness.
But the EPIC study published in 2010, tracking the eating habits of 478,000 Europeans suggested that consuming lots of fruits and vegetables has little if any effect on preventing cancer.[39]
Modifiable factors
The vast majority of cancer risk factors are environmental or lifestyle-related, leading to the claim that cancer is a largely preventable disease.[40] Examples of modifiable cancer risk factors include alcohol consumption (associated with increased risk of oral, esophageal, breast, and other cancers), smoking (80% of women with lung cancer have smoked in the past, and 90% of men[41]), physical inactivity (associated with increased risk of colon, breast, and possibly other cancers), and being overweight / obese (associated with colon, breast, endometrial, and possibly other cancers). Based on epidemiologic evidence, it is now thought that avoiding excessive alcohol consumption may contribute to reductions in risk of certain cancers; however, compared with tobacco exposure, the magnitude of effect is modest or small and the strength of evidence is often weaker. Other lifestyle and environmental factors known to affect cancer risk (either beneficially or detrimentally) include certain sexually transmitted diseases (such as those conveyed by the human papillomavirus), the use of exogenous hormones, exposure to ionizing radiation and ultraviolet radiation from the sun or from tanning beds, and certain occupational and chemical exposures.
Every year, at least 200,000 people die worldwide from cancer related to their workplace.[42] Millions of workers run the risk of developing cancers such as lung cancer and mesothelioma from inhaling asbestos fibers and tobacco smoke, or leukemia from exposure to benzene at their workplaces.[42] Currently, most cancer deaths caused by occupational risk factors occur in the developed world.[42] It is estimated that approximately 20,000 cancer deaths and 40,000 new cases of cancer each year in the U.S. are attributable to occupation.[43]
Diet
The consensus on diet and cancer is that obesity increases the risk of developing cancer. Particular dietary practices often explain differences in cancer incidence in different countries (e.g. gastric cancer is more common in Japan, while colon cancer is more common in the United States. In this example the preceding consideration of Haplogroups are excluded). Studies have shown that immigrants develop the risk of their new country, often within one generation, suggesting a substantial link between diet and cancer.[44] Whether reducing obesity in a population also reduces cancer incidence is unknown.
Despite frequent reports of particular substances (including foods) having a beneficial or detrimental effect on cancer risk, few of these have an established link to cancer. These reports are often based on studies in cultured cell media or animals. Public health recommendations cannot be made based on these studies until they have been validated in an observational (or occasionally a prospective interventional) trial in humans.
Proposed dietary interventions for primary cancer risk reduction generally gain support from epidemiological association studies. Examples of such studies include reports that reduced meat consumption is associated with decreased risk of colon cancer,[45] and reports that consumption of coffee is associated with a reduced risk of liver cancer.[46] Studies have linked consumption of grilled meat to an increased risk of stomach cancer,[47] colon cancer,[48] breast cancer,[49] and pancreatic cancer,[50] a phenomenon which could be due to the presence of carcinogens such as benzopyrene in foods cooked at high temperatures.
A recent study analysed the correlation between many factors and cancer and concluded that the major contributory dietary factor was animal protein, whereas plant protein did not have an effect. Animal studies confirmed the mechanism by showing that reducing the proportion of animal protein switched off both the initiation and promotion stages. [51]
A 2005 secondary prevention study showed that consumption of a plant-based diet and lifestyle changes resulted in a reduction in cancer markers in a group of men with prostate cancer who were using no conventional treatments at the time.[52] These results were amplified by a 2006 study. Over 2,400 women were studied, half randomly assigned to a normal diet, the other half assigned to a diet containing less than 20% calories from fat. The women on the low fat diet were found to have a markedly lower risk of breast cancer recurrence, in the interim report of December, 2006.[53]
Recent[when?] studies have also demonstrated potential links between some forms of cancer and high consumption of refined sugars and other simple carbohydrates.[54][55][56][57][58] Although the degree of correlation and the degree of causality is still debated,[59][60][61] some organizations have in fact begun to recommend reducing intake of refined sugars and starches as part of their cancer prevention regimens.[62][63][64]
In November 2007, the American Institute for Cancer Research (AICR), in conjunction with the World Cancer Research Fund (WCRF), published Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective, "the most current and comprehensive analysis of the literature on diet, physical activity and cancer".[65] The WCRF/AICR Expert Report lists 10 recommendations that people can follow to help reduce their risk of developing cancer, including the following dietary guidelines: (1) reducing intake of foods and drinks that promote weight gain, namely energy-dense foods and sugary drinks, (2) eating mostly foods of plant origin, (3) limiting intake of red meat and avoiding processed meat, (4) limiting consumption of alcoholic beverages, and (5) reducing intake of salt and avoiding mouldy cereals (grains) or pulses (legumes).[66][67]
Some mushrooms offer an anti-cancer effect, which is thought to be linked to their ability to up-regulate the immune system. Some mushrooms known for this effect include, Reishi,[68][69] Agaricus blazei,[70] Maitake,[71] and Trametes versicolor.[72] Research suggests the compounds in medicinal mushrooms most responsible for up-regulating the immune system and providing an anti-cancer effect, are a diverse collection of polysaccharide compounds, particularly beta-glucans. Beta-glucans are known as "biological response modifiers", and their ability to activate the immune system is well documented. Specifically, beta-glucans stimulate the innate branch of the immune system. Research has shown beta-glucans have the ability to stimulate macrophage, NK cells, T cells, and immune system cytokines. The mechanisms in which beta-glucans stimulate the immune system is only partially understood. One mechanism in which beta-glucans are able to activate the immune system, is by interacting with the Macrophage-1 antigen (CD18) receptor on immune cells.[73]
Vitamins
As of 2010 vitamins have not been found to be effective at preventing cancer.[74] While low levels of vitamin D is correlated with increased cancer risk.[75][76] Whether this relationship is causal and vitamin D supplementation is protective is yet to be determined.[77] Beta-carotene supplementation has been found to increase slightly, but not significantly risks of lung cancer.[78] Folic acid supplementation has not been found effective in preventing colon cancer and may increase colon polyps.[79]
Chemoprevention
The concept that medications could be used to prevent cancer is an attractive one, and many high-quality clinical trials support the use of such chemoprevention in defined circumstances.
Daily use of tamoxifen, a selective estrogen receptor modulator (SERM), typically for 5 years, has been demonstrated to reduce the risk of developing breast cancer in high-risk women by about 50%. A recent[when?] study reported that the selective estrogen receptor modulator raloxifene has similar benefits to tamoxifen in preventing breast cancer in high-risk women, with a more favorable side effect profile.[80]
Raloxifene is a SERM like tamoxifen; it has been shown (in the STAR trial) to reduce the risk of breast cancer in high-risk women equally as well as tamoxifen. In this trial, which studied almost 20,000 women, raloxifene had fewer side effects than tamoxifen, though it did permit more DCIS to form.[80]
Finasteride, a 5-alpha-reductase inhibitor, has been shown to lower the risk of prostate cancer, though it seems to mostly prevent low-grade tumors.[81] The effect of COX-2 inhibitors such as rofecoxib and celecoxib upon the risk of colon polyps have been studied in familial adenomatous polyposis patients[82] and in the general population.[83][84] In both groups, there were significant reductions in colon polyp incidence, but this came at the price of increased cardiovascular toxicity.
