Bedaquiline
 | |
| Clinical data | |
|---|---|
| Trade names | Sirturo |
| Other names | TMC207; R207910; AIDS222089 |
| License data |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
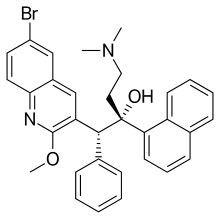
| Formula | C32H31BrN2O2 |
| Molar mass | 555.5 g mol g·mol−1 |
| 3D model (JSmol) | |
| |
Bedaquiline (also known as Sirturo, TMC207 or R207910) is an diarylquinoline anti-tuberculosis drug, which was discovered by a team led by the Belgian Koen Andries at Janssen Pharmaceutica.[1] It was described for the first time in 2004 at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) meeting Late-Breaker Session, after the drug had been in development for over seven years,[2] and a trial of 47 patients showed that it is effective in the treatment of M. tuberculosis.[3] It is the first new medicine to fight the infection in more than forty years.[4][5] Sirturo is the first medicine specifically designed for treating multi-drug-resistant tuberculosis — an increasingly common form of the disease that cannot be treated with at least two of the four primary antibiotics used to treat tuberculosis. The standard drugs used to fight the disease were developed in the 1950s and 1960s.
Roughly one-third of the world's population is estimated to be infected with the bacteria causing tuberculosis.
Bedaquiline was successfully applied in a phase II efficacy study published in 2010; it was developed by Tibotec and the TB Alliance.[6]
It is manufactured by Johnson & Johnson (J&J), who sought accelerated approval of the drug, a type of temporary approval for diseases lacking viable treatment options, such as multi-drug-resistant tuberculosis.[7] According to J&J, "the commercial opportunity is very limited [and] is part of [the company's effort to] advance innovative medicines that help address serious public-health issues."[7] By gaining approval for a drug that treats a neglected disease, J&J will be able to request expedited FDA review of a future drug.[8]
It was formally approved for use by the U.S. Food and Drug Administration (FDA) for use in tuberculosis (TB) treatment- but it is to be used normally only in cases of multi-drug-resistant tuberculosis, and in an even more resistant category, extensively drug resistant tuberculosis. Multi-drug resistant tuberculosis is defined as tuberculosis cases that do not respond to at least two of the four primary (first-line) antibiotics, developed mostly in the 1950s and 1960s, that are used to treat tuberculosis. The drug has been given a black-box warning for arrhythmias which may cause cardiac arrest[9].
Mode of action
Bedaquiline affects the proton pump for ATP synthase, which is unlike the quinolones, whose target is DNA gyrase.[10]
References
- ^ de Jonge MR, Koymans LH, Guillemont JE, Koul A, Andries K (2007). "A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910". Proteins. 67 (4): 971–80. doi:10.1002/prot.21376. PMID 17387738.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Protopopova M, Bogatcheva E, Nikonenko B, Hundert S, Einck L, Nacy CA (2007). "In search of new cures for tuberculosis" (PDF). Med Chem. 3 (3): 301–16. doi:10.2174/157340607780620626. PMID 17504204.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, Pistorius C, Krause R, Bogoshi M, Churchyard G, Venter A, Allen J, Palomino JC, De Marez T, van Heeswijk RP, Lounis N, Meyvisch P, Verbeeck J, Parys W, de Beule K, Andries K, Mc Neeley DF (2009). "The Diarylquinoline TMC207 for Multidrug-Resistant Tuberculosis". N. Engl. J. Med. 360 (23): 2397–2405. doi:10.1056/NEJMoa0808427. PMID 19494215.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "FDA Approves 1st New Tuberculosis Drug in 40 Years". ABC News. Retrieved 31 December 2012.
- ^ "F.D.A. Approves New Tuberculosis Drug". New York Times. Retrieved 31 December 2012.
- ^ Matteelli A, Carvalho AC, Dooley KE, Kritski A (2010). "TMC207: the first compound of a new class of potent anti-tuberculosis drugs". Future Microbiol. 5 (6): 849–58. doi:10.2217/fmb.10.50. PMC 2921705. PMID 20521931.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Walker, Joseph; Tadena, Nathalie (December 31, 2012). "J&J Tuberculosis Drug Gets Fast-Track Clearance". Wall Street Journal. Retrieved 2013-01-01.
- ^ Edney, Anna (December 31, 2012). "J&J&J Sirturo Wins FDA Approval to Treat Drug-Resistant TB". Bloomberg. Retrieved 2013-01-01.
- ^ http://news.msn.com/science-technology/fda-approves-1st-new-tuberculosis-drug-in-40-years-1?
- ^ Kotz J (2005). "Targeting tuberculosis". Nature Chemical Biology. doi:10.1038/nchembio002.
{{cite journal}}: Unknown parameter|month=ignored (help)
See also
- Multi-drug-resistant tuberculosis (MDR-TB)
