User:Neilsmith38/sandbox
Kallmann syndrome (KS) is a genetic disorder that prevents a person from starting or fully completing puberty. If left untreated people with Kallmann syndrome will have poorly defined secondary sexual characteristics, show signs of hypogonadism, almost invariably be infertile and be at increased risk of developing osteoporosis.[1]
Kallmann syndrome is a form of a group of conditions termed hypogonadotropic hypogonadism. Kallmann syndrome has an additional symptom of a total lack of sense of smell or a reduced sense of sense of smell which distinguishes it from other forms of hypogonadotropic hypogonadism.[1]
The underlying cause of the condition is a failure in the correct production or activity of the hormone normally produced by the hypothalamus called GnRH. This failure can lead to problems with the normal progression of puberty, failure of the reproductive cycle and hypogonadism. Hypogonadism is characterised by low levels of the sex hormones testosterone in males or oestrogen and progesterone in females. A range of other physical symptoms affecting the face, hands and skeletal system can also occur in some cases of Kallmann syndrome or hypogonadotropic hypogonadism. Diagnosis normally occurs during teenage years when puberty fails to start. Treatment for both males and females is normally required life long. Hormone replacement therapy (HRT) is the major form of treatment with the aim to replace the missing testosterone or oestrogen / progesterone. Specialised fertility treatments are also available.[2][3][4]
A 2011 study of the Finnish population produced an estimated incidence of 1 in 48,000 people overall, with 1 in 30,000 for males and 1 in 125,000 for females.[5] The condition is more commonly diagnosed in males than in females.[6] Kallmann syndrome was first described by name in a paper published in 1944 by Franz Josef Kallmann, a German-American geneticist.[7][8] The link between anosmia and hypogonadism had already been noted by the Spanish doctor Aureliano Maestre de San Juan in 1856.[9]
It is normally difficult to distinguish a case of KS / HH from a straightforward constitutional delay of puberty. However, if puberty has not started by either age 14 (girls) or 15 (boys) and one or more of the non-reproductie features mentioned belowe is present then a referral to reproductive endocrinologist might be advisable.[10]
The features of Kallmann syndrome (KS) and other forms of hypogonadotropic hypogonadism (HH) can be split into two different categories; "reproductive" and "non reproductive".[3][11][4][12][2]
- Failure to start or fully complete puberty in both men and women
- Lack of testicle development in men (size < 4 ml, whereas the normal range is between 12 and 25 ml)
- Primary amenorrhoea (failure to start menstruation)
- Poorly defined secondary sexual characteristics in both men and women.
- Micropenis in 5-10% of male cases
- Cryptorchidism (undescended testicles) at birth.
- Low levels of the gonadotropins LH and FSH
- Hypogonadism due to low levels of testosterone in men or oestrogen / progesterone in females
- Infertility
- Total lack of sense of smell (anosmia) or markedly reduced sense of smell (hyposmia). This is the defining feature of Kallmann syndrome; it is not seen in other cases of HH. Approximately 50% of HH cases occur with anosmia and can be termed as Kallmann syndrome.[2]
- Cleft palate, hare lip or other midline cranio-facial defects.[3]
- Neural hearing impairment[2]
- Absence of one of the kidneys (unilateral renal agenesis)[2]
- Skeletal defects including split hand/foot (ectrodactyly), shortened middle finger (metacarpal) or scoliosis[2]
- Manual synkinesis (mirror movements of hands)[2]
- Missing teeth (hypodontia)[2]
- Poor balance or coordination due to cerebral ataxia
- Eye movement abnormalities
The exact genetic nature of each particular case of KS / HH will determine which, if any, of the non-reproductive features will occur. The severity of the symptoms will also vary from case to case. Even family members will not show the same range or severity of symptoms.[2]
KS / HH is most often present from birth but adult onset versions are found in both males and females. The hypothalamic-pituitary-gonadal axis (HPG axis) functions normally at birth and well into adult life giving normal puberty and normal reproductive function. The HPG axis then either fails totally or is reduced to a very low level of GnRH release, in adult life with no obvious cause such as a pituitary tumour. This will lead to a fall in testosterone or oestrogen levels and infertility.
Functional hypothalamic amenorrhoea is seen in females where the HPG axis is suppressed in response to physical or psychological stress or malnutrition. It is reversible with the removal of the stressor.
Some cases of KS / HH appear to reverse during adult life where the HPG axis resumes its normal function and GnRH, LH, and FSH levels return to normal levels. This occurs in an estimated 10 to 20% of cases, primarily normosmic CHH cases rather than KS cases and only found in patients who have undergone some form of testosterone replacement therapy. It is only normally discovered when testicular volume increases while on testosterone treatment alone and testosterone levels return to normal when treatment is stopped. This type of KS/CHH rarely occurs in cases where males have had a history of un-descended testes.
Affected individuals with KS and other forms of HH are almost invariably born with normal sexual differentiation; i.e., they are physically male or female. This is due to the human chorionic gonadotrophin (hCG) produced by placenta at approximately 12 to 20 weeks gestation (pregnancy) which is normally unaffected by having KS or CHH.
People with KS/CHH lack the surge of GnRH, LH, and FSH that normally occurs between birth and six months of age. This surge is particularly important in infant boys as it helps with testicular descent into the scrotum. The surge of GnRH/LH/FSH in non KS/HH children gives detectable levels of testosterone in boys and oestrogen & progesterone in girls. The lack of this surge can sometimes be used as a diagnostic tool if KS/CHH is suspected in a newborn boy, but is not normally distinct enough for diagnosis in girls.[3]
Taken together, it is likely that testosterone has direct effects on bone quality via the androgen receptor as well as indirect effects via conversion to estrogen by aromatase. Bisphosphonates should be first-line therapy in the treatment of male hypogonadism-related osteoporosis, with the consideration for the addition of testosterone replacement therapy.
One possible side effect of having KS/CHH is the increased risk of developing secondary osteoporosis or osteopenia. Oestrogen (females) or testosterone (males) is essential for maintaining bone density.[16] Deficiency in either testosterone or oestrogen can increase the rate of bone resorption while at the same time slowing down the rate of bone formation. Overall this can lead to weakened, fragile bones which have a higher tendency to fracture.
Even a short time with low oestrogen or testosterone, as in cases of delayed diagnosis of KS/CHH can lead to an increased risk of developing osteoporosis but other risk factors are involved so the risk of developing it will vary from person to person.
People with KS/CHH should have a bone density scan at least every five years, even if they are on constant hormone replacement therapy. This interval will be shortened to three years if the patient is already in the at-risk zone (osteopenia) or yearly if the patient has osteoporosis already.
