Evolution
In biology, evolution is the change in the inherited traits of a population from generation to generation. These traits are the expression of genes that are copied and passed on to offspring during reproduction. Mutations, and other random changes in these genes, can produce new or altered traits, resulting in heritable differences (genetic variation) between organisms. New traits can also come from transfer of genes between populations, as in migration or horizontal gene transfer. Evolution occurs when these heritable differences become more common or rare in a population, either nonrandomly through natural selection or randomly through genetic drift.
Natural selection is a process that causes heritable traits that are helpful for survival and reproduction to become more common, and harmful traits to become rarer. This occurs because organisms with advantageous traits pass on more copies of the traits to the next generation.[1][2] Over many generations, adaptations occur through a combination of successive, small, random changes in traits, and the natural selection of the variants best-suited for their environment.[3] In contrast with this, genetic drift produces random changes in the frequency of traits in a population. Genetic drift arises from the element of chance involved in which individuals succeed in reproducing.
A species is a group of organisms that can reproduce with one another. However, when a species is separated into populations that are prevented from interbreeding, mutations, genetic drift, and the favoring of different traits by different environments result in the accumulation of differences over generations and the emergence of these populations as new species.[4] The similarities between organisms suggest that all known species are descended from a single ancestral species through this process of gradual divergence.[1]
The theory of evolution by natural selection was first proposed by Charles Darwin and Alfred Russel Wallace and set out in detail in Darwin's 1859 book On the origin of species.[5] In the 1930s, Darwinian natural selection was combined with Mendelian inheritance to form the modern evolutionary synthesis,[3] in which the connection between the units of evolution (genes) and the mechanism of evolution (natural selection) was made. This powerful explanatory and predictive theory has become the central organizing principle of modern biology, providing a unifying explanation for the diversity of life on Earth.[6]
Heredity

Inheritance in organisms occurs through discrete traits – particular characteristics of an organism. In humans, for example, eye color is an inherited characteristic, which individuals can inherit from one of their parents.[7] Inherited traits are controlled by genes and the complete set of genes within an organism's genome is called its genotype.[8]
The complete set of observable traits that make up the structure and behavior of an organism is called its phenotype. These traits come from the interaction of its genotype with the environment.[9] As a result, not every aspect of an organism's phenotype is inherited. Suntanned skin results from the interaction between a person's genotype and sunlight; thus, a suntan is not hereditary. However, people have different responses to sunlight, arising from differences in their genotype; a striking example is individuals with the inherited trait of albinism, who do not tan and are highly sensitive to sunburn.[10]
Genes are regions within DNA molecules that contain genetic information.[8] DNA is a long molecule with four types of bases attached along its length. Different genes have different sequences of bases; it is the sequence of these bases that encodes genetic information. Within cells, the long strands of DNA associate with proteins to form structures called chromosomes. A specific location within a chromosome is known as a locus. If the DNA sequence at a locus varies between individuals, the different forms of this sequence are called alleles. DNA sequences can change through mutations, producing new alleles. If a mutation occurs within a gene, the new allele may affect the trait that the gene controls, altering the phenotype of the organism. However, while this simple correspondence between an allele and a trait works in some cases, most traits are more complex and are controlled by multiple interacting genes.[11][12]
Variation
Because an individual's phenotype results from the interaction of their genotype with the environment, the variation in phenotypes in a population reflects the variation in these organisms' genotypes.[12] The modern evolutionary synthesis defines evolution as the change over time in this genetic variation.[13] The frequency of one particular allele will fluctuate, becoming more or less prevalent relative to other forms of that gene. Evolutionary forces act by driving these changes in allele frequency in one direction or another. Variation disappears when an allele reaches the point of fixation — when it either disappears from the population or replaces the ancestral allele entirely.[14]
Variation comes from mutations in genetic material, migration between populations (gene flow), and the reshuffling of genes through sexual reproduction. Variation also comes from exchanges of genes between different species, through horizontal gene transfer in bacteria, and hybridization in plants.[15] Despite the constant introduction variation through these processes, most sites in the genome of a species remain identical in all individuals of the species.[16] However, even relatively small changes in genotype can lead to dramatic changes in phenotype; for example, chimpanzees and humans differ in only about 5% of their genomes.[17]
Mutation
Genetic variation comes from random mutations that occur in the genomes of organisms. Mutations are changes in the DNA sequence of a cell's genome and are caused by radiation, viruses, transposons and mutagenic chemicals, as well as errors that occur during meiosis or DNA replication.[18][19][20] These mutagens produce several different types of change in DNA sequences; these can either have no effect, alter the product of a gene, or prevent the gene from functioning. Due to the damaging effects that these mutations can have on cells, organisms have evolved mechanisms such as DNA repair to remove mutations.[18] Therefore, the optimal mutation rate for an species is a trade-off between short-term costs, such as the risk of cancer, and the long-term benefits of advantageous mutations.[21]

Large sections of DNA can also be duplicated, which is a major source of raw material for evolving new genes, with tens to hundreds of genes duplicated in animal genomes every million years.[22] Most genes belong to larger families of genes of shared ancestry.[23] Novel genes are produced either through duplication and mutation of an ancestral gene, or by recombining parts of different genes to form new combinations with new functions.[24][25] For example, the human eye uses four genes to make structures that sense light: three for color vision and one for night vision; all four arose from a single ancestral gene.[26] An advantage of duplicating a gene (or even an (entire genome) is that overlapping or redundant functions in multiple genes allows alleles to be retained that would otherwise be harmful, thus increasing genetic diversity.[27]
Changes in chromosome number may also involve the breakage and rearrangement of DNA within chromosomes. For example, two chromosomes in the Homo genus fused to produce human chromosome 2; this fusion did not occur in the chimpanzee lineage and chimpanzees retain these separate chromosomes.[28] In evolution, the most important role of such chromosomal rearrangements may be to accelerate the divergence of a population into new species by preserving genetic differences within populations.[29]
Sequences of DNA that can move about the genome, such as transposons, make up a major fraction of the genetic material of plants and animals, and may have been important in the evolution of genomes.[30] For example, more than a million copies of the Alu sequence are present in the human genome, and these sequences have now been recruited to perform functions such as regulating gene expression.[31] Another effect of these mobile DNA sequences is that when they move within a genome, they can mutate or delete existing genes and thereby produce genetic diversity.[32]
Recombination
In asexual organisms, genes are inherited together, or linked, as they cannot mix with genes in other organisms during reproduction. However, the offspring of sexual organisms contain a random mixture of their parents' chromosomes that is produced through independent assortment. In the related process of genetic recombination, sexual organisms can also exchange DNA between two matching chromosomes.[33] These shuffling processes can allow even alleles that are close together in a strand of DNA to be inherited independently. However, as only about one recombination event occurs per million base pairs in humans, genes close together on a chromosome may not be shuffled away from each other, and tend to be inherited together.[34] This tendency is measured by finding how often two alleles occur together, which is called their linkage disequilibrium. A set of alleles that is usually inherited in a group is called a haplotype, and this co-inheritance can indicate that the locus is under positive selection (see below).[35]
Recombination in sexual organisms helps to remove harmful mutations and retain beneficial mutations.[36] Consequently, when alleles cannot be separated by recombination – such as in mammalian Y chromosomes – harmful mutations accumulate.[37][38] In addition, recombination can produce individuals with new and advantageous gene combinations. These positive effects of recombination are balanced by the fact that this process can cause mutations and separate beneficial combinations of genes.[36] The optimal rate of recombination for a species is therefore a trade-off between conflicting factors.