Genetic testing
Genetic testing for high-risk individuals is already available for certain cancer-related genetic mutations. Carriers of genetic mutations that increase risk for cancer incidence can undergo enhanced surveillance, chemoprevention, or risk-reducing surgery. Early identification of inherited genetic risk for cancer, along with cancer-preventing interventions such as surgery or enhanced surveillance, can be lifesaving for high-risk individuals.
| Gene | Cancer types | Availability |
|---|---|---|
| BRCA1, BRCA2 | Breast, ovarian, pancreatic | Commercially available for clinical specimens |
| MLH1, MSH2, MSH6, PMS1, PMS2 | Colon, uterine, small bowel, stomach, urinary tract | Commercially available for clinical specimens |
Vaccination
Prophylactic vaccines have been developed to prevent infection by oncogenic infectious agents such as viruses, and therapeutic vaccines are in development to stimulate an immune response against cancer-specific epitopes.[85]
As reported above, a preventive human papillomavirus vaccine exists that targets certain sexually transmitted strains of human papillomavirus that are associated with the development of cervical cancer and genital warts. The only two HPV vaccines on the market as of October 2007 are Gardasil and Cervarix.[85] There is also a hepatitis B vaccine, which prevents infection with the hepatitis B virus, an infectious agent that can cause liver cancer.[85] A canine melanoma vaccine has also been developed.[86][87]
Screening
Cancer screening is an attempt to detect unsuspected cancers in an asymptomatic population. In this sense screening is not a means of prevention. Whereas prevention is designed to reduce the incidence of cancer, screening is designed to increase the incidence of early cancer which, it is argued, should be more effectively treatable. Screening tests suitable for large numbers of healthy people must be relatively affordable, safe, noninvasive procedures with acceptably low rates of false positive results. If signs of cancer are detected, more definitive and invasive follow up tests are performed to confirm the diagnosis.
Screening for cancer can lead to earlier diagnosis in specific cases. Early diagnosis may lead to extended life, but may also falsely prolong the lead time to death through lead time bias or length time bias.[88]
A number of different screening tests have been developed for different malignancies. Breast cancer screening can be done by breast self-examination, though this approach was discredited by a 2005 study in over 300,000 Chinese women. Screening for breast cancer with mammograms has been shown to reduce the average stage of diagnosis of breast cancer in a population. Stage of diagnosis in a country has been shown to decrease within ten years of introduction of mammographic screening programs. Colorectal cancer can be detected through fecal occult blood testing and colonoscopy, which reduces both colon cancer incidence and mortality, presumably through the detection and removal of pre-malignant polyps. Similarly, cervical cytology testing (using the Pap smear) leads to the identification and excision of precancerous lesions. Over time, such testing has been followed by a dramatic reduction of cervical cancer incidence and mortality. Testicular self-examination is recommended for men beginning at the age of 15 years to detect testicular cancer. Prostate cancer can be screened using a digital rectal exam along with prostate specific antigen (PSA) blood testing, though some authorities (such as the US Preventive Services Task Force) recommend against routinely screening all men.
Screening for cancer is controversial in cases when it is not yet known if the test actually saves lives. The controversy arises when it is not clear if the benefits of screening outweigh the risks of follow-up diagnostic tests and cancer treatments. For example: when screening for prostate cancer, the PSA test may detect small cancers that would never become life threatening, but once detected will lead to treatment. This situation, called overdiagnosis, puts men at risk for complications from unnecessary treatment such as surgery or radiation. Follow up procedures used to diagnose prostate cancer (prostate biopsy) may cause side effects, including bleeding and infection. Prostate cancer treatment may cause incontinence (inability to control urine flow) and erectile dysfunction (erections inadequate for intercourse). This situation was summarised in an editorial commenting on recent randomised controlled trials. [89]. Similarly, for breast cancer, there have recently[when?] been criticisms that breast screening programs in some countries cause more problems than they solve. This is because screening of women in the general population will result in a large number of women with false positive results which require extensive follow-up investigations to exclude cancer, leading to having a high number-to-treat (or number-to-screen) to prevent or catch a single case of breast cancer early.[90]
One difficulty with demonstrating the benefits of mammography screening is that proof of benefit requires not only a reduction in breast cancer mortality among women offered screening compared with those in the control group in randomised controlled trials, but also a reduction in deaths from all causes.[91]. In most screening trials the observed reduction in deaths from the particular cancer was accompanied by a comparable increase in deaths from other causes, presumably as a result of harm caused by post-screening treatments, giving no significant reduction in deaths from all causes.[92] Even in the large breast and prostate cancer screening trials the power of the trials is inadequate to confirm the significance of the lack of reduction in overall deaths. Despite the reduction in harm caused by post-screening treatments in recent years there is still a significant number of deaths due to treatment. [93]
Cervical cancer screening via the Pap smear has the best cost-benefit profile of all the forms of cancer screening from a public health perspective as, largely caused by a virus, it has clear risk factors (sexual contact), and the natural progression of cervical cancer is that it normally spreads slowly over a number of years therefore giving more time for the screening program to catch it early. Moreover, the test is easy to perform and relatively cheap.
For these reasons, it is important that the benefits and risks of diagnostic procedures and treatment be taken into account when considering whether to undertake cancer screening.
Use of medical imaging to search for cancer in people without clear symptoms is similarly marred with problems. There is a significant risk of detection of what has been recently[when?] called an incidentaloma - a benign lesion that may be interpreted as a malignancy and be subjected to potentially dangerous investigations. Recent[when?] studies of CT scan-based screening for lung cancer in smokers have had equivocal results, and systematic screening is not recommended as of July 2007. Randomized clinical trials of plain-film chest X-rays to screen for lung cancer in smokers have shown no benefit for this approach.
Canine cancer detection has shown promise, but is still in the early stages of research.
Diagnosis

Most cancers are initially recognized either because signs or symptoms appear or through screening. Neither of these lead to a definitive diagnosis, which usually requires the opinion of a pathologist, a type of physician (medical doctor) who specializes in the diagnosis of cancer and other diseases. People with suspected cancer are investigated with medical tests. These commonly include blood tests, X-rays, CT scans and endoscopy.
Pathology
A cancer may be suspected for a variety of reasons, but the definitive diagnosis of most malignancies must be confirmed by histological examination of the cancerous cells by a pathologist. Tissue can be obtained from a biopsy or surgery. Many biopsies (such as those of the skin, breast or liver) can be done in a doctor's office. Biopsies of other organs are performed under anesthesia and require surgery in an operating room.