The bone density scan is known as a dual energy X-ray absorptiometry scan (DEXA or DXA scan). It is a very simple straightforward test, taking less than 15 minutes to perform. It involves taking a specialised X-ray picture of the spine and hips and measuring the bone mineral density and comparing the result to the average value for a young healthy adult in the general population.[17]
Adequate calcium levels, and probably more importantly vitamin D levels are essential for healthy bone density. Some patients with KS/CHH will have their levels checked and may be prescribed extra vitamin D tablets or injections to try to prevent the condition getting worse. The role of vitamin D for general overall health is under close scrutiny at the moment with some researchers claiming vitamin D deficiency is prevalent in many populations and can be linked to other disease states.
Some people with severe osteoporosis might be prescribed bisphosphonates to preserve bone mass. Exercise, especially weight bearing and resistance exercise, is known to reduce the risk of osteoporosis.
Cite error: The <ref> tag has too many names (see the help page).
[14]
Cite error: A <ref> tag is missing the closing </ref> (see the help page).
Cite error: A <ref> tag is missing the closing </ref> (see the help page).
[18]. Thereafter, the same named reference may be reused any number of times either before or after the defining use by typing just [18].
In males with KS / CHH infertility is primarily due the lack of sperm production within the testes. Sperm production can be achieved through either the use of GnRH administered via a micro infusion pump or through the use of gonadotropin injections (hCG,FSH,hMG). The time taken to achieve adequate sperm production for natural conception will vary from person to person. If the pre treatment testes are very small and there has been a history of undescended testes it might take longer to achieve sperm production. In these cases assisted reproductive technology such as sperm retrieval using testicular sperm extraction (TESE) and / or intracytoplasmic sperm injection (ICSI) might be required.[27]
In females with KS / CHH infertility is primarily due to the lack of maturation of eggs located within the ovaries. Ovulation induction can be achieved either with pulsatile GnRH therapy or alternatively with gonadotropin injections (hCG,FSH,hMG) given at set intervals to trigger the maturation and release of the egg to allow for natural conception.[27]
The gonadotrophin deficiency in men and women with nCHH/KS is a cause of infertility resulting from failed gamete production and/or maturation (5-9). In men with CHH/KS, infertility is due to absent sperm production (5,6,27,28). However, spermatogenesis can be induced in most cases by either long-term pulsatile GnRH administration via a microinfusion pump, or by exogenous gonadotropin injections 5,6,27,28). A number of studies conducted over the past 30 years have clearly demonstrated that these treatments can be effective (5-9,27,28). In the most difficult cases (patients with very small testes and/or with cryptorchidism)(29,30), longer treatment may be required to conceive as well as use of assisted reproductive techniques (ART) i.e. microsurgical testicular sperm extraction (micro-TESE) and/or intracytoplasmic sperm injection (ICSI) (27,28,31,32).
[28] [28] [28] [28] [28] [28] [28]
PMCID: PMC5341390
Abnormalities in various genes have be shown to disrupt the ability of the hypothalamus to produce gonadotrophin releasing hormone GnRH which in turn causes the pituitary to fail to release sufficient levels of follicle-stimulating hormone (FSH) and luteinising hormone (LH). LH and FSH have a direct action on the testes in men and ovaries in women.[12]
Sixteen known gene defects have so far been shown to cause a disruption in GnRH production.[37]These gene defects can be split into two separate groups depending on their resluting action on the hypothalamus.
One group of gene defects disrupt the ability of the hypothalamus itself to produce or release GnRH, leading to a case of HH with an unaffected sense of smell, sometimes called normosmic hypogonadotrophic hypogonadism (nHH). The other major group of gene defects affect the migration of GnRH neurones into the hypothalmus during embryonic development. Since the GnRH neurones and olfactory neurones travel along the same pathways any impairment in GnRH neurone migration also prevents olfactory neurone migration leading to the anosmia or lack of sense of smell seen in Kallmann syndrome.
Table of known genes responsible for cases of Kallmann syndrome and other forms of hypogonadotropic hypogonadism. Listed are the estimated prevalence of cases caused by the specific gene, additional associated symptoms and the form of inheritance.[6][2]Between 35-45% of cases of KS / CHH have an unknown genetic cause.[26]
| Prevalence (%) | OMIM | Name | Gene | Locus | Clinical features | Syndromes Associated | Inheritance pattern |
|---|---|---|---|---|---|---|---|
| 5[6], 5-10[2] | Template:OMIM2 | KAL1 (ANOS1) | KAL1 | Xp22.3 | Anosmia. Bimanual synkinesis. Renal agenesis. | x-linked | |
| 10[6][2] | Template:OMIM2 | KAL2 | FGFR1 | 8p11.23 | Cleft lip and / or cleft palate. Septo-optic dysplasia. Skeletal anomomalies. Bimanual synkinesis. Hand / foot malformations such as ectrodactyly. Combined pituitary hormone deficiency. | Hartsfield syndrome | Autosomal dominant |