Mechanisms
There are three basic mechanisms of evolutionary change: natural selection, genetic drift, and gene flow. Natural selection favors genes that improve capacity for survival and reproduction. Genetic drift is the random sampling of a generation's genes during reproduction, causing random changes in the frequency of alleles, and gene flow is the transfer of genes within and between populations. The relative importance of natural selection and genetic drift in a population varies depending on the strength of the selection and the effective population size, which is the number of individuals capable of breeding.[39] Natural selection usually predominates in large populations, while genetic drift dominates in small populations. As a result, changing population size can dramatically influence the course of evolution. Population bottlenecks, where the population shrinks temporarily and therefore loses genetic variation, result in a more uniform population.[14] Bottlenecks also result from alterations in gene flow such as decreased migration, expansions into new habitats, or population subdivision.[39]
Natural selection

Natural selection is the process by which genetic mutations that enhance reproduction become, and remain, more common in successive generations of a population. It has often been called a "self-evident" mechanism because it necessarily follows from the following facts:
- Heritable variation exists within populations of organisms
- Organisms produce more offspring than can survive
- These offspring vary in their ability to survive and reproduce
- Consequently, successful reproducers pass advantageous traits on, while unsuccessful reproducers do not pass disadvantageous traits on to the next generation.
The central concept of natural selection is the evolutionary fitness of an organism. This measures the organism's genetic contribution to the next generation. However, this is not the same as the total number of offspring: instead fitness measures the proportion of subsequent generations that carry an organism's genes.[40] Consequently, if an allele increases fitness more than the other alleles of that gene, then with each generation this allele will become more common within the population. These traits are said to be "selected for". Examples of traits that can increase fitness are enhanced survival, and increased fecundity. Conversely, the lower fitness caused by having a less beneficial or deleterious allele results in this allele becoming rarer — they are "selected against".[2] Importantly, the fitness of an allele is not a fixed characteristic, if the environment changes, previously neutral or harmful traits may become beneficial and previously beneficial traits become harmful.[1]
Natural selection within a population for a trait that can vary across a range of values, such as height, can be categorized into three different types. The first is directional selection, which is a shift in the average value of a trait over time — for example organisms slowly getting taller.[41] Secondly, disruptive selection is selection for extreme trait values and often results in two different values becoming most common, with selection against the average value. This would be when either short or tall organisms had an advantage, but not those of medium height. Finally, in stabilizing selection there is selection against extreme trait values on both ends, which causes a decrease in variance around the average value.[42] This would, for example, cause organisms to slowly become all the same height.
A special case of natural selection is sexual selection, which is selection for any trait that increases mating success by increasing the attractiveness of an organism to potential mates.[43] Traits that evolved through sexual selection are particularly prominent in males of some animal species, despite traits such as cumbersome antlers, mating calls or bright colors that attract predators, decreasing the survival of individual males.[44] This survival disadvantage is balanced by higher reproductive success in males that show these hard to fake, sexually selected traits.[45]
An active area of research is the unit of selection, with natural selection being proposed to work at the level of genes, cells, individual organisms, groups of organisms and even species.[46][47] None of these models are mutually-exclusive and selection may act on multiple levels simultaneously.[48] Below the level of the individual, genes called transposons try to copy themselves throughout the genome. [49] Selection at a level above the individual, such as group selection, may allow the evolution of co-operation, as discussed below.[50]

Genetic drift
Genetic drift is the change in allele frequency from one generation to the next that occurs because alleles in the offspring generation are a random sample of those in the parent generation, and are thus subject to sampling error.[14] As a result, when selective forces are absent or relatively weak, allele frequencies tend to "drift" upward or downward in a random walk. This drift halts when an allele eventually becomes fixed, either by disappearing from the population, or replacing the other alleles entirely. Genetic drift may therefore eliminate some alleles from a population due to chance alone, and two separate populations that began with the same genetic structure can drift apart by random fluctuation into two divergent populations with different sets of alleles.[51] The time for an allele to become fixed by genetic drift depends on population size, with fixation occurring more rapidly in smaller populations.[52]
Although natural selection is responsible for adaptation, the relative importance of the two forces of natural selection and genetic drift in driving evolutionary change in general is an area of current research in evolutionary biology.[53] These investigations were prompted by the neutral theory of molecular evolution, which proposed that most evolutionary changes are the result the fixation of neutral mutations that do not have any immediate effects on the fitness of an organism.[54] Hence, in this model, most genetic changes in a population are the result of constant mutation pressure and genetic drift.[55]
Gene flow

Gene flow is the exchange of genes between populations, which are usually of the same species.[56] Examples of gene flow within a species include the migration and then breeding of organisms, or the exchange of pollen. Gene transfer between species includes the formation of hybrid organisms and horizontal gene transfer.