The tissue diagnosis given by the pathologist indicates the type of cell that is proliferating, its histological grade, genetic abnormalities, and other features of the tumor. Together, this information is useful to evaluate the prognosis of the patient and to choose the best treatment. Cytogenetics and immunohistochemistry are other types of testing that the pathologist may perform on the tissue specimen. These tests may provide information about the molecular changes (such as mutations, fusion genes, and numerical chromosome changes) that has happened in the cancer cells, and may thus also indicate the future behavior of the cancer (prognosis) and best treatment.
-
Typical macroscopic appearance of cancer. This invasive ductal carcinoma of the breast (pale area at the center) shows an oval tumor surrounded by spikes of whitish scar tissue in the surrounding yellow fatty tissue. The silhouette vaguely resembles a crab.
-
An invasive colorectal carcinoma (top center) in a colectomy specimen.
-
A squamous cell carcinoma (the whitish tumor) near the bronchi in a lung specimen.
-
A large invasive ductal carcinoma in a mastectomy specimen.
Management
Many management options for cancer exist including: chemotherapy, radiation therapy, surgery, immunotherapy, monoclonal antibody therapy and other methods. Which are used depends upon the location and grade of the tumor and the stage of the disease, as well as the general state of a person's health. Experimental cancer treatments are also under development.
Complete removal of the cancer without damage to the rest of the body is the goal of treatment. Sometimes this can be accomplished by surgery, but the propensity of cancers to invade adjacent tissue or to spread to distant sites by microscopic metastasis often limits its effectiveness. Surgery often required the removal of a wide surgical margin or a free margin. The width of the free margin depends on the type of the cancer, the method of removal (CCPDMA, Mohs surgery, POMA, etc.). The margin can be as little as 1 mm for basal cell cancer using CCPDMA or Mohs surgery, to several centimeters for aggressive cancers. The effectiveness of chemotherapy is often limited by toxicity to other tissues in the body. Radiation can also cause damage to normal tissue.
Because "cancer" refers to a class of diseases,[94][95] it is unlikely that there will ever be a single "cure for cancer" any more than there will be a single treatment for all infectious diseases.[96] Angiogenesis inhibitors were once thought to have potential as a "silver bullet" treatment applicable to many types of cancer, but this has not been the case in practice.[97]
Prognosis
Cancer has a reputation as a deadly disease. While this certainly applies to certain particular types, the truths behind the historical connotations of cancer are increasingly overturned by advances in medical care. Some types of cancer have a prognosis that is substantially better than nonmalignant diseases such as heart failure and stroke.
Progressive and disseminated malignant disease has a substantial impact on a cancer patient's quality of life, and many cancer treatments (such as chemotherapy) may have severe side-effects. In the advanced stages of cancer, many patients need extensive care, affecting family members and friends. Palliative care solutions may include permanent or "respite" hospice nursing.
Emotional impact
Many local organizations offer a variety of practical and support services to people with cancer. Support can take the form of support groups, counseling, advice, financial assistance, transportation to and from treatment, films or information about cancer. Neighborhood organizations, local health care providers, or area hospitals may have resources or services available.
Counseling can provide emotional support to cancer patients and help them better understand their illness. Different types of counseling include individual, group, family, peer counseling, bereavement, patient-to-patient, and sexuality.
Many governmental and charitable organizations have been established to help patients cope with cancer. These organizations are often involved in cancer prevention, cancer treatment, and cancer research.
Epidemiology

As of 2004[update], worldwide cancer caused 13% of all deaths (7.4 million). The leading causes were: lung cancer (1.3 million deaths/year), stomach cancer (803,000 deaths), colorectal cancer (639,000 deaths), liver cancer (610,000 deaths), and breast cancer (519,000 deaths).[99] Greater than 30% of cancer is preventable via avoiding risk factors including: tobacco, overweight or obesity, low fruit and vegetable intake, physical inactivity, alcohol, sexually transmitted infections, and air pollution.[38]
In the United States, cancer is responsible for 25% of all deaths with 30% of these from lung cancer. The most commonly occurring cancer in men is prostate cancer (about 25% of new cases) and in women is breast cancer (also about 25%). Cancer can occur in children and adolescents, but it is uncommon (about 150 cases per million in the U.S.), with leukemia the most common.[100] In the first year of life the incidence is about 230 cases per million in the U.S., with the most common being neuroblastoma.[101]
In the developed world, one in three people will develop cancer during their lifetimes. If all cancer patients survived and cancer occurred randomly, the lifetime odds of developing a second primary cancer would be one in nine.[102] However, cancer survivors have an increased risk of developing a second primary cancer, and the odds are about two in nine.[102] About half of these second primaries can be attributed to the normal one-in-nine risk associated with random chance.[102] The increased risk is believed to be primarily due to the same risk factors that produced the first cancer (such as the person's genetic profile, alcohol and tobacco use, obesity, and environmental exposures), and partly due to the treatment for the first cancer, which typically includes mutagenic chemotherapeutic drugs or radiation.[102] Cancer survivors may also be more likely to comply with recommended screening, and thus may be more likely than average to detect cancers.[102]
-
Most common cancers in males, by occurrence[100]
-
in females, by occurrence[100]
-
in males, by mortality[100]
-
in females, by mortality[100]
History
Today, the Greek term carcinoma is the medical term for a malignant tumor derived from epithelial cells. It is Celsus who translated carcinos into the Latin cancer, also meaning crab. Galen used "oncos" to describe all tumours, the root for the modern word oncology.[103]
Hippocrates described several kinds of cancers. Galen called benign tumours oncos, Greek for swelling, and malignant tumours carcinos, Greek for crab or crayfish. This name comes from the appearance of the cut surface of a solid malignant tumour, with "the veins stretched on all sides as the animal the crab has its feet, whence it derives its name".[104] He later added the suffix -oma, Greek for swelling, giving the name carcinoma. Since it was against Greek tradition to open the body, Hippocrates only described and made drawings of outwardly visible tumors on the skin, nose, and breasts. Treatment was based on the humor theory of four bodily fluids (black and yellow bile, blood, and phlegm). According to the patient's humor, treatment consisted of diet, blood-letting, and/or laxatives. Through the centuries it was discovered that cancer could occur anywhere in the body, but humor-theory based treatment remained popular until the 19th century with the discovery of cells.