| 6-16[6], 5-10[2] | Template:OMIM2 | GNRHR | GNRHR | 4q13.2 | Autosomal recessive | ||
| 6[6], 5-10[2] | Template:OMIM2 | CHD7 | CHD7 | 8q12.2 | Congenital hearing loss. Semicircular canal hypoplasia. | CHARGE syndrome | Autosomal dominant |
| 3-6[6], <2[2] | Template:OMIM2 | KAL4 | PROK2 | 3p13 | Autosomal recessive | ||
| 3-6[6], 5[2] | Template:OMIM2 | KAL3 | PROKR2 | 20p12.3 | Combined pituitary hormone deficiency. | Morning Glory syndrome | Autosomal recessive |
| 3[6], 2-5[2] | Template:OMIM2 | IL17RD | IL17RD | 3p14.3 | Congenital hearing loss. | Autosomal recessive | |
| 2[6], 2-5[2] | Template:OMIM2 | SOX10 | SOX10 | 22q13.1 | Congenital hearing loss. | Waardenburg syndrome | Autosomal dominant |
| 2[6], <2[2] | Template:OMIM2 | KISS1 | KiSS-1 | 1q32.1 | Autosomal recessive | ||
| 2[6], <2[2] | Template:OMIM2 | KISS1R (GPR54) | GPR54 | 19p13.3 | Autosomal recessive | ||
| <2[2] | Template:OMIM2 | FGF8 | FGF8 | 10q24.32 | Cleft lip and / or cleft palate. Skeletal anomomolies. Bimanual synkinesis. Combined pituitary hormone deficiency. | Autosomal dominant | |
| <2[6], 1 report[2] | Template:OMIM2 | FGF17 | FGF17 | 8p21.3 | Dandy-Walker syndrome | Autosomal dominant | |
| <2[6] | Template:OMIM2 | LEP | LEP | 7q32.1 | Early onset of morbid obesity. | Autosomal recessive | |
| <2[6] | Template:OMIM2 | LEPR | LEPR | 1p31.3 | Early onset of morbid obesity. | Autosomal recessive | |
| <2[6] | Template:OMIM2 | PCSK1 | PCSK1 | 5q15 | Early onset of morbid obesity. | Autosomal recessive | |
| Rare[6], 1 report[2] | Template:OMIM2 | FEZF1 | FEZF1 | 7q31.32 | Autosomal recessive | ||
| Rare[6], 1 report[2] | Template:OMIM2 | CCDC141 | CCDC141 | 2q31.2 | Unknown | ||
| Rare[6], <2[2] | Template:OMIM2 | SEMA3A | SEMA3A | 7q21.11 | Autosomal dominant | ||
| 1 report[2] | Template:OMIM2 | SEMA3E | SEMA3E | 7q21.11 | CHARGE syndrome | Autosomal dominant | |
| Rare[6] | Template:OMIM2 | SEMA7A | SEMA7A | 15q24.1 | Autosomal dominant | ||
| Rare[6], <2[2] | Template:OMIM2 | HS6ST1 | HS6ST1 | 2q14.3 | Cleft lip and / or cleft palate. Skeletal anomalies. | Autosomal dominant | |
| Rare[6], 1 report[2] | Template:OMIM2 | WDR11 | WDR11 | 10q26.12 | Combined pituitary hormone deficiency. | Autosomal dominant | |
| Rare[6] | Template:OMIM2 | NELF (NSMF) | NELF | 9q34.3 | Autosomal dominant | ||
| Rare[6] | Template:OMIM2 | IGSF10 | IGSF10 | 3q24 | Autosomal dominant | ||
| Rare[6], <2[2] | Template:OMIM2 | GNRH1 | GNRH1 | 8p21.2 | Autosomal recessive | ||
| Rare[6], <2[2] | Template:OMIM2 | TAC3 | TAC3 | 12q3 | Autosomal recessive | ||
| Rare[6], 5[2] | Template:OMIM2 | TACR3 | TACR3 | 4q24 | Autosomal recessive | ||
| Rare[6] | Template:OMIM2 | OTUD4 | OTUD4 | 4q31.21 | Cerebellar ataxia. | Gordon Holmes syndrome | Autosomal recessive |
| Rare[6] | Template:OMIM2 | RNF216 | RNF216 | 7p22.1 | Cerebellar ataxia. | Gordon Holmes syndrome | Autosomal recessive |
| Rare[6] | Template:OMIM2 | PNPLA6 | PNPLA6 | 19p13.2 | Cerebellar ataxia. | Gordon Holmes syndrome | Autosomal recessive |
| 1 report[2] | Template:OMIM2 | AXL | AXL | 19q13.2 | Unknown | ||
| Rare[6] | Template:OMIM2 | DMXL2 | DMXL2 | 15q21.2 | Polyendocrine deficiencies and polyneuropathy. | Autosomal recessive | |
| Rare[6] | Template:OMIM2 | NR0B1 (DAX1) | NR0B1 | Xp21.2 | Adrenal hypoplasia. | x-linked | |
| 1 report[2] | Template:OMIM2 | DUSP6 | DUSP6 | 12q21.33 | Autosomal dominant | ||
| 1 report[2] | Template:OMIM2 | POLR3B | POLR3B | 12q23.3 | Autosomal recessive | ||
| 1 report[2] | Template:OMIM2 | SPRY4 | SPRY4 | 5q31.3 | Autosomal dominant | ||
| 1 report[2] | Template:OMIM2 | FLRT3 | FLRT3 | 20p12.1 | Autosomal dominant | ||
| 1 report[2] | Template:OMIM2 | SRA1 | SRA1 | 19q13.33 | Unknown | ||
| Rare[6] | Template:OMIM2 | HESX1 | HESX1 | 3p14.3 | Septo-optic dysplasia. Combined pituitary hormone deficiency. | Autosomal recessive and dominant |
704 is consensus guidelines
Diagnosis of KS / CHH normal involves a range of clinical, biochemical and radiological tests to exclude other conditions that can cause similar symptoms.
- Comparing height to standard growth charts.
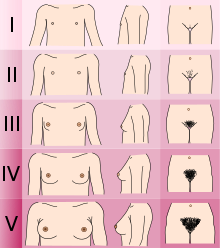
- Determining the Tanner stage of sexual development.
- Checking for micropenis and undescended testes (cryptorchidism) in males.
- Measuring testicular volume.
- Checking for breast development and age at menarche in females.
- Checking sense of smell using odorant panel or University of Pennsylvania Smell Identification Test (UPSIT)
- Checking for hearing impairment.
- Checking for missing teeth or presence of cleft lip and/or cleft palate.
- Checking for pigmentation of skin and hair.
- Checking for mirror movements of the hands or signs of neurodevelopmental delay.
- Early morning hormonal testing including FSH, LH, testosterone, oestrogen and prolactin.
- GnRH and / or hCG stimulation test to determine activity of hypothalamus and pituitary.
- Sperm test
- Liver function, renal function and inflammation marker testing.
- Karyotype to check for chromosomal abnormalities.
- Performing wrist x-ray to determine bone age.
- Brain MRI to rule out any structural abnormalities in the hypothalamus or pituitary and to check for presence of olfactory bulbs.
- Ultrasound of kidneys to rule of unilateral renal agenesis.
- Bone density scan (DXA) to check for osteoporosis or osteopenia.