Migration into or out of a population can change allele frequencies. Immigration may add new genetic material to the established gene pool of a population. Conversely, emigration may remove genetic material. As barriers to reproduction between two diverging populations are required for the populations to become new species, gene flow may slow this process by spreading genetic differences between the populations. Gene flow is hindered by mountain ranges, oceans and deserts or even man-made structures such as the Great Wall of China, which has hindered the flow of plant genes.[57]
Depending on how far two species have diverged since their last common ancestor, it may still be possible for them to produce offspring, as with horses and donkeys mating to produce mules.[58] Such hybrids are generally infertile, due to the two different sets of chromosomes being unable to pair up during meiosis. In this case, closely-related species may regularly interbreed, but hybrids will be selected against and the species will remain distinct. However, viable hybrids are occasionally formed and these new species can either have properties intermediate between their parent species, or possess a totally new phenotype.[59] Hybridization rarely leads to new species in animals, although this has been seen in Gray tree frog.[60] Hybridization is, however, an important means of speciation in plants, since polyploidy (having more than two copies of each chromosome) is tolerated in plants more readily than in animals.[61] Polyploidy is important in hybrids as it allows reproduction, with the two different sets of chromosomes each being able to pair with an identical partner during meiosis.[62] Polyploids also have more genetic diversity, which allows them to avoid inbreeding depression in small populations.[63]
Horizontal gene transfer is the transfer of genetic material from one organism to another organism that is not its offspring, this is most common among bacteria.[64] In medicine, this contributes to the spread of antibiotic resistance, as when one bacteria acquires resistance genes it can rapidly transfer them to other species.[65] Horizontal transfer of genes from bacteria to eukaryotes such as the yeast Saccharomyces cerevisiae and the adzuki bean beetle Callosobruchus chinensis may also have occurred.[66][67] Viruses can also carry DNA between organisms, allowing transfer of genes even across biological domains.[68] Gene transfer has also occurred within eukaryotic cells, from the chloroplast and mitochondrial genomes to nuclear genomes.[69]
Outcomes
Evolution influences every aspect of the form and behavior of organisms. Most prominent are the specific behavioral and physical adaptations that are the outcome of natural selection. These adaptations increase fitness by aiding activities such as finding food, avoiding predators or attracting mates. Organisms can also respond to selection by co-operating with each other, usually by aiding their relatives or engaging in mutually-beneficial partnerships. In the longer-term, evolution produces new species through splitting ancestral populations of organisms into new groups that are unable to breed with one another.
These outcomes of evolution are sometimes divided into macroevolution, which is evolution that occurs at or above the level of species, such as speciation, and microevolution, which is smaller evolutionary changes, such as adaptations, within a species or population. In general, macroevolution is the outcome of long periods of microevolution.[70] Thus, the distinction between micro- and macroevolution is not a fundamental one - the difference is simply the time involved.[71] However, in macroevolution, the traits of the entire species are important. For instance, a large amount of variation between individuals allows a species to rapidly adapt to new habitats, lessening the chance of it going extinct, while a wide geographic range increases the chance of speciation, by making it more likely that part of the population will become isolated. In this sense, microevolution and macroevolution can sometimes be separate.[72]
A common misconception is that evolution is "progressive", but natural selection has no long-term goal and does not necessarily produce greater complexity.[73] Although complex species have evolved, this occurs as a side-effect of the overall number of organisms increasing, and simple forms of life remain more common.[74] For example, the overwhelming majority of species are microscopic prokaryotes, which form about half the world's biomass despite their small size,[75] and constitute the vast majority of Earth's biodiversity.[76] Simple organisms therefore remain the dominant form of life on Earth, and complex life appears more diverse only because it is more noticeable.[77]
Adaptation
Adaptations are structures or behaviors that enhance a specific function, causing organisms to become better at surviving and reproducing.[5] They are produced by a combination of the continuous production of small, random changes in traits, followed by natural selection of the variants best-suited for their environment.[78] This process can cause either the gain of a new feature, or the loss of an ancestral feature. An example that shows both types of change is bacterial adaptation to antibiotic selection, with mutations causing antibiotic resistance by either modifying the target of the drug, or removing the transporters that allow the drug into the cell.[79] However, many traits that appear to be simple adaptations are in fact exaptations: structures originally adapted for one function, but which coincidentally became somewhat useful for some other function in the process.[80] One example is the African lizard Holapsis guentheri, which developed an extremely flat head for hiding in crevices, as can be seen by looking at its near relatives. However, in this species, the head has become so flattened that it assists in gliding from tree to tree - an exaptation.[80]

As adaptation occurs through the gradual modification of existing structures, structures with similar internal organization may have very different functions in related organisms. This is the result of a single ancestral structure being adapted to function in different ways. The bones within bat wings, for example, are structurally similar to both human hands and seal flippers, due to the common descent of these structures from an ancestor that also had 5 digits at the end of each forelimb. Other idiosyncratic anatomical features, such as bones in the wrist of the panda being formed into a false "thumb", indicate that an organism's evolutionary lineage can limit what adaptations are possible.[81]
During adaption, some structures may lose their original function and become vestigial structures.[82] Such structures may have little or no function in a current species, yet have a clear function in ancestral species, or other closely-related species. Examples include the non-functional remains of eyes in blind cave-dwelling fish,[83] wings in flightless birds,[84] and the presence of hip bones in whales and snakes.[85] Examples of vestigial structures in humans include wisdom teeth,[86] the coccyx,[82] and the vermiform appendix.[82]
An area of current investigation in evolutionary developmental biology is the developmental basis of adaptations and exaptations.[87] This research addresses the origin and evolution of embryonic development and how modifications of development and developmental processes produce novel features.[88] These studies have shown that evolution can alter development to create new structures, such as embryonic bone structures that develop into the jaw in other animals instead forming part of the middle ear in mammals.[89] It is also possible for structures that have been lost in evolution to reappear due to changes in developmental genes, such as a mutation in chickens causing embryos to grow teeth similar to those of crocodiles.[90]
Co-evolution and co-operation
Interactions between organisms can produce both conflict and co-operation. When the interaction is between pairs of species, such as a pathogen and a host, or a predator and its prey, these species can develop matched sets of adaptations. Here, the evolution of one species causes adaptations in a second species. These changes in the second species then, in turn, cause new adaptations in the first species. This cycle of selection and response is called co-evolution.[91] An example is the production of tetrodotoxin in the rough-skinned newt and the evolution of tetrodotoxin resistance in its predator, the common garter snake. In this predator-prey pair, an evolutionary arms race has produced high levels of toxin in the newt and correspondingly high levels of resistance in the snake.[92]