The oldest known description and surgical treatment of cancer was discovered in Egypt and dates back to approximately 1600 BC. The Papyrus describes 8 cases of ulcers of the breast that were treated by cauterization, with a tool called "the fire drill." The writing says about the disease, "There is no treatment."[105]
Another very early surgical treatment for cancer was described in the 1020s by Avicenna (Ibn Sina) in The Canon of Medicine. He stated that the excision should be radical and that all diseased tissue should be removed, which included the use of amputation or the removal of veins running in the direction of the tumor. He also recommended the use of cauterization for the area treated if necessary.[106]
In the 16th and 17th centuries, it became more acceptable for doctors to dissect bodies to discover the cause of death. The German professor Wilhelm Fabry believed that breast cancer was caused by a milk clot in a mammary duct. The Dutch professor Francois de la Boe Sylvius, a follower of Descartes, believed that all disease was the outcome of chemical processes, and that acidic lymph fluid was the cause of cancer. His contemporary Nicolaes Tulp believed that cancer was a poison that slowly spreads, and concluded that it was contagious.[107]
The first cause of cancer was identified by British surgeon Percivall Pott, who discovered in 1775 that cancer of the scrotum was a common disease among chimney sweeps. The work of other individual physicians led to various insights, but when physicians started working together they could make firmer conclusions.
With the widespread use of the microscope in the 18th century, it was discovered that the 'cancer poison' spread from the primary tumor through the lymph nodes to other sites ("metastasis"). This view of the disease was first formulated by the English surgeon Campbell De Morgan between 1871 and 1874.[108] The use of surgery to treat cancer had poor results due to problems with hygiene. The renowned Scottish surgeon Alexander Monro saw only 2 breast tumor patients out of 60 surviving surgery for two years. In the 19th century, asepsis improved surgical hygiene and as the survival statistics went up, surgical removal of the tumor became the primary treatment for cancer. With the exception of William Coley who in the late 19th century felt that the rate of cure after surgery had been higher before asepsis (and who injected bacteria into tumors with mixed results), cancer treatment became dependent on the individual art of the surgeon at removing a tumor. During the same period, the idea that the body was made up of various tissues, that in turn were made up of millions of cells, laid rest the humor-theories about chemical imbalances in the body. The age of cellular pathology was born.
The genetic basis of cancer was recognised in 1902 by the German zoologist Theodor Boveri, professor of zoology at Munich and later in Würzburg.[109] He discovered a method to generate cells with multiple copies of the centrosome, a structure he discovered and named. He postulated that chromosomes were distinct and transmitted different inheritance factors. He suggested that mutations of the chromosomes could generate a cell with unlimited growth potential which could be passed onto its descendants. He proposed the existence of cell cycle check points, tumour suppressor genes and oncogenes. He speculated that cancers might be caused or promoted by radiation, physical or chemical insults or by pathogenic microorganisms.
When Marie Curie and Pierre Curie discovered radiation at the end of the 19th century, they stumbled upon the first effective non-surgical cancer treatment. With radiation also came the first signs of multi-disciplinary approaches to cancer treatment. The surgeon was no longer operating in isolation, but worked together with hospital radiologists to help patients. The complications in communication this brought, along with the necessity of the patient's treatment in a hospital facility rather than at home, also created a parallel process of compiling patient data into hospital files, which in turn led to the first statistical patient studies.
A founding paper of cancer epidemiology was the work of Janet Lane-Claypon, who published a comparative study in 1926 of 500 breast cancer cases and 500 control patients of the same background and lifestyle for the British Ministry of Health. Her ground-breaking work on cancer epidemiology was carried on by Richard Doll and Austin Bradford Hill, who published "Lung Cancer and Other Causes of Death In Relation to Smoking. A Second Report on the Mortality of British Doctors" followed in 1956 (otherwise known as the British doctors study). Richard Doll left the London Medical Research Center (MRC), to start the Oxford unit for Cancer epidemiology in 1968. With the use of computers, the unit was the first to compile large amounts of cancer data. Modern epidemiological methods are closely linked to current concepts of disease and public health policy. Over the past 50 years, great efforts have been spent on gathering data across medical practise, hospital, provincial, state, and even country boundaries to study the interdependence of environmental and cultural factors on cancer incidence.
Cancer patient treatment and studies were restricted to individual physicians' practices until World War II, when medical research centers discovered that there were large international differences in disease incidence. This insight drove national public health bodies to make it possible to compile health data across practises and hospitals, a process that many countries do today. The Japanese medical community observed that the bone marrow of victims of the atomic bombings of Hiroshima and Nagasaki was completely destroyed. They concluded that diseased bone marrow could also be destroyed with radiation, and this led to the discovery of bone marrow transplants for leukemia. Since World War II, trends in cancer treatment are to improve on a micro-level the existing treatment methods, standardize them, and globalize them to find cures through epidemiology and international partnerships.
Research
Cancer research is the intense scientific effort to understand disease processes and discover possible therapies. The improved understanding of molecular biology and cellular biology due to cancer research has led to a number of new, effective treatments for cancer since President Nixon declared "War on Cancer" in 1971. Since 1971 the United States has invested over $200 billion on cancer research; that total includes money invested by public and private sectors and foundations.[110] Despite this substantial investment, the country has seen a five percent decrease in the cancer death rate (adjusting for size and age of the population) between 1950 and 2005.[111]
Leading cancer research organizations and projects include the American Association for Cancer Research, the American Cancer Society (ACS), the American Society of Clinical Oncology, the European Organisation for Research and Treatment of Cancer, the National Cancer Institute, the National Comprehensive Cancer Network, and The Cancer Genome Atlas project at the NCI.
Glossary
The following closely related terms may be used to designate abnormal growths:
- Tumor or tumour: originally, it meant any abnormal swelling, lump or mass. In current English, however, the word tumor has become synonymous with neoplasm, specifically solid neoplasm. Note that some neoplasms, such as leukemia, do not form tumors.
- Neoplasm: the scientific term to describe an abnormal proliferation of genetically altered cells. Neoplasms can be benign or malignant:
- Malignant neoplasm or malignant tumor: synonymous with cancer.
- Benign neoplasm or benign tumor: a tumor (solid neoplasm) that stops growing, does not invade other tissues and does not form metastases.
- Invasive tumor is another synonym of cancer. The name refers to invasion of surrounding tissues.
- Pre-malignancy, pre-cancer or non-invasive tumor: A neoplasm that is not invasive but has the potential to progress to cancer (become invasive) if left untreated. These lesions are, in order of increasing potential for cancer, atypia, dysplasia and carcinoma in situ.
The following terms can be used to describe a cancer:
- Screening: a test done on healthy people to detect tumors before they become apparent. A mammogram is a screening test.
- Diagnosis: the confirmation of the cancerous nature of a lump. This usually requires a biopsy or removal of the tumor by surgery, followed by examination by a pathologist.
- Surgical excision: the removal of a tumor by a surgeon.
- Surgical margins: the evaluation by a pathologist of the edges of the tissue removed by the surgeon to determine if the tumor was removed completely ("negative margins") or if tumor was left behind ("positive margins").
- Grade: a number (usually on a scale of 3) established by a pathologist to describe the degree of resemblance of the tumor to the surrounding benign tissue.