Early treatment in male neonates involves lowering the testes into the correct position in the case of cryptorchidism and treating micropenis if present. There is no early treatment required for female neo
At around the ages of 14 or 15, or earlier if specific additional symptoms such as anosmia are present treatment can be given to both male and
Micropenis: testosterone, DHT or gonadotropin therapy (patients aged 1–6 months) Penile growth
Adolescent
Patients aged 14–15 years (earlier in the presence of specific signs such as anosmia) Virilization or estrogenization
Sexual function
Growth and bone health
Gonadal maturation and future fertility
Psychological wellbeing Male patients: testosterone (oral, injectable or transdermal)
Gonadotropins? Genital development
Growth and epiphyseal closure
Virilization
Sexual function
Wellbeing
Adherence
Reversibility Morning serum testosterone levels (trough levels for injections), LH, FSH and inhibin B levels, haemocrit Testosterone treatment will not induce testicular growth or fertility
Female patients: estradiol (oral or transdermal) followed by estradiol + progesterone or progestin Breast development
Growth and epiphyseal closure
Estrogenization
Feminized body
Menses
Sexual function
Bone health
Wellbeing
Adherence
Reversibility No specific Estradiol must be increased slowly before the combination of estradiol + progesterone or progestin to maximize breast development and avoid areolar protrusion
Adulthood
All patients Sexual function
Fertility
Limiting comorbidities
Psychological wellbeing
Puberty induction Male patients:
Testosterone (injectable or transdermal)
hCG ± FSH
FSH, FSH + hCG
GnRH pump Pubertal development
Sexual function and libido
Bone health
Wellbeing
Adherence
Fertility
Reversibility Tough serum testosterone levels, LH, FSH and inhibin B levels, haematocrit, PSA levels Sex steroid replacement will not induce fertility
Female patients:
Estradiol (oral or transdermal)
Progesterone or progestin
FSH + hCG or GnRH pump Pubertal development
Sexual function and libido
Bone health
Wellbeing
Adherence
Fertility
Reversibility Serum levels of estradiol, LH, FSH, inhibin B and AMH
In the 1950s De Morsier and Gauthier reported the partial or complete absence of the olfactory bulb in the brains of men with hypogonadism.[59][11]
(from PDB: 1YY1)
To date at least twenty five different genes have been implicated in causing Kallmann syndrome or other forms of HH through a disruption in the production or activity of GnRH. These genes involved cover all forms of inheritance and no one gene defect has been shown to be common to all cases which makes genetic testing and inheritance prediction difficult.[37][60]
The number of genes known to cause cases of KS / CHH is still increasing.[12] In addition it is thought that some cases of KS / CHH are caused by two separate gene defects occurring at the same time.[6]
Some of the genes known to be involved in cases of KS / CHH are listed in the Online Mendelian Inheritance in Man ((OMIM)) table at the end of this article.
| OMIM | Name | Gene | Locus | Description |
|---|---|---|---|---|
| Template:OMIM2 | KAL1 | KAL1 (ANOS1) | Xp22.3 | Kallmann syndrome can be inherited as an X-linked recessive trait, in which case there is a defect in the KAL1 (ANOS1) gene, which maps at chromosome Xp22.3.[61][62]
This genetic form may include synkinesis and renal agenesis. ANOS1 encodes an extracellular matrix glycoprotein, anosmin-1, present in various embryonic tissues including the presumptive olfactory bulbs in the rostral forebrain. The protein is required to promote the embryonic migration of olfactory nerve fibres and GnRH neurons from the olfactory epithelium of the nose into the brain.[63][64] |
| Template:OMIM2 and Template:OMIM2 | KAL2 | FGFR1 and FGF8 | 8p11.23 and 10q24.32 | Autosomal dominant mutations of FGFR1, encoding fibroblast growth factor receptor 1, or FGF8, encoding one of its ligands (fibroblast growth factor 8), cause about 10% of KS/CHH cases. These genetic forms may include cleft lip and / or palate, hypodontia, hearing impairment, or ectrodactyly (FGFR1 mutations).[65][66][67] |
| Template:OMIM2 and Template:OMIM2 | KAL3 | PROKR2 and PROK2 | 20p12.3 and 3p13 | Mutations of PROKR2, encoding prokineticin receptor-2, or PROK2, encoding one of its ligands (prokineticin 2), are involved in autosomal recessive forms of KS (where both alleles of the gene are mutated), but most people carrying mutations in either gene only have one mutated allele, suggesting that they carry at least one additional mutation in another, as yet unidentified in most cases, KS gene (oligogenic forms).[55] |
| Template:OMIM2 | FEZF1 | FEZF1 | 7q31.32 | Mutations of FEZF1, encoding a (zinc finger)-domain containing protein, are involved in an autosomal recessive form of KS. The protein is required for the passage of growing olfactory nerve fibres and GnRH releasing neurones into the brain.[68] |
| Template:OMIM2 | CHD7 | CHD7 | 8q12.2 | Mutations of CHD7 have first been reported in CHARGE syndrome, a severe developmental disease affecting multiple organs, which often includes KS. CHD7 encodes a transcriptional regulator that binds to enhancer elements in the nucleoplasm.[22][69] |
| Template:OMIM2 | SOX10 | SOX10 | 22q13.1 | Mutations of SOX10 have first been reported in Waardenburg syndrome, which may include KS in addition to deafness. SOX10 encodes a transcription factor expressed by olfactory ensheathing cells, glial cells of neural crest origin that are permissive for the elongation and targeting of olfactory nerve fibres.[70] |
| Template:OMIM2 | SEMA3A | SEMA3A | 7q21.11 | Mutations of SEMA3A, encoding semaphorin 3A (ligand of plexin A1 receptor), involved in the guidance of olfactory nerve fibers into the brain, are thought to be involved in oligogenic forms of KS.[71] |
| Template:OMIM2 | NELF | NELF | 9q34.3 | Associated with the migration of the olfactory axons and GnRH neurones during development. |
| Template:OMIM2 | FLRT3 | FLRT3 | 20p12.1 | Encodes fibronectin-like domain-containing leucine rich transmembrane protein 3. Protein associated with the function of the KAL2 genes (FGFR1 and FGF8) which allows for the migration of both olfactory axons and GnRH releasing neurones during early embryonic development.[36] |
| Template:OMIM2 | FGF17 | FGF17 | 8p21.3 | Encodes fibroblast growth factor 17. Protein associated with the function of the KAL2 genes (FGFR1 and FGF8) which allows for the migration of both olfactory axons and GnRH releasing neurones during early embryonic development.[36] |
| Template:OMIM2 | IL17RD | IL17RD | 3p14.3 | Encodes interleukin receptor 17 D. Protein associated with the function of the KAL2 genes (FGFR1 and FGF8) which allows for the migration of both olfactory axons and GnRH releasing neurones during early embryonic development.[36] |
| Template:OMIM2 | DUSP6 | DUSP6 | 12q21.33 | Encodes dual specificity phosphate-6. Protein associated with the function of the KAL2 genes (FGFR1 and FGF8) which allows for the migration of both olfactory axons and GnRH releasing neurones during early embryonic development.[36] |
| Template:OMIM2 | SPRY4 | SPRY4 | 5q31.3 | Encodes sprouty, Drosphila, homolog of, 4. Protein associated with the function of the KAL2 genes (FGFR1 and FGF8) which allows for the migration of both olfactory axons and GnRH releasing neurones during early embryonic development.[36] |
| Template:OMIM2 and Template:OMIM2 | GNRHR and GNRH1 | GNRHR and GNRH1 | 4q13.2 and 8p21.2 | Biallelic mutations of GNRHR or GNRH1, encoding the GnRH receptor and the hormone GnRH1, respectively, cause normosmic CHH or partial CHH. Binding of GnRH1 to its receptor allows FSH/LH secretion by the pituitary gland.[72][73] |