However, not all interactions involve conflict — genes, cells, and organisms can also cooperate to aid their survival and reproduction.[93] Extreme cooperation occurs between plants and the mycorrhizal fungi that grow on their roots and aid the plant in absorbing nutrients from the soil.[94] This is a reciprocal relationship as the plants provide the fungi with sugars from photosynthesis. Here, the fungi actually grow inside plant cells, allowing them to exchange nutrients with their hosts, while sending signals that suppress the plant immune system.[95] Coalitions between organisms of the same species are highly developed in the social insects, such as bees, termites and ants, where sterile insects feed and guard the small number of organisms in a colony that are able to reproduce. On an even smaller scale, the somatic cells that make up the body of an animal control their own reproduction to allow the animal's germ cells to produce offspring. Here, somatic cells respond to specific signals that instruct them to either grow or kill themselves. If cells ignore these signals and attempt to multiply inappropriately, their uncontrolled growth causes cancer.[18]
These examples of cooperation within species are usually produced through kin selection, which is where one organism acts to aid a relative.[96] This provides an evolutionary advantage to alleles promoting cooperation as the two relatives will share genetic material.[97] Other forces that may drive cooperation include group selection, where cooperation provides benefits to a group of organisms.[98]
Speciation

Speciation is the process where a species diverges into two descendant species.[99] It has been observed multiple times under both controlled laboratory conditions and in nature.[100] In sexually-reproducing organisms, speciation results from reproductive isolation followed by genealogical divergence. There are four mechanisms for speciation. The most common in animals is allopatric speciation, which occurs in populations initially isolated geographically, such as by habitat fragmentation or migration. As selection and drift act independently in isolated populations, separation will eventually produce organisms that cannot interbreed.[101]
The second mechanism of speciation is peripatric speciation, which occurs when small populations of organisms become isolated in a new environment. This differs from allopatric speciation in that the isolated populations are numerically much smaller than the parental population. Here, the founder effect causes rapid speciation through both rapid genetic drift and selection on a small gene pool.[102]
The third mechanism of speciation is parapatric speciation. This is similar to peripatric speciation in that a small population enters a new habitat, this type of speciation differs as there is no physical separation between these two populations. Instead, speciation results from the evolution of mechanisms that reduce gene flow between the two populations.[99] Generally this occurs when there has been a drastic change in the environment within the parental species' habitat. One example is the grass Anthoxanthum odoratum, which can undergo parapatric speciation in response to localized metal pollution from mines.[103] Here, plants evolve that have resistance to high levels of metals in the soil. Selection against interbreeding with the metal-sensitive parental population produces a change in flowering time of the metal-resistant plants, causing reproductive isolation. Selection against hybrids between the two populations may cause reinforcement, which is the evolution of traits that promote mating within a species, as well as character displacement, which is when two species become more distinct.[104]
Finally, in sympatric speciation species diverge without geographic isolation or changes in habitat. This form is rare since even a small amount of gene flow may remove genetic differences between parts of a population.[105] Generally, sympatric speciation in animals requires the evolution of both genetic differences and non-random mating, to allow reproductive isolation to evolve.[106] Cross-breeding of two related species to produce a new one is fairly common, but viable hybrids are more common in plants than in animals, as plants can double their number of chromosomes, to form polyploids. This allows the chromosomes from both species to form a matching pair, as needed for reproduction.[107] Indeed, chromosome doubling can itself cause reproductive isolation, as half the doubled chromosomes will be unmatched when breeding with undoubled organisms.[108]
Speciation events are important in the theory of punctuated equilibrium, which accounts for the pattern in the fossil record of short "bursts" of evolution interspersed with relatively long periods of stasis, where species remain relatively unchanged.[109] In this theory, speciation and rapid evolution are linked, with natural selection and genetic drift acting most strongly on organisms undergoing speciation in novel habitats or small populations. As a result, the periods of stasis in the fossil record correspond to the parental population, and the organisms undergoing speciation and rapid evolution are found in small populations or geographically-restricted habitats, and therefore rarely being preserved as fossils.[110]
Extinction
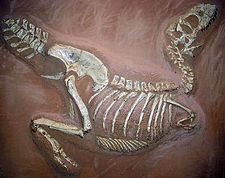
Extinction is the disappearance of entire species. Extinction is not an unusual event as species regularly appear through speciation, and disappear through extinction.[111] Indeed, virtually all animal and plant species that have lived on earth are now extinct.[112] These extinctions have happened continuously throughout the history of life, although the rate of extinction spikes in occasional mass extinction events.[113] As it extinguished the dinosaurs, the Cretaceous-Tertiary extinction event is the most well-known, but the earlier Permian-Triassic extinction event was even more severe, with approximately 96% of species driven to extinction.[113] The Holocene extinction event is an ongoing mass extinction associated with humanity's expansion across the globe over the last few thousand years. Present-day extinction rates are currently 100-1000 times greater than the background rate, and up to 30% species may be extinct by the mid 21st century.[114] Human activities are now the primary cause of the ongoing extinction event;[115] global warming may further accelerate it in the future.[116]
The role of extinction in evolution depends on which type is considered. The cause of the continuous "low-level" extinction events, which form the majority of extinctions, are not well understood and may be the result of competition between species for shared resources.[117] If competition from other species does alter the probability that a species will become extinct, this could produce species selection as a level of natural selection.[46] The intermittent mass extinctions are also important, but instead of acting as a selective force, they drastically reduce diversity in a non-specific manner and promote a burst of rapid evolution and speciation in survivors.[113]
Evolutionary history of life
Origin of life
The origin of life is a necessary precursor for biological evolution, but understanding that evolution has occurred and investigating how this happens does not depend on understanding exactly how life began.[118] The current scientific consensus is that the complex biochemistry that makes up life came from simpler chemical reactions, but disputes over the definition of life makes it unclear at which point a complex set of reactions would became an organism.[119] Not much is certain about the earliest developments in life, the structure of the first living things, or the identity of the last universal common ancestor.[120][121] Consequently, there is no scientific consensus on how life began, but proposals include self-replicating molecules such as RNA,[122] and the assembly of simple cells.[123]
Common descent
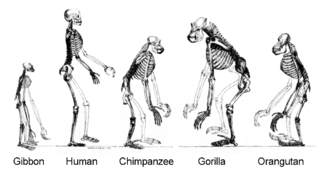
All organisms on Earth are descended from a common ancestor.[124] Current species are a stage in the process of evolution, with their diversity the product of a long series of speciation and extinction events.[125] The common descent of organisms was first deduced from four simple facts about organisms: Firstly, they have geographic distributions that cannot be explained by local adaptation. Secondly, the diversity of life is not a set of completely unique organisms, but organisms that share morphological similarities. Thirdly, vestigial traits with no clear purpose resemble functional ancestral traits, and finally, that organisms can be classified using these similarities into a hierarchy of nested groups.[5]
Past species have also left records of their evolutionary history. Fossils, along with the comparative anatomy of present-day organisms, constitute the morphological, or anatomical, record.[126] By comparing the anatomies of both modern and extinct species, paleontologists can infer the lineages of those species. However, this approach is most successful for organisms that had hard body parts, such as shells, bones or teeth. Furthermore, as prokaryotes such as bacteria and archaea share a limited set of common morphologies, their fossils do not provide information on their ancestry.