- Stage: a number (usually on a scale of 4) established by the oncologist to describe the degree of invasion of the body by the tumor.
- Recurrence: new tumors that appear at the site of the original tumor after surgery.
- Metastasis: new tumors that appear far from the original tumor.
- Median survival time: a period, often measured in months or years, over which 50% of the cancer patients are expected to be alive.[112]
- Transformation: the concept that a low-grade tumor transforms to a high-grade tumor over time. Example: Richter's transformation.
- Chemotherapy: treatment with drugs.
- Radiation therapy: treatment with radiations.
- Adjuvant therapy: treatment, either chemotherapy or radiation therapy, given after surgery to kill the remaining cancer cells.
- Neoadjuvant therapy: treatment either chemotherapy or radiation therapy, given before surgery to shrink a tumor to make its resection easier.
- Palliative care: treatment that does other than cure the disease i.e. reduces severity of disease, relieve suffering and improves quality of life.
- Prognosis: the probability of cure/remission after the therapy. It is usually expressed as a probability of survival five years after diagnosis. Alternatively, it can be expressed as the number of years when 50% of the patients are still alive. Both numbers are derived from statistics accumulated with hundreds of similar patients to give a Kaplan-Meier curve.
- Cure: A cancer patient is "cured" or "in remission" if they live past the time by which 95% of treated patients live after the date of their diagnosis of cancer. This period varies among different types of cancer; for example, in the case of Hodgkin's disease this period is 10 years, whereas for Burkitt's lymphoma this period would be 1 year.[113] The phrase "cure" used in oncology is based upon the statistical concept of a median survival time and disease-free median survival time.[114]
See also
- The Cancer Prevention and Education Society (in the UK)
- Risk factors in pregnancy
- Cancer-related fatigue
Notes
- ^ Cancer Research UK (2007). "UK cancer incidence statistics by age". Retrieved 2007-06-25.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ WHO (2006). "Cancer". World Health Organization. Retrieved 2007-06-25.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ American Cancer Society (2007). "Report sees 7.6 million global 2007 cancer deaths". Reuters. Retrieved 2008-08-07.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ a b c d Anand P, Kunnumakkara AB, Kunnumakara AB; et al. (2008). "Cancer is a preventable disease that requires major lifestyle changes". Pharm. Res. 25 (9): 2097–116. doi:10.1007/s11095-008-9661-9. PMC 2515569. PMID 18626751.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kinzler, Kenneth W.; Vogelstein, Bert (2002). "Introduction". The genetic basis of human cancer (2nd, illustrated, revised ed.). New York: McGraw-Hill, Medical Pub. Division. p. 5. ISBN 978-0-07-137050-9.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Parenteral Drug Association, October 2010, "Scientists Suggest that Cancer is Purely Man-Made," http://pda.physorg.com/news/2010-10-scientists-cancer-purely-man-made.html citing the journal Nature Reviews Cancer, 10, 728-733, October 2010, "Cancer: An Old Disease, A New Disease Or Something in Between?," by David and Zimmerman, http://www.nature.com/nrc/journal/v10/n10/full/nrc2914.html
- ^ a b Sasco AJ, Secretan MB, Straif K (2004). "Tobacco smoking and cancer: a brief review of recent epidemiological evidence". Lung cancer (Amsterdam, Netherlands). 45 Suppl 2: S3–9. doi:10.1016/j.lungcan.2004.07.998. PMID 15552776.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Biesalski HK, Bueno de Mesquita B, Chesson A; et al. (1998). "European Consensus Statement on Lung Cancer: risk factors and prevention. Lung Cancer Panel". CA: a cancer journal for clinicians. 48 (3): 167–76, discussion 164–6. doi:10.3322/canjclin.48.3.167. PMID 9594919.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ O'Reilly KM, Mclaughlin AM, Beckett WS, Sime PJ (2007). "Asbestos-related lung disease". American family physician. 75 (5): 683–8. PMID 17375514.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Seitz HK, Pöschl G, Simanowski UA (1998). "Alcohol and cancer". Recent developments in alcoholism : an official publication of the American Medical Society on Alcoholism, the Research Society on Alcoholism, and the National Council on Alcoholism. 14: 67–95. PMID 9751943.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kuper H, Boffetta P, Adami HO (2002). "Tobacco use and cancer causation: association by tumour type". Journal of internal medicine. 252 (3): 206–24. doi:10.1046/j.1365-2796.2002.01022.x. PMID 12270001.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Kuper H, Adami HO, Boffetta P (2002). "Tobacco use, cancer causation and public health impact". Journal of internal medicine. 251 (6): 455–66. doi:10.1046/j.1365-2796.2002.00993.x. PMID 12028500.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Proctor RN (2004). "The global smoking epidemic: a history and status report". Clinical lung cancer. 5 (6): 371–6. doi:10.3816/CLC.2004.n.016. PMID 15217537.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Irigaray P, Newby JA, Clapp R; et al. (2007). "Lifestyle-related factors and environmental agents causing cancer: an overview". Biomed. Pharmacother. 61 (10): 640–58. doi:10.1016/j.biopha.2007.10.006. PMID 18055160.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ English DR, Armstrong BK, Kricker A, Fleming C (1997). "Sunlight and cancer". Cancer causes & control : CCC. 8 (3): 271–83. doi:10.1023/A:1018440801577. PMID 9498892.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Berrington de González A, Mahesh M, Kim KP; et al. (2009). "Projected cancer risks from computed tomographic scans performed in the United States in 2007". Arch. Intern. Med. 169 (22): 2071–7. doi:10.1001/archinternmed.2009.440. PMID 20008689.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Brenner DJ, Hall EJ (2007). "Computed tomography--an increasing source of radiation exposure". N. Engl. J. Med. 357 (22): 2277–84. doi:10.1056/NEJMra072149. PMID 18046031.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Feychting M, Ahlbom A, Kheifets L (2005). "EMF and health". Annual review of public health. 26: 165–89. doi:10.1146/annurev.publhealth.26.021304.144445. PMID 15760285.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pagano JS, Blaser M, Buendia MA; et al. (2004). "Infectious agents and cancer: criteria for a causal relation". Semin. Cancer Biol. 14 (6): 453–71. doi:10.1016/j.semcancer.2004.06.009. PMID 15489139.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Pagano JS, Blaser M, Buendia MA; et al. (2004). "Infectious agents and cancer: criteria for a causal relation". Semin. Cancer Biol. 14 (6): 453–71. doi:10.1016/j.semcancer.2004.06.009. PMID 15489139.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ zur Hausen H (1991). "Viruses in human cancers". Science. 254 (5035): 1167–73. doi:10.1126/science.1659743. PMID 1659743.
- ^ Peter S, Beglinger C (2007). "Helicobacter pylori and gastric cancer: the causal relationship". Digestion. 75 (1): 25–35. doi:10.1159/000101564. PMID 17429205.