| Template:OMIM2 and Template:OMIM2 | KISS1R and KISS1 | KiSS-1 and KiSS-1R | 19p13.3 and 1q32.1 | Biallelic mutations of KISS1R or KISS1, encoding the kisspeptin receptor 1 and the ligand kisspeptin 1, respectively, cause normosmic CHH. Kisspeptin, produced in the hypothalamus, is essential for pulsatile GnRH secretion, and is thought to be involved in the timing of the onset of puberty.[74][75] |
| Template:OMIM2 and Template:OMIM2 | TACR3 and TAC3 | TACR3 and TAC3 | 4q24 and 12q13.3 | Biallelic mutations of TACR3 or TAC, encoding the receptor of neurokinin B and the ligand neurokinin B, respectively, cause normosmic CHH (usually severe HH with high incidence of micropenis). They are associated with a higher rate of reversible HH than mutations of other CHH genes. Neurokinin B, produced in the hypothalamus, is crucial for GnRH secretion.[76] |
| Template:OMIM2 | LEP | LEP | 7q31.2 | Encodes leptin, the ligand of the receptor LEPR. Involved in pulsatile GnRH secretion. |
| Template:OMIM2 | DAX1/NROB1 | DAX1 | Xp21.2 | Encodes a nuclear receptor with no known ligand. Known to be a transcription inhibitor. Mutations in DAX1 are thought to cause X-linked recessive forms of CHH in both males and occasionally females. Known to cause pubertal delay in females. |


==============================
Treatment

For both males and females the initial aim for treatment is the development of the secondary sexual characteristics normally seen at puberty.[2][45][77][78][58] Once this has been achieved continued hormone replacement therapy is required for both males and females to maintain sexual function, bone health, libido and general wellbeing.[3]In males testosterone replacement therapy is required for the maintenance of normal muscle mass.[2]
Early treatment is sometimes required for male infants with suspected KS / CHH to correct un-descended testes and micropenis if present with the use or surgery or gonadotropin or DHT treatment. Females with KS / CHH normally do not require any treatment before the age of adolescence. Currently no treatments exist for the lack of sense of smell, mirror movement of the hands or the absence of one kidney.[3]
Treatment for both males and females with KS / CHH is normally comprised of one of three options[2][3]
- Sex hormone replacement (testosterone or oestrogen & progesterone).
- Gonadotropin therapy (medications that replicate the activity of FSH and LH).
- GnRH pulsatile therapy.
Hormone replacement therapy
The method and dose of treatment will vary depending on the individual being treated. Initial treatment is normally made with lower doses in younger patients in order to develop the secondary sexual characteristics before adult doses are reached.[2]
For males with KS / CHH the types of testosterone delivery include daily patches, daily gel use, daily capsules, sub cutaneous or intramuscular injections or six monthly implants. Different formulations of testosterone are used to ensure both the anabolic and androgenic effects of testosterone are achieved.[3][4]Nasal testosterone delivery methods have been developed but their use in KS / CHH treatment has not been formally evaluated.[2]
Gonadotropin therapy in the form of human chorionic gonadotropin (hCG) injections with or without the use of FSH can also be used in male patients to induce secondary sexual characteristic development along with possible fertility induction at the same time.[3]
For females hormone replacement involves the use of oestrogen and progesterone. In females oestrogen only is used first in tablet or gel form in order to maximise breast development before a combination of oestrogen and progesterone is used.[3][2]Cyclical progesterone is normally required used to help keep the endometrium (lining of the uterus healthy).[2]
In males the monitoring of treatment normally requires the measurement of serum testosterone, inhibin B, haematocrit and prostate-specific antigen (PSA). If injections are used trough levels are taken to ensure an adequate level of testosterone is achieved throughout the injection cycle.[3]
In females monitoring normally comprises of measurement of oestrogen, FSH, LH, inhibin B and anti-Müllerian hormone (AMH).[3]
Standard hormone replacement therapy will not normally induce fertility in either males or females, with no testicular growth in males. Early treatment as adolescents can help with psychological well being of people with KS / CHH.[3]
Gonadotropin therapy
For males with KS / CHH the types of testosterone delivery include daily patches, daily gel use, daily capsules, sub cutaneous or intramuscular injections or six monthly implants. Different formulations of testosterone are used to ensure both the anabolic and androgenic effects of testosterone are achieved.[3][4]
For females hormone replacement involves the use of oestrogen and progesterone. In females oestrogen only is used first before a combination of oestrogen and progesterone.[3]
For both males and females the initial aim for treatment is the development of the majority of the secondary sexual characteristics normally seen at puberty. Once this has been achieved continued hormone replacement therapy is required for both males and females to maintain sexual function, bone health, libido and general wellbeing.[3]In males testosterone replacement therapy is required for the maintenance of normal muscle mass.[2]
For both males and females standard hormone replacement therapy will not induce fertility or testicular growth in males.[3]
- ^ a b c d e f https://ghr.nlm.nih.gov/condition/kallmann-syndrome
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az Balasubramanian R, Crowley WF Jr (2017). "Isolated Gonadotropin-Releasing Hormone (GnRH) Deficiency". SourceGeneReviews® [Internet]. PMID 20301509.
- ^ a b c d e f g h i j k l m n o p q r s Boehm U, Bouloux PM, Dattani MT, et al. (2015). "Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism-pathogenesis, diagnosis and treatment". Nat Rev Endocrinol. 11 (Jul 21): 547–64. doi:10.1038/nrendo.2015.112. PMID 26194704. Cite error: The named reference "pmid:26194704" was defined multiple times with different content (see the help page).
- ^ a b c d e Dunkel L, Quinton R (2014). "Transition in endocrinology: induction of puberty". Eur J Endocrinol. 170 (6): R229-39. doi:10.1530/EJE-13-0894. PMID 24836550. Cite error: The named reference "pmid:24836550" was defined multiple times with different content (see the help page).
- ^ a b Laitinen EM1, Vaaralahti K, Tommiska J, Eklund E, Tervaniemi M, Valanne L, Raivio T. (2011). "Incidence, phenotypic features and molecular genetics of Kallmann syndrome in Finland". Orphanet J Rare Dis. 6:41 (Jun 17): 41. doi:10.1186/1750-1172-6-41.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) CS1 maint: unflagged free DOI (link) Cite error: The named reference "pmid: 21682876" was defined multiple times with different content (see the help page). - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq Lima Amato LG, Latronico AC, Gontijo Silveira LF (2017). "Molecular and Genetic Aspects of Congenital Isolated Hypogonadotropic Hypogonadism". Endocrinol Metab Clin North Am. 46 (2): 283–303. doi:10.1016/j.ecl.2017.01.010. PMID 28476224.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid:28476224" was defined multiple times with different content (see the help page). - ^ Kallmann FJ, Schönfeld WA, Barrera SE (1943–1944). "The genetic aspects of primary eunuchoidism". Am J Ment Defic. 48: 203–236.
- ^ synd/2549 at Who Named It?