More recently, evidence for common descent has come from the study of biochemical similarities between organisms. For example, all living cells use the same nucleic acids and amino acids.[127] The development of molecular genetics has revealed the record of evolution left in organisms' genomes: dating when species diverged through the molecular clock produced by mutations.[128] For example, these DNA sequence comparisons have revealed the close genetic similarity between humans and chimpanzees and shed light on when the common ancestor of these species existed.[129]
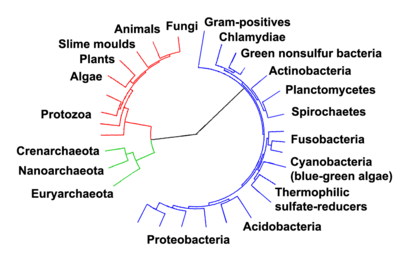
Evolution of life
Despite the uncertainty on how life began, it is clear that microorganisms were the first organisms to inhabit Earth,[131] approximately 3–4 billion years ago.[132] No obvious changes occurred in these organisms over the next few billion years and their morphology is strikingly similar to their modern relatives.[133]
The eukaryotes were the next major innovation in evolution. These came from ancient bacteria being engulfed by the ancestors of eukaryotic cells, in an cooperative association called endosymbiosis.[134][135] The engulfed bacteria and the host cell then underwent co-evolution, with the bacteria evolving into either mitochondria or hydrogenosomes.[136] An independent second engulfment of cyanobacterial-like organisms led to the formation of chloroplasts in algae and plants.[137]
As these early eukaryotes and prokaryotes were microscopic, most of the history of life describes simple microorganisms. It is only about a billion years ago in the Ediacaran period that complex multicellular organisms began to appear in the oceans.[131][138] The first multicellular organisms may have evolved from species similar to slime moulds, which live as single cells but aggregate as part of reproduction.[139]
Soon after the emergence of the first multicellular organisms, a remarkable amount of biological diversity appeared in only about 10 million years in an event called the Cambrian explosion. Here, the majority of types of modern animals evolved, as well as unique lineages that subsequently became extinct.[140] Various triggers for the Cambrian explosion have been proposed, including the accumulation of oxygen in the atmosphere from photosynthesis.[141] About 500 million years ago, plants and fungi colonized the land, and were soon followed by arthropods and other animals.[142] Amphibians first appeared around 300 million years ago, followed by early amniotes, then mammals around 200 million years ago and birds around 100 million years ago (both from "reptile"-like lineages). However, despite the evolution of these large animals, smaller organisms similar to the types that evolved early in this process continue to be highly successful and dominate the Earth, with the majority of species prokaryotes and the majority of animals insects.[143]
History of evolutionary thought

Evolutionary ideas such as common descent and the transmutation of species have existed since at least the 6th century BC, when they were expounded by the Greek philosopher Anaximander,[144] and were developed by other early thinkers, including the Greek philosopher Empedocles, the Roman philosopher Lucretius, and the Arab biologist Al-Jahiz.[145] As biological knowledge grew in the 18th century, a variety of such ideas developed, beginning with Pierre Maupertuis in 1745, and with contributions from natural philosophers such as Erasmus Darwin and Jean-Baptiste Lamarck.[146] In 1858, Charles Darwin and Alfred Russel Wallace jointly proposed the theory of evolution by natural selection to the Linnean Society of London in separate papers.[147] Shortly after, Darwin's publication of The Origin of Species provided detailed support for the theory and led to increasingly wide acceptance of the occurrence of evolution.
Nonetheless, Darwin's specific ideas about evolution, such as gradualism and the mechanisms of natural selection, were strongly contested at first. Lamarckists argued that transmutation of species occurred as parents passed on adaptations acquired during their lifetimes.[148] Eventually, when experiments failed to support it, this idea was abandoned in favor of Darwinism.[149] More significantly, Darwin could not account for how traits were passed down from generation to generation. A mechanism was provided in 1865 by Gregor Mendel, who found that traits were inherited in a predictable manner.[150] When Mendel's work was rediscovered in 1900, disagreements over the rate of evolution predicted by early geneticists and biometricians led to a rift between the Mendelian and Darwinian models of evolution.

This contradiction was reconciled in the 1930s by biologists such as Ronald Fisher. The end result was a combination of evolution by natural selection and Mendelian inheritance, the modern evolutionary synthesis, or "Neo-Darwinism".[151] In the 1940s, the identification of DNA as the genetic material by Oswald Avery and colleagues and the subsequent publication of the structure of DNA by James Watson and Francis Crick in 1953, demonstrated the physical basis for inheritance. Since then, genetics and molecular biology have become core parts of evolutionary biology and have revolutionized the field of phylogenetics.[117]
In its early history, evolutionary biology primarily drew in scientists from traditional taxonomically-oriented disciplines, whose specialist training in particular organisms addressed general questions in evolution. As evolutionary biology expanded as an academic discipline, particularly after the development of the modern evolutionary synthesis, it began to draw more widely from the biological sciences.[117] Currently the study of evolutionary biology involves scientists from fields as diverse as biochemistry, ecology, genetics and physiology, and evolutionary concepts are used in even more distant disciplines such as psychology, medicine, philosophy and computer science.