- ^ Wang C, Yuan Y, Hunt RH (2007). "The association between Helicobacter pylori infection and early gastric cancer: a meta-analysis". Am. J. Gastroenterol. 102 (8): 1789–98. doi:10.1111/j.1572-0241.2007.01335.x. PMID 17521398.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cheung TK, Xia HH, Wong BC (2007). "Helicobacter pylori eradication for gastric cancer prevention". J. Gastroenterol. 42 Suppl 17: 10–5. doi:10.1007/s00535-006-1939-2. PMID 17238019.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wood C, Harrington W (2005). "AIDS and associated malignancies". Cell Res. 15 (11–12): 947–52. doi:10.1038/sj.cr.7290372. PMID 16354573.
- ^ Mellemkjaer L, Hammarstrom L, Andersen V; et al. (2002). "Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study". Clin. Exp. Immunol. 130 (3): 495–500. doi:10.1046/j.1365-2249.2002.02004.x. PMC 1906562. PMID 12452841.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Tolar J, Neglia JP (2003). "Transplacental and other routes of cancer transmission between individuals". J Pediatr Hematol Oncol. 25 (6): 430–4. doi:10.1097/00043426-200306000-00002. PMID 12794519.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Dingli D, Nowak MA (2006). "Cancer biology: infectious tumour cells". Nature. 443 (7107): 35–6. doi:10.1038/443035a. PMC 2711443. PMID 16957717.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Cancer Spread By Transplantation Extremely Rare: In Very Rare Case, Woman Develops Leukemia from Liver Transplant".
- ^ "The Nobel Prize in Physiology or Medicine 1980".
- ^ Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA (2006). "Clonal origin and evolution of a transmissible cancer". Cell. 126 (3): 477–87. doi:10.1016/j.cell.2006.05.051. PMC 2593932. PMID 16901782.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Croce CM (2008). "Oncogenes and cancer". The New England journal of medicine. 358 (5): 502–11. doi:10.1056/NEJMra072367. PMID 18234754.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Knudson AG (2001). "Two genetic hits (more or less) to cancer". Nature reviews. Cancer. 1 (2): 157–62. doi:10.1038/35101031. PMID 11905807.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E (2004). "Hypoxia and defective apoptosis drive genomic instability and tumorigenesis". Genes & Development. 18 (17): 2095–107. doi:10.1101/gad.1204904. PMC 515288. PMID 15314031.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Merlo LM, Pepper JW, Reid BJ, Maley CC (2006). "Cancer as an evolutionary and ecological process". Nat. Rev. Cancer. 6 (12): 924–35. doi:10.1038/nrc2013. PMID 17109012.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Cancer prevention: 7 steps to reduce your risk". Mayo Clinic. 2008-09-27. Retrieved 2010-01-30.
- ^ a b "Cancer Cancer". World Health Organization.
- ^ Boffetta P, Couto E, Wichmann J; et al. (2010). "Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)". J Natl Cancer Inst,. 8 (102): 529–37. doi:10.1093/jnci/djq072. PMID 20371762.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M (2005). "Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors". Lancet. 366 (9499): 1784–93. doi:10.1016/S0140-6736(05)67725-2. PMID 16298215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Lung Cancer in American Women: Facts". Retrieved 2007-01-19.
- ^ a b c "WHO calls for prevention of cancer through healthy workplaces" (Press release). World Health Organization. 2007-04-27. Retrieved 2007-10-13.
- ^ "National Institute for Occupational Safety and Health- Occupational Cancer". United States National Institute for Occupational Safety and Health. Retrieved 2007-10-13.
- ^ Buell P, Dunn JE (1965). "Cancer mortality among Japanese Issei and Nisei of California". Cancer. 18: 656–64. doi:10.1002/1097-0142(196505)18:5<656::AID-CNCR2820180515>3.0.CO;2-3. PMID 14278899.
- ^ Slattery ML, Boucher KM, Caan BJ, Potter JD, Ma KN (1998). "Eating patterns and risk of colon cancer". Am. J. Epidemiol. 148 (1): 4–16. PMID 9663397.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Larsson SC, Wolk A (2007). "Coffee consumption and risk of liver cancer: a meta-analysis". Gastroenterology. 132 (5): 1740–5. doi:10.1053/j.gastro.2007.03.044. PMID 17484871.
- ^ Ward MH, Sinha R, Heineman EF; et al. (1997). "Risk of adenocarcinoma of the stomach and esophagus with meat cooking method and doneness preference". Int. J. Cancer. 71 (1): 14–9. doi:10.1002/(SICI)1097-0215(19970328)71:1<14::AID-IJC4>3.0.CO;2-6. PMID 9096659.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Sinha R, Peters U, Cross AJ; et al. (2005). "Meat, meat cooking methods and preservation, and risk for colorectal adenoma". Cancer Res. 65 (17): 8034–41. doi:10.1158/0008-5472.CAN-04-3429. PMID 16140978.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Steck SE, Gaudet MM, Eng SM; et al. (2007). "Cooked meat and risk of breast cancer--lifetime versus recent dietary intake". Epidemiology (Cambridge, Mass.). 18 (3): 373–82. doi:10.1097/01.ede.0000259968.11151.06. PMID 17435448.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Anderson KE, Kadlubar FF, Kulldorff M; et al. (2005). "Dietary intake of heterocyclic amines and benzo(a)pyrene: associations with pancreatic cancer". Cancer Epidemiol. Biomarkers Prev. 14 (9): 2261–5. doi:10.1158/1055-9965.EPI-04-0514. PMID 16172241.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Campbell, T Colin and Campbell, Thomas M. The China Study: Startling implications for Diet, Weight Loss and Long-Term Health. Wakefield Press: South Australia 2007
- ^ Ornish D; et al. (2005). "Intensive lifestyle changes may affect the progression of prostate cancer". The Journal of Urology. 174 (3): 1065–9, discussion 1069–70. doi:10.1097/01.ju.0000169487.49018.73. PMID 16094059.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Chlebowski RT, Blackburn GL, Thomson CA; et al. (2006). "Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study". J. Natl. Cancer Inst. 98 (24): 1767–76. doi:10.1093/jnci/djj494. PMID 17179478.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Romieu I, Lazcano-Ponce E, Sanchez-Zamorano LM, Willett W, Hernandez-Avila M (1 August 2004). "Carbohydrates and the risk of breast cancer among Mexican women". Cancer Epidemiol Biomarkers Prev. 13 (8): 1283–9. PMID 15298947.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Francesca Bravi, Cristina Bosetti, Lorenza Scotti, Renato Talamini, Maurizio Montella, Valerio Ramazzotti, Eva Negri, Silvia Franceschi, and Carlo La Vecchia (2006). "Food Groups and Renal Cell Carcinoma: A Case-Control Study from Italy". International Journal of Cancer. 355:1991-2002 (3): 681. doi:10.1002/ijc.22225. PMID 17058282.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM (2005). "Fasting serum glucose level and cancer risk in Korean men and women". JAMA. 293 (2): 194–202. doi:10.1001/jama.293.2.194. PMID 15644546.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Michaud DS, Liu S, Giovannucci E, Willett WC, Colditz GA, Fuchs CS (2002). "Dietary sugar, glycemic load, and pancreatic cancer risk in a prospective study". J Natl Cancer Inst. 94 (17): 1293–300. doi:10.1093/jnci/94.17.1293. PMID 12208894.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Venkateswaran V, Haddad AQ, Fleshner NE; et al. (2007). "Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts". J Natl Cancer Inst. 99 (23): 1793–800. doi:10.1093/jnci/djm231. PMID 18042933.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Friebe, Richard: Can a High-Fat Diet Beat Cancer?, Time Magazine, Sep. 17, 2007
- ^ Hitti, Miranda: High Blood Sugar Linked to Cancer Risk, WebMD, 22 February 2008
- ^ Moynihan, Timothy:Cancer causes: Popular myths about the causes of cancer, MayoClinic.com, retrieved 22 Feb 2008
- ^ Avoid Sugary Drinks. Limit Consumption of Energy-Dense Foods, American Institute for Cancer Research, retrieved 20 Feb 2008
- ^ High sugar levels increase cancer and mortality risk, The Nation's Health: The Official Newspaper of the American Public Health Association, February 2005
- ^ Kushi LH, Byers T, Doyle C; et al. (2006). "American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity". CA Cancer J Clin. 56 (5): 254–81, quiz 313–4. doi:10.3322/canjclin.56.5.254. PMID 17005596.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Historical Overview" dietandcancerreport.org. Retrieved on 27 August 2008.