- ^ Maestre de San Juan, Aureliano (1856). "Teratolagia: falta total de los nervios olfactorios con anosmia en un individuo en quien existia una atrofia congenita de los testiculos y miembro viril". El Siglo Médico. 3: 211–221.
- ^ McCabe MJ, Bancalari RE, Dattani MT (2014). "Diagnosis and evaluation of hypogonadism". Pediatr Endocrinol Rev. 11 (Feb): Suppl 2:214–29. PMID 24683946.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Soo-Hyun Kim (2015). "Congenital Hypogonadotropic Hypogonadism and Kallmann Syndrome: Past, Present, and Future". Endocrinol Metab (Seoul). 30 (4): 456–466. doi:10.3803/EnM.2015.30.4.456. PMID 4722398. Cite error: The named reference "pmid:PMC4722398" was defined multiple times with different content (see the help page).
- ^ a b c d Mitchell AL, Dwyer A, Pitteloud N, Quinton R (2011). "Genetic basis and variable phenotypic expression of Kallmann syndrome: towards a unifying theory". Trends Endocrinol Metab. 22 (7): 249–58. PMID 21511493.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid21511493" was defined multiple times with different content (see the help page). - ^ Golds G, Houdek D, Arnason T (2017). "Male Hypogonadism and Osteoporosis: The Effects, Clinical Consequences, and Treatment of Testosterone Deficiency in Bone Health". Int J Endocrinol. : (2017:4602129). doi:10.1155/2017/4602129. PMID PMC5376477.
{{cite journal}}: Check|pmid=value (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Cite error: The named reference
NIH Genetics Home Referencewas invoked but never defined (see the help page). - ^ https://ghr.nlm.nih.gov/condition/kallmann-syndrome%7C"Kallmann syndrome. National Institutes for Health. US National Library of Medicine. Genetics Home Reference. December 2017"
- ^ synd/2549 at Who Named It?
- ^ a b "Kallmann syndrome". Rare Diseases. National Organisation for Rare Disorders (NORD). 2012. Retrieved December 16, 2017.
- ^ a b text of the citation
- ^ Sperling, Mark (2014). Pediatric Endocrinology E-Book. Elsevier Health Sciences. p. 136. ISBN 1455759732, 9781455759736.
{{cite book}}: Check|isbn=value: invalid character (help) - ^ Eckalbar WL, Fisher RE, Rawls A, Kusumi K (2012). "Scoliosis and segmentation defects of the vertebrae". Wiley Interdiscip Rev Dev Biol. 1 (3): 401–23. doi:10.1002/wdev.34. PMID 23801490.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Quaynor SD, Bosley ME, Duckworth CG, Porter KR, Kim SH, Kim HG, Chorich LP, Sullivan ME, Choi JH, Cameron RS, Layman LC (2016). "Targeted next generation sequencing approach identifies eighteen new candidate genes in normosmic hypogonadotropic hypogonadism and Kallmann syndrome". Mol Cell Endocrinol. 5 (437): 86–96. doi:10.1016/j.mce.2016.08.007. PMID 27502037.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Kim HG, Kurth I, Lan F, Meliciani I, Wenzel W, Eom SH, Kang GB, Rosenberger G, Tekin M, Ozata M, Bick DP, Sherins RJ, Walker SL, Shi Y, Gusella JF, Layman LC (2008). "Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome". Am J Hum Genet. 83 (4): 511–9. doi:10.1016/j.ajhg.2008.09.005. PMID PMC2561938.
{{cite journal}}: Check|pmid=value (help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid:18834967" was defined multiple times with different content (see the help page). - ^ Andrew A Dwyer, Richard Quinton, Nelly Pitteloud, and Diane Morin (2015). "Psychosexual Development in Men with Congenital Hypogonadotropic Hypogonadism on Long-Term Treatment: A Mixed Methods Study". Sex Med. 3 (1): 32–41. doi:10.1002/sm2.50. PMC 4380912.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Sidhoum VF, Chan YM, Lippincott MF; et al. (2014). "Reversal and Relapse of Hypogonadotropic Hypogonadism: Resilience and Fragility of the Reproductive Neuroendocrine System". J Clin Endocrinol Metab. 99 (3): 861–70. doi:10.1210/jc.2013-2809. PMC 3942233.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid:24423288" was defined multiple times with different content (see the help page). - ^ Wimalawansa SJ, Razzaque DMS, Al-Daghri NM (2017). "Calcium and Vitamin D in Human Health: Hype or Real?". J Steroid Biochem Mol Biol. Dec 16. doi:10.1016/j.jsbmb.2017.12.009. PMID 29258769.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Vezzoli V, Duminuco P, Bassi I, Guizzardi F, Persani L, Bonomi M (2016). "The complex genetic basis of congenital hypogonadotropic hypogonadism". Minerva Endocrinol. 41 (2): 223–39. PMID 26934720.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Maione L, Dwyer AA, Francou B, Guiochon-Mantel A, Binart N, Bouligand J, Young J (2018). "GENETICS IN ENDOCRINOLOGY: Genetic counseling for congenital hypogonadotropic hypogonadism and Kallmann syndrome: new challenges in the era of oligogenism and next-generation sequencing". Eur J Endocrinol. doi:10.1530/EJE-17-0749 [Epub ahead of print]. PMID 29330225.
{{cite journal}}: Check|doi=value (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h
{{cite journal}}: Empty citation (help) Cite error: The named reference "pmid" was defined multiple times with different content (see the help page). - ^ Dwyer AA, Raivio T, Pitteloud N (2016). "MANAGEMENT OF ENDOCRINE DISEASE: Reversible hypogonadotropic hypogonadism". Eur J Endocrinol. 174 (6): R267-74. doi:10.1530/EJE-15-1033. Epub 2016 Jan 20.
{{cite journal}}: Check|doi=value (help)CS1 maint: multiple names: authors list (link) - ^ Shaw ND, Seminara SB, Welt CK, Au MG, Plummer L, Hughes VA, Dwyer AA, Martin KA, Quinton R, Mericq V, Merino PM, Gusella JF, Crowley WF Jr, Pitteloud N, Hall JE (2011). "Expanding the phenotype and genotype of female GnRH deficiency". J Clin Endocrinol Metab. 96 (3): E566-76. doi:10.1210/jc.2010-2292. PMID 21209029.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jayasena CN, Nijher GM, Abbara A, Murphy KG, Lim A, Patel D, Mehta A, Todd C, Donaldson M, Trew GH; et al. (2010). "Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea". Clin Pharmacol Ther. 88 (6): 840–847. doi:10.1038/clpt.2010.204. PMID 20980998.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Jyothis T. George and Stephanie B. Seminara. "Kisspeptin and the Hypothalamic Control of Reproduction: Lessons from the Human". Endocrinology. 153 (11): 5130–5136. doi:10.1210/en.2012-1429. PMID PMC3473216.