Social and religious controversies

Even before the publication of The Origin of Species, the idea that life had evolved was a source of controversy and evolution is still the subject of contention. Debate has generally centered on the philosophical, social and religious implications of evolution, not on the science itself; the proposition that biological evolution occurs through the mechanism of natural selection is completely uncontested in the scientific literature.[152]
As Darwin recognized early on, the most controversial aspect of evolutionary thought is its application to humans. Specifically, some people object to the idea that humans arose through natural processes without supernatural intervention. Although many religions and denominations have reconciled their beliefs with evolution through theological development, several denominations contain creationists who object to evolution, as it contradicts their literal interpretation of origin beliefs.[153] In some countries – notably the United States – these tensions between scientific and religious teachings have fueled the ongoing creation–evolution controversy, a religious conflict focusing on politics and public education.[154] While other scientific fields such as cosmology[155] and earth science[156] also conflict with literal interpretations of many religious texts, evolutionary biology has borne the brunt of religious objection.
Evolution has also attracted controversy because it has been used to support philosophical positions that promote discrimination and racism. For example, the eugenic ideas of Francis Galton were developed into arguments that the human gene pool should be improved by selective breeding policies, including incentives for those considered "good stock" to reproduce, and the compulsory sterilization, prenatal testing, birth control, and even killing, of those considered bad stock.[157] Another example of an extension of evolutionary theory that is now widely regarded as unwarranted is "Social Darwinism", a term given to the 19th-century Whig Malthusian theory developed by Herbert Spencer into ideas about "survival of the fittest" in commerce and human societies as a whole, and by others into claims that social inequality, racism, and imperialism were justified.[158] However, contemporary scientists and philosophers consider these ideas to have been neither mandated by evolutionary theory nor supported by data.[159]
Uses in technology
A major technological application of the power of evolution is artificial selection, which is the intentional selection of certain traits in a population of organisms. Humans have used artificial selection for thousands of years in the domestication of plants and animals.[160] More recently, such selection has become a vital part of genetic engineering, with selectable markers such as antibiotic resistance genes being used to manipulate DNA in molecular biology.
As evolution can produce highly optimized processes and networks, it has many applications in computer science. Here, simulations of evolution using evolutionary algorithms and artificial life started with the work of Nils Aall Barricelli in the 1960s, and was extended by Alex Fraser, who published a series of papers on simulation of artificial selection.[161]. Artificial evolution became a widely recognized optimization method as a result of the work of Ingo Rechenberg in the 1960s and early 1970s, who used evolution strategies to solve complex engineering problems.[162] Genetic algorithms in particular became popular through the writing of John Holland.[163] As academic interest grew, dramatic increases in the power of computers allowed practical applications. Evolution algorithms are now used to solve multi-dimensional problems more quickly than software produced by human designers, and also to optimize the design of systems.[164]
Further reading
Introductory reading
- Jones S (2001). Almost Like a Whale: The Origin of Species Updated. (American title: Darwin's Ghost). New York: Ballantine Books. ISBN 0-345-42277-5.
- Dawkins R (2006). The Blind Watchmaker. London: Penguin Books Ltd. ISBN 0-141-02616-2.
- Charlesworth CB, Charlesworth D (2003). Evolution. Oxfordshire: Oxford University Press. ISBN 0-192-80251-8.
- Gould SJ (1989). Wonderful Life: The Burgess Shale and the Nature of History. New York: W.W. Norton. ISBN 0-393-30700-X.
- Carroll S (2005). Endless Forms Most Beautiful. New York: W.W. Norton. ISBN 0-393-06016-0.
History of evolutionary thought
- Larson EJ (2004). Evolution: The Remarkable History of a Scientific Theory. New York: Modern Library. ISBN 0-679-64288-9.
- Zimmer C (2001). Evolution: The Triumph of an Idea. London: HarperCollins. ISBN 0-060-19906-7.
Advanced reading
- Gould SJ (2002). The Structure of Evolutionary Theory. Cambridge: Belknap Press (Harvard University Press). ISBN 0-674-00613-5.
- Futuyma DJ (2005). Evolution. Sunderland: Sinauer Associates. ISBN 0-878-93187-2.
- Mayr E (2001). What Evolution Is. New York: Basic Books. ISBN 0-465-04426-3.
- Coyne JA, Orr HA (2004). Speciation. Sunderland: Sinauer Associates. ISBN 0-878-93089-2.
- Smith JM, Szathmáry E (1997). The Major Transitions in Evolution. Oxfordshire: Oxford University Press. ISBN 0-198-50294-X.
References
- ^ a b c Futuyma, Douglas J. (2005). Evolution. Sunderland, Massachusetts: Sinauer Associates, Inc. ISBN 0-87893-187-2.
- ^ a b Lande R, Arnold SJ (1983). "The measurement of selection on correlated characters". Evolution. 37: 1210–26}. doi:10.2307/2408842.
- ^ a b "Mechanisms: the processes of evolution". Understanding Evolution. University of California, Berkeley. Retrieved 2006-07-14.
- ^ Gould, Stephen J. (2002). The Structure of Evolutionary Theory. Belknap Press. ISBN 0-674-00613-5.
- ^ a b c Darwin, Charles (1859). On the Origin of Species (1st ed.). London: John Murray. pp. p. 1.
{{cite book}}:|pages=has extra text (help). Related earlier ideas were acknowledged in Darwin, Charles (1861). On the Origin of Species (3rd ed.). London: John Murray. pp. p. xiii.{{cite book}}:|pages=has extra text (help). - ^ "IAP Statement on the Teaching of Evolution" (PDF). The Interacademy Panel on International Issues. 2006. Retrieved 2007-04-25.
*"Statement on the Teaching of Evolution" (PDF). American Association for the Advancement of Science. 2006. Retrieved 2007-04-25. - ^ Sturm RA, Frudakis TN (2004). "Eye colour: portals into pigmentation genes and ancestry". Trends Genet. 20 (8): 327–32. PMID 15262401.
- ^ a b Pearson H (2006). "Genetics: what is a gene?". Nature. 441 (7092): 398–401. PMID 16724031.