- ^ "Recommendations". dietandcancerreport.org. Retrieved on 27 August 2008.
- ^ Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Chapter 12 World Cancer Research Fund (2007). ISBN 978-0-9722522-2-5.
- ^ Yuen JW, Gohel MD (2005). "Anticancer effects of Ganoderma lucidum: a review of scientific evidence". Nutr Cancer. 53 (1): 11–7. doi:10.1207/s15327914nc5301_2. ISSN 0163-5581. PMID 16351502.
- ^ Hsu SC, Ou CC, Li JW; et al. (2008). "Ganoderma tsugae extracts inhibit colorectal cancer cell growth via G(2)/M cell cycle arrest". J Ethnopharmacol. 120 (3): 394. doi:10.1016/j.jep.2008.09.025. ISSN 0378-8741. PMID 18951965.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://www3.interscience.wiley.com/cgi-bin/fulltext/121398729/PDFSTART?CRETRY=1&SRETRY=0
- ^ http://www.ncbi.nlm.nih.gov/pubmed/12126464
- ^ http://www.ncbi.nlm.nih.gov/pubmed/7606203
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15084502, please use {{cite journal}} with
|pmid=15084502instead. (review) - ^ "Vitamins and minerals: not for cancer or cardiovascular prevention". Prescrire Int. 19 (108): 182. 2010. PMID 20939459.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Giovannucci E, Liu Y, Rimm EB; et al. (2006). "Prospective study of predictors of vitamin D status and cancer incidence and mortality in men". J. Natl. Cancer Inst. 98 (7): 451–9. doi:10.1093/jnci/djj101. PMID 16595781.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Vitamin D Has Role in Colon Cancer Prevention". Archived from the original on December 4, 2006. Retrieved 2007-07-27.
- ^ Schwartz GG, Blot WJ (2006). "Vitamin D status and cancer incidence and mortality: something new under the sun". J. Natl. Cancer Inst. 98 (7): 428–30. doi:10.1093/jnci/djj127. PMID 16595770.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Questions and answers about beta carotene chemoprevention trials" (PDF). National Cancer Institute. 1997-06-27. Retrieved 2009-04-23.
- ^ Cole BF, Baron JA, Sandler RS; et al. (2007). "Folic acid for the prevention of colorectal adenomas: a randomized clinical trial". JAMA. 297 (21): 2351–9. doi:10.1001/jama.297.21.2351. PMID 17551129.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Vogel V, Costantino J, Wickerham D, Cronin W, Cecchini R, Atkins J, Bevers T, Fehrenbacher L, Pajon E, Wade J, Robidoux A, Margolese R, James J, Lippman S, Runowicz C, Ganz P, Reis S, McCaskill-Stevens W, Ford L, Jordan V, Wolmark N (2006). "Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial". JAMA. 295 (23): 2727–41. doi:10.1001/jama.295.23.joc60074. PMID 16754727.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thompson I, Goodman P, Tangen C, Lucia M, Miller G, Ford L, Lieber M, Cespedes R, Atkins J, Lippman S, Carlin S, Ryan A, Szczepanek C, Crowley J, Coltman C (2003). "The influence of finasteride on the development of prostate cancer". N Engl J Med. 349 (3): 215–24. doi:10.1056/NEJMoa030660. PMID 12824459.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hallak A, Alon-Baron L, Shamir R, Moshkowitz M, Bulvik B, Brazowski E, Halpern Z, Arber N (2003). "Rofecoxib reduces polyp recurrence in familial polyposis". Dig Dis Sci. 48 (10): 1998–2002. doi:10.1023/A:1026130623186. PMID 14627347.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Baron J, Sandler R, Bresalier R, Quan H, Riddell R, Lanas A, Bolognese J, Oxenius B, Horgan K, Loftus S, Morton D (2006). "A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas". Gastroenterology. 131 (6): 1674–82. doi:10.1053/j.gastro.2006.08.079. PMID 17087947.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bertagnolli M, Eagle C, Zauber A, Redston M, Solomon S, Kim K, Tang J, Rosenstein R, Wittes J, Corle D, Hess T, Woloj G, Boisserie F, Anderson W, Viner J, Bagheri D, Burn J, Chung D, Dewar T, Foley T, Hoffman N, Macrae F, Pruitt R, Saltzman J, Salzberg B, Sylwestrowicz T, Gordon G, Hawk E (2006). "Celecoxib for the prevention of sporadic colorectal adenomas". N Engl J Med. 355 (9): 873–84. doi:10.1056/NEJMoa061355. PMID 16943400.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c "Cancer Vaccine Fact Sheet". NCI. 2006-06-08. Retrieved 2008-11-15.
- ^ Liao JC, Gregor P, Wolchok JD, Orlandi F, Craft D, Leung C, Houghton AN, Bergman PJ. (2006). "Vaccination with human tyrosinase DNA induces antibody responses in dogs with advanced melanoma". Cancer Immun. 6: 8. PMC 1976276. PMID 16626110.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "USDA Grants Conditional Approval for First Therapeutic Vaccine to Treat Cancer" (Press release). Animal Medical Centre. 2007-03-26. Retrieved 2009-06-06.