{{cite journal}}: Check|pmid=value (help); Cite has empty unknown parameter:|year2012=(help) - ^ Skorupskaite K, George JT, Anderson RA (2014). "The kisspeptin-GnRH pathway in human reproductive health and disease". Hum Reprod Update. 20 (4): 485–500. doi:10.1093/humupd/dmu009. PMID 24615662.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Dwyer AA, Quinton R, Morin D, Pitteloud N. (2014). "Identifying the unmet health needs of patients with congenital hypogonadotropic hypogonadism using a web-based needs assessment: implications for online interventions and peer-to-peer support". Orphanet J Rare Dis. 83 (9): 1750–1172. doi:10.1186/1750-1172-9-83. PMID 24915927.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Eberhard Nieschlag, Pierre-Marc G. Bouloux, Barbara J. Stegmann, R. Ravi Shankar, Yanfen Guan, Anjela Tzontcheva, Christine McCrary Sisk, and Hermann M. Behre (2017). "An open-label clinical trial to investigate the efficacy and safety of corifollitropin alfa combined with hCG in adult men with hypogonadotropic hypogonadism". Reprod Biol Endocrinol. 15 (17). doi:10.1186/s12958-017-0232-y. PMID 5341390.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b c d e f Miraoui H1, Dwyer AA, Sykiotis GP, Plummer L, Chung W, Feng B, Beenken A, Clarke J, Pers TH, Dworzynski P, Keefe K, Niedziela M, Raivio T, Crowley WF Jr, Seminara SB, Quinton R, Hughes VA, Kumanov P, Young J, Yialamas MA, Hall JE, Van Vliet G, Chanoine JP, Rubenstein J, Mohammadi M, Tsai PS, Sidis Y, Lage K, Pitteloud N. (2013). "Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism". Am J Hum Genet. 92 (5): 725–43. doi:10.1016/j.ajhg.2013.04.008.. PMID 23643382.
{{cite journal}}: Check|doi=value (help)CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) Cite error: The named reference "pmid:23643382" was defined multiple times with different content (see the help page). - ^ a b c Layman L. (2013). "Clinical Testing for Kallmann Syndrome". J Clin Endocrinol Metab. 98 (5): 1860–1862. doi:10.1210/jc.2013-1624. PMID 23650337. Cite error: The named reference "pmid:23650337" was defined multiple times with different content (see the help page).
- ^ Pitteloud N. (2012). "Managing delayed or altered puberty in boys". BMJ. 345 (Dec 3): e7913. doi:10.1136/bmj.e7913. PMID 23207503.
- ^ Quinton R. (2005). "Adolescent development: advice in ABC of adolescence is potentially misleading". BMJ. 330 (7494) (Apr 2): 789. PMID 15802728.
- ^ Smith N, Quinton R. (2012). "Kallmann syndrome". BMJ. 345:e6971 (Dec 3). doi:10.1136/bmj.e6971. PMID 23207501.
- ^ Chadwick PM, Liao LM, Boyle ME. (2005). "Size matters: experiences of atypical genital and sexual development in males". J Health Psychol. 10 (4): 529–43. PMID 16014390.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Peter A. Lee, Christopher P. Houk. The Smallest Kid in School: Evaluating Delayed Puberty. Medscape. Aug 13, 2012.
- ^ Fromantin M; et al. (1973). "[Impuberism and hypogonadism at induction into military service. Statistical study]". Probl Actuels Endocrinol Nutr. 16 (May): 179–99. PMID 4147392.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Bry-Gauillard H; et al. (May 2010 (Epub)). "Congenital hypogonadotropic hypogonadism in females: clinical spectrum, evaluation and genetics". Ann Endocrinol (Paris). 71 (3): 158–162. doi:10.1016/j.ando.2010.02.024. PMID 20363464.
{{cite journal}}: Check date values in:|year=(help); Explicit use of et al. in:|author=(help)CS1 maint: year (link) - ^ a b Bouvattier C; et al. (2011). "Neonatal gonadotropin therapy in male congenital hypogonadotropic hypogonadism". Nat Rev Endocrinol. 18, 8 (3): 172–82. PMID 22009162.
{{cite journal}}: Explicit use of et al. in:|author=(help) Cite error: The named reference "pmid22009162" was defined multiple times with different content (see the help page). - ^ Young J (2012). "Approach to the Male Patient with Congenital Hypogonadotropic Hypogonadism". J Clin Endocrinol Metab. 97 (3): 707–718. PMID 22392951.
- ^ Yousem DM, Geckle RJ, Bilker W, McKeown DA, Doty RL. (1996). "MR evaluation of patients with congenital hyposmia or anosmia". AJR Am J Roentgenol. 166 (2): 439–43. PMID 8553963.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J; et al. (2007). "Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism". J Clin Invest. 117 (2): 457–63. PMID 17235395.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Laitinen EM, Tommiska J, Sane T, Vaaralahti K, Toppari J, Raivio T. (Epub 2012). "Reversible congenital hypogonadotropic hypogonadism in patients with CHD7, FGFR1 or GNRHR mutations". PLoS One. 7 (6): e39450. PMID 22724017.
{{cite journal}}: Check date values in:|year=(help)CS1 maint: multiple names: authors list (link) CS1 maint: year (link) - ^ Caronia LM, Martin C, Welt CK, Sykiotis GP; et al. (2011). "A genetic basis for functional hypothalamic amenorrhea". N Engl J Med. 20, 364 (3): 215–25. PMID 21247312.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Guo CY, Jones TH, Eastell R (1997). "Treatment of isolated hypogonadotropic hypogonadism effect on bone mineral density and bone turnover". J Clin Endocrinol Metab. 82 (2): 658–65. PMID 9024272.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Laitinen EM, Hero M, Vaaralahti K, Tommiska J, Raivio T (2012). "Bone mineral density, body composition and bone turnover in patients with congenital hypogonadotropic hypogonadism". Int J Androl. 35 (4): 534–40. PMID 22248317.
{{cite journal}}: Cite has empty unknown parameter:|doi10.1111/j.1365-2605.2011.01237.x=(help)CS1 maint: multiple names: authors list (link) - ^ Maestre de San Juan, Aureliano (1856). "Teratolagia: falta total de los nervios olfactorios con anosmia en un individuo en quien existia una atrofia congenita de los testiculos y miembro viril". El Siglo Médico. 3: 211–221.
- ^ Kallmann FJ, Schönfeld WA, Barrera SE (1943–1944). "The genetic aspects of primary eunuchoidism". Am J Ment Defic. 48: 203–236.