- ^ Peaston AE, Whitelaw E (2006). "Epigenetics and phenotypic variation in mammals". Mamm. Genome. 17 (5): 365–74. PMID 16688527.
- ^ Oetting WS, Brilliant MH, King RA (1996). "The clinical spectrum of albinism in humans". Molecular medicine today. 2 (8): 330–35. PMID 8796918.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mayeux R (2005). "Mapping the new frontier: complex genetic disorders". J. Clin. Invest. 115 (6): 1404–07. PMID 15931374.
- ^ a b Wu R, Lin M (2006). "Functional mapping - how to map and study the genetic architecture of dynamic complex traits". Nat. Rev. Genet. 7 (3): 229–37. PMID 16485021.
- ^ Stoltzfus A (2006). "Mutationism and the dual causation of evolutionary change". Evol. Dev. 8 (3): 304–17. PMID 16686641.
- ^ a b c Harwood AJ (1998). "Factors affecting levels of genetic diversity in natural populations". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 353 (1366): 177–86. PMID 9533122.
- ^ Draghi J, Turner P (2006). "DNA secretion and gene-level selection in bacteria". Microbiology (Reading, Engl.). 152 (Pt 9): 2683–8. PMID 16946263.
*Mallet J (2007). "Hybrid speciation". Nature. 446 (7133): 279–83. PMID 17361174., - ^ Butlin RK, Tregenza T (1998). "Levels of genetic polymorphism: marker loci versus quantitative traits". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 353 (1366): 187–98. PMID 9533123.
- ^ Wetterbom A, Sevov M, Cavelier L, Bergström TF (2006). "Comparative genomic analysis of human and chimpanzee indicates a key role for indels in primate evolution". J. Mol. Evol. 63 (5): 682–90. PMID 17075697.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Bertram J (2000). "The molecular biology of cancer". Mol. Aspects Med. 21 (6): 167–223. PMID 11173079.
- ^ Aminetzach YT, Macpherson JM, Petrov DA (2005). "Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila". Science. 309 (5735): 764–67. doi:10.1126/science.1112699. PMID 16051794.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Burrus V, Waldor M (2004). "Shaping bacterial genomes with integrative and conjugative elements". Res. Microbiol. 155 (5): 376–86. PMID 15207870.
- ^ Sniegowski P, Gerrish P, Johnson T, Shaver A (2000). "The evolution of mutation rates: separating causes from consequences". Bioessays. 22 (12): 1057–66. PMID 11084621.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Carroll SB, Grenier J, Weatherbee SD (2005). From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Second Edition. Oxford: Blackwell Publishing. ISBN 1-4051-1950-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Harrison P, Gerstein M (2002). "Studying genomes through the aeons: protein families, pseudogenes and proteome evolution". J Mol Biol. 318 (5): 1155–74. PMID 12083509.
- ^ Orengo CA, Thornton JM (2005). "Protein families and their evolution-a structural perspective". Annu. Rev. Biochem. 74: 867–900. PMID 15954844.
- ^ Pál C, Papp B, Lercher MJ (2006). "An integrated view of protein evolution". Nat. Rev. Genet. 7 (5): 337–48. PMID 16619049.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bowmaker JK (1998). "Evolution of colour vision in vertebrates". Eye (London, England). 12 (Pt 3b): 541–47. PMID 9775215.
- ^ Gregory TR, Hebert PD (1999). "The modulation of DNA content: proximate causes and ultimate consequences". Genome Res. 9 (4): 317–24. PMID 10207154.
- ^ Zhang J, Wang X, Podlaha O (2004). "Testing the chromosomal speciation hypothesis for humans and chimpanzees". Genome Res. 14 (5): 845–51. PMID 15123584.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ayala FJ, Coluzzi M (2005). "Chromosome speciation: humans, Drosophila, and mosquitoes". Proc. Natl. Acad. Sci. U.S.A. 102 Suppl 1: 6535–42. PMID 15851677.
- ^ Hurst GD, Werren JH (2001). "The role of selfish genetic elements in eukaryotic evolution". Nat. Rev. Genet. 2 (8): 597–606. PMID 11483984.
- ^ Häsler J, Strub K (2006). "Alu elements as regulators of gene expression". Nucleic Acids Res. 34 (19): 5491–97. PMID 17020921.
- ^ Aminetzach YT, Macpherson JM, Petrov DA (2005). "Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila". Science. 309 (5735): 764–67. PMID 16051794.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Radding C (1982). "Homologous pairing and strand exchange in genetic recombination". Annu. Rev. Genet. 16: 405–37. PMID 6297377.
- ^ Lien S, Szyda J, Schechinger B, Rappold G, Arnheim N (2000). "Evidence for heterogeneity in recombination in the human pseudoautosomal region: high resolution analysis by sperm typing and radiation-hybrid mapping". Am. J. Hum. Genet. 66 (2): 557–66. PMID 10677316.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sabeti P, Schaffner S, Fry B, Lohmueller J, Varilly P, Shamovsky O, Palma A, Mikkelsen T, Altshuler D, Lander E (2006). "Positive natural selection in the human lineage". Science. 312 (5780): 1614–20. PMID 16778047.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Otto S (2003). "The advantages of segregation and the evolution of sex". Genetics. 164 (3): 1099–118. PMID 12871918.
- ^ Muller H (1964). "The relation of recombination to mutational advance". Mutat. Res. 106: 2–9. PMID 14195748.
- ^ Charlesworth B, Charlesworth D (2000). "The degeneration of Y chromosomes". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 355 (1403): 1563–72. PMID 11127901.
- ^ a b Whitlock M (2003). "Fixation probability and time in subdivided populations". Genetics. 164 (2): 767–79. PMID 12807795.
- ^ Haldane J (1959). "The theory of natural selection today". Nature. 183 (4663): 710–13. PMID 13644170.
- ^ Hoekstra H, Hoekstra J, Berrigan D, Vignieri S, Hoang A, Hill C, Beerli P, Kingsolver J (2001). "Strength and tempo of directional selection in the wild". Proc. Natl. Acad. Sci. U.S.A. 98 (16): 9157–60. PMID 11470913.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Felsenstein (1979). "Excursions along the Interface between Disruptive and Stabilizing Selection". Genetics. 93 (3): 773–95. PMID 17248980.