- ^ Black W, Haggerstrom D and Welch HG. All-cause Mortality in Randomized Trials of Cancer Screening. J Natl Cancer Inst, 6 Feb 2002; 94(3):167-73.
- ^ Boyle P and Brawley OW. Prostate Cancer: Current Evidence Weighs Against Population Screening (Editorial). CA Cancer J Clin (Jun 29) 2009; 59:220-224.
- ^ Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA (21 Oct) 2009; 302 (15): 1685-92.
- ^ Black W, Haggerstrom D and Welch HG. All-cause Mortality in Randomized Trials of Cancer Screening. J Natl Cancer Inst, 6 Feb 2002; 94(3):167-73
- ^ Benjamin, DJ. The efficacy of surgical treatment of breast cancer. Medical Hypotheses 1996; 47 (5): 389-97; Gøtzsche, P and Nielsen, M. Screening for breast cancer with mammography. Cochrane Database of Systematic Reviews, 2009. Issue 4 Art No.:CD001877
- ^ Brown BW, Brauner C, Minnotte MC. Non-cancer deaths in White Adult Cancer Patients. JNCI 1993; 85 (12): 979-987; Cuzick J, Stewart H, Rutqvist L et al. Cause-Specific Mortality in Long-term Survivors of Breast Cancer Who Participated in Trials of Radiotherapy. J. Clin Oncol 1994; 12 (3): 447-453
- ^ "What Is Cancer?". National Cancer Institute. Retrieved 2009-08-17.
- ^ "Cancer Fact Sheet". Agency for Toxic Substances & Disease Registry. 2002-08-30. Retrieved 2009-08-17.
- ^ Wanjek, Christopher (2006-09-16). "Exciting New Cancer Treatments Emerge Amid Persistent Myths". Retrieved 2009-08-17.
- ^ Hayden, Erika C. (2009-04-08). "Cutting off cancer's supply lines". Nature. 458 (7239): 686–687. doi:10.1038/458686b. PMID 19360048. [dead link]
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved Nov. 11, 2009.
{{cite web}}: Check date values in:|accessdate=(help) - ^ "Cancer" (PDF). World Health Organization.
- ^ a b c d e Jemal A, Siegel R, Ward E; et al. (2008). "Cancer statistics, 2008". CA Cancer J Clin. 58 (2): 71–96. doi:10.3322/CA.2007.0010. PMID 18287387.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Gurney JG, Smith MA, Ross JA (1999). "Cancer among infants". In Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR (eds) (ed.). Cancer Incidence and Survival among Children and Adolescents, United States SEER program 1975–1995. NIH Pub. No 99-4649. Bethesda, MD: National Cancer Institute, SEER Program. pp. 149–56.
{{cite book}}:|editor=has generic name (help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Rheingold, Susan; Neugut, Alfred; Meadows, Anna (2003). "156: Secondary Cancers: Incidence, Risk Factors, and Management". In Frei, Emil; Kufe, Donald W.; Holland, James F. (ed.). Cancer medicine 6. Hamilton, Ont: BC Decker. p. 2399. ISBN 1-55009-213-8.
{{cite book}}:|access-date=requires|url=(help); Check date values in:|accessdate=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Karpozilos A, Pavlidis N (2004). "The treatment of cancer in Greek antiquity". European Journal of Cancer. 40 (14): 2033–40. doi:10.1016/j.ejca.2004.04.036. PMID 15341975.
- ^ Moss, Ralph W. (2004). "Galen on Cancer". CancerDecisions.
- ^ "The History of Cancer". American Cancer Society. 2009.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Patricia Skinner (2001), Unani-tibbi, Encyclopedia of Alternative Medicine
- ^ Marilyn Yalom "A history of the breast" 1997. New York: Alfred A. Knopf. ISBN 0-679-43459-3
- ^ Grange JM, Stanford JL, Stanford CA (2002). "Campbell De Morgan's 'Observations on cancer', and their relevance today". Journal of the Royal Society of Medicine. 95 (6): 296–9. doi:10.1258/jrsm.95.6.296. PMC 1279913. PMID 12042378.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Boveri, Theodor (2008). "Concerning The Origin of Malignant Tumours". Journal of Cell Science. 121 (Supplement 1): 1–84. doi:10.1242/jcs.025742. PMID 18089652.
- ^ Sharon Begley (2008-09-16). "Rethinking the War on Cancer". Newsweek. Retrieved 2008-09-08.
- ^ Kolata, Gina (April 23, 2009). "Advances Elusive in the Drive to Cure Cancer". The New York Times. Retrieved 2009-05-05.
- ^ http://www.cancer.gov/templates/db_alpha.aspx?CdrID=44158
- ^ "Definition of Cure for Hodgkin's Disease." Cancer Research 31 1970 p 1828-1833
- ^ http://jco.ascopubs.org/cgi/content/full/23/34/8564
References
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ, Eds. Cancer Management: A Multidisciplinary Approach. 11th ed. 2009.
- The Basic Science of Oncology 4th ed. Tannock IF, Hill RP et al. (eds.) (2005). McGraw-Hill. ISBN 0-07138-774-9.
- Principles of Cancer Biology. Kleinsmith, LJ (2006). Pearson Benjamin Cummings. ISBN 0-80534-003-3.
- Parkin D, Bray F, Ferlay J, Pisani P (2005). "Global cancer statistics, 2002". CA Cancer J Clin. 55 (2): 74–108. doi:10.3322/canjclin.55.2.74. PMID 15761078.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. World Cancer Research Fund (2007). ISBN 978-0-9722522-2-5. Full text
- Cancer Medicine, 6th Edition—Textbook
- Encyclopedia of Cancer—4 volume reference work
- Weinberg, Robert A. (1996). "How Cancer Arises; An explosion of research is uncovering the long-hidden molecular underpinnings of cancer—and suggesting new therapies" (PDF). Scientific American: 62–70.
Introductory explanation of cancer biology in layman's language
{{cite journal}}: Unknown parameter|month=ignored (help)




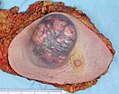
![Most common cancers in males, by occurrence[100]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/37/Most_common_cancers_-_male%2C_by_occurrence.png/147px-Most_common_cancers_-_male%2C_by_occurrence.png)
![in females, by occurrence[100]](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c5/Most_common_cancers_-_female%2C_by_occurrence.png/146px-Most_common_cancers_-_female%2C_by_occurrence.png)
![in males, by mortality[100]](http://upload.wikimedia.org/wikipedia/commons/thumb/a/a7/Most_common_cancers_-_male%2C_by_mortality.png/133px-Most_common_cancers_-_male%2C_by_mortality.png)
![in females, by mortality[100]](http://upload.wikimedia.org/wikipedia/commons/thumb/a/a4/Most_common_cancers_-_female%2C_by_mortality.png/141px-Most_common_cancers_-_female%2C_by_mortality.png)