{{cite journal}}: CS1 maint: date format (link) CS1 maint: multiple names: authors list (link) - ^ a b Dodé C, Teixeira L, Levilliers J; et al. (2006). "Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2". PLoS Genet. 20, 2 (10): e175. PMID 17054399.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid17054399" was defined multiple times with different content (see the help page). - ^ MacColl G, Bouloux P, Quinton R (2002). "Kallmann syndrome: adhesion, afferents, and anosmia". Neuron. 34 (5): 675–8. doi:10.1016/S0896-6273(02)00720-1. PMID 12062015.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Quinton R, Cheow HK, Tymms DJ, Bouloux PM, Wu FC, Jacobs HS (1999). "Kallmann's syndrome: is it always for life?". Clin Endocrinol (Oxf). 50 (4): 481–5. PMID 10468907.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Han TS, Bouloux PM (2010). "What is the optimal therapy for young males with hypogonadotropic hypogonadism?". Clin Endocrinol (Oxf). 72 (6): 731–7. PMID 19912242. Cite error: The named reference "pmid19912242" was defined multiple times with different content (see the help page).
- ^ De Morsier,G Gauthier, G (1963). "[OLFACTO-GENITAL DYSPLASIA]". Pathol Biol (11): 1267–72. PMID 14099201.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Valdes-Socin H, Rubio Almanza M, Tomé Fernández-Ladreda M, Debray FG, Bours V, Beckers A (2014). "Reproduction, smell, and neurodevelopmental disorders: genetic defects in different hypogonadotropic hypogonadal syndromes". Front Endocrinol (Lausanne). 5 (109). doi:10.3389/fendo.2014.00109. PMC 4088923.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Legouis R, Hardelin JP, Levilliers J, Claverie JM, Compain S, Wunderle V, Millasseau P, Le Paslier D, Cohen D, Caterina D; et al. (1991). "The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules". Cell. 67 (2): 423–35. PMID 1913827.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, Brown CJ, Willard HF, Lawrence C, Graziella Persico M, Camerino G, Ballabio A. (1991). "A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules". Nature. 353 (6344): 529–36. doi:10.1038/353529a0. PMID 1922361.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schwanzel-Fukuda, M; Bick, D; Pfaff, DW (1989). "Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome". Brain Res Mol Brain Res. 6 (4): 311–26. doi:10.1016/0169-328x(89)90076-4. PMID 2687610.
- ^ Hardelin J-P & Dodé C (2016) FGFR1, FGF8, PROKR2, PROK2, ANOS1, and the olfactogenital (Kallmann) syndrome. Chapter 64 in Epstein’s Inborn errors of development: the molecular basis of clinical disorders of morphogenesis. 3rd edition. Erickson RP, Wynshaw-Boris A (eds) Oxford University Press. New York. pp 485-492./doi=10.1093/med/9780199934522.003.0064
- ^ Dodé C et al. (2003) Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet, 33, 463-465
- ^ Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N (2008). "Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice". J Clin Invest. 118 (8): 2822–31. doi:10.1172/JCI34538. PMC 2441855. PMID 18596921.
- ^ Villanueva C (2015). "Congenital hypogonadotropic hypogonadism with split hand/foot malformation: a clinical entity with a high frequency of FGFR1 mutations". Genet Med. 17 (8): 651–659. doi:10.1038/gim.2014.166.
- ^ Kotan, L. D.; Hutchins, B. I.; Ozkan, Y; Demirel, F; Stoner, H; Cheng, P. J.; Esen, I; Gurbuz, F; Bicakci, Y. K.; Mengen, E; Yuksel, B; Wray, S; Topaloglu, A. K. (2014). "Mutations in FEZF1 Cause Kallmann Syndrome". The American Journal of Human Genetics. 95 (3): 326–31. doi:10.1016/j.ajhg.2014.08.006. PMC 4157145. PMID 25192046.
- ^ MCJ Jongmans, CMA van Ravenswaaij-Arts, N Pitteloud, T Ogata,d N Sato, HL Claahsen-van der Grinten, K van der Donk, S Seminara, JEH Bergman, HG Brunner, WF Crowley, Jr, and LH Hoefsloota (2009). "CHD7 mutations in patients initially diagnosed with Kallmann syndrome – the clinical overlap with CHARGE syndrome". Clin Genet. 75 (1): 65–71. doi:10.1111/j.1399-0004.2008.01107.x. PMC 2854009.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pingault, Veronique; Bodereau, Virginie; Baral, Viviane; Marcos, Severine; Watanabe, Yuli; Chaoui, Asma; Fouveaut, Corinne; Leroy, Chrystel; Vérier-Mine, Odile; Francannet, Christine; Dupin-Deguine, Delphine; Archambeaud, Françoise; Kurtz, François-Joseph; Young, Jacques; Bertherat, Jérôme; Marlin, Sandrine; Goossens, Michel; Hardelin, Jean-Pierre; Dodé, Catherine; Bondurand, Nadege (May 2013). "Loss-of-Function Mutations in SOX10 Cause Kallmann Syndrome with Deafness". The American Journal of Human Genetics. 92 (5): 707–724. doi:10.1016/j.ajhg.2013.03.024.
- ^ Young, J; Metay, C; Bouligand, J; Tou, B; Francou, B; Maione, L; Tosca, L; Sarfati, J; Brioude, F; Esteva, B; Briand-Suleau, A; Brisset, S; Goossens, M; Tachdjian, G; Guiochon-Mantel, A (May 2012). "SEMA3A deletion in a family with Kallmann syndrome validates the role of semaphorin 3A in human puberty and olfactory system development". Human reproduction (Oxford, England). 27 (5): 1460–5. doi:10.1093/humrep/des022. PMID 22416012.
- ^ de Roux N1, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E (1997). "A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor". N Engl J Med. 22 (337): 1597–602. doi:10.1056/NEJM199711273372205. PMID 9371856.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Bouligand, J., Ghervan, C., Tello, J.A., Brailly-Tabard, S., Salenave, S., Chanson, P., Lombes, M., Millar, R.P., Guiochon-Mantel, A. and Young, J (2009). "Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation". N Engl J Med (360): 2742–2748. doi:10.1056/NEJMoa0900136. PMID 19535795.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E (2003). "Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54". Proc Natl Acad Sci U S A. 100 (19): 10972–6. doi:10.1073/pnas.1834399100. PMC 196911. PMID 12944565.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. (2003). "The GPR54 gene as a regulator of puberty". N Engl J Med. 349 (17): 164–27. doi:10.1056/NEJMoa035322. PMID 14573733.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Topaloglu AK; et al. (2009). "TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction". Nat Genet. 41 (3): 354–358. doi:10.1038/ng.306. PMC 4312696.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Cite error: The named reference
Oxford Endocrinology Library 2008was invoked but never defined (see the help page). - ^ Cite error: The named reference
Male Hypogonadism 2004was invoked but never defined (see the help page).