- ^ Andersson M, Simmons L (2006). "Sexual selection and mate choice". Trends Ecol. Evol. (Amst.). 21 (6): 296–302. PMID 16769428.
- ^ Kokko H, Brooks R, McNamara J, Houston A (2002). "The sexual selection continuum". Proc. Biol. Sci. 269 (1498): 1331–40. PMID 12079655.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hunt J, Brooks R, Jennions M, Smith M, Bentsen C, Bussière L (2004). "High-quality male field crickets invest heavily in sexual display but die young". Nature. 432 (7020): 1024–27. PMID 15616562.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Gould SJ (1998). "Gulliver's further travels: the necessity and difficulty of a hierarchical theory of selection". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 353 (1366): 307–14. PMID 9533127.
- ^ Mayr E (1997). "The objects of selection". Proc. Natl. Acad. Sci. U.S.A. 94 (6): 2091–94. PMID 9122151.
- ^ Maynard Smith J (1998). "The units of selection". Novartis Found. Symp. 213: 203–11, discussion 211–17. PMID 9653725.
- ^ Hickey DA (1992). "Evolutionary dynamics of transposable elements in prokaryotes and eukaryotes". Genetica. 86 (1–3): 269–74. PMID 1334911.
- ^ Gould SJ, Lloyd EA (1999). "Individuality and adaptation across levels of selection: how shall we name and generalize the unit of Darwinism?". Proc. Natl. Acad. Sci. U.S.A. 96 (21): 11904–09. PMID 10518549.
- ^ Lande R (1989). "Fisherian and Wrightian theories of speciation". Genome. 31 (1): 221–27. PMID 2687093.
- ^ Otto S, Whitlock M (1997). "The probability of fixation in populations of changing size". Genetics. 146 (2): 723–33. PMID 9178020.
- ^ Nei M (2005). "Selectionism and neutralism in molecular evolution". Mol. Biol. Evol. 22 (12): 2318–42. PMID 16120807.
- ^ Kimura M (1991). "The neutral theory of molecular evolution: a review of recent evidence". Jpn. J. Genet. 66 (4): 367–86. PMID 1954033.
- ^ Kimura M (1989). "The neutral theory of molecular evolution and the world view of the neutralists". Genome. 31 (1): 24–31. PMID 2687096.
- ^ Morjan C, Rieseberg L (2004). "How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles". Mol. Ecol. 13 (6): 1341–56. PMID 15140081.
- ^ Su H, Qu L, He K, Zhang Z, Wang J, Chen Z, Gu H (2003). "The Great Wall of China: a physical barrier to gene flow?". Heredity. 90 (3): 212–19. PMID 12634804.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Short RV (1975). "The contribution of the mule to scientific thought". J. Reprod. Fertil. Suppl. (23): 359–64. PMID 1107543.
- ^ Gross B, Rieseberg L (2005). "The ecological genetics of homoploid hybrid speciation". J. Hered. 96 (3): 241–52. PMID 15618301.
- ^ Vrijenhoek RC (2006). "Polyploid hybrids: multiple origins of a treefrog species". Curr. Biol. 16 (7): R245–47. PMID 16581499.
- ^ Wendel J (2000). "Genome evolution in polyploids". Plant Mol. Biol. 42 (1): 225–49. PMID 10688139.
- ^ Comai L (2005). "The advantages and disadvantages of being polyploid". Nat. Rev. Genet. 6 (11): 836–46. PMID 16304599.
- ^ Soltis P, Soltis D (2000). "The role of genetic and genomic attributes in the success of polyploids". Proc. Natl. Acad. Sci. U.S.A. 97 (13): 7051–57. PMID 10860970.
- ^ Boucher Y, Douady CJ, Papke RT, Walsh DA, Boudreau ME, Nesbo CL, Case RJ, Doolittle WF (2003). "Lateral gene transfer and the origins of prokaryotic groups". Annu Rev Genet. 37: 283–328. PMID 14616063.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Walsh T (2006). "Combinatorial genetic evolution of multiresistance". Curr. Opin. Microbiol. 9 (5): 476–82. PMID 16942901.
- ^ Kondo N, Nikoh N, Ijichi N, Shimada M, Fukatsu T (2002). "Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect". Proc. Natl. Acad. Sci. U.S.A. 99 (22): 14280–85. PMID 12386340.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sprague G (1991). "Genetic exchange between kingdoms". Curr. Opin. Genet. Dev. 1 (4): 530–33. PMID 1822285.
- ^ Baldo A, McClure M (1999). "Evolution and horizontal transfer of dUTPase-encoding genes in viruses and their hosts". J. Virol. 73 (9): 7710–21. PMID 10438861.
- ^ Poole A, Penny D (2007). "Evaluating hypotheses for the origin of eukaryotes". Bioessays. 29 (1): 74–84. PMID 17187354.
- ^ Hendry AP, Kinnison MT (2001). "An introduction to microevolution: rate, pattern, process". Genetica. 112–113: 1–8. PMID 11838760.
- ^ Leroi AM (2000). "The scale independence of evolution". Evol. Dev. 2 (2): 67–77. PMID 11258392.
- ^ Gould, Stephen J. (2002). The Structure of Evolutionary Theory. Belknap Press. ISBN 0-674-00613-5. pp. 657–58
- ^ Scientific American; Biology: Is the human race evolving or devolving?, see also biological devolution.
- ^ Carroll SB (2001). "Chance and necessity: the evolution of morphological complexity and diversity". Nature. 409 (6823): 1102–09. PMID 11234024.
- ^ Whitman W, Coleman D, Wiebe W (1998). "Prokaryotes: the unseen majority". Proc Natl Acad Sci U S A. 95 (12): 6578–83. PMID 9618454.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schloss P, Handelsman J (2004). "Status of the microbial census". Microbiol Mol Biol Rev. 68 (4): 686–91. PMID 15590780.
- ^ Nealson K (1999). "Post-Viking microbiology: new approaches, new data, new insights". Orig Life Evol Biosph. 29 (1): 73–93. PMID 11536899.
- ^ Orr H (2005). "The genetic theory of adaptation: a brief history". Nat. Rev. Genet. 6 (2): 119–27. PMID 15716908.
- ^ Nakajima A, Sugimoto Y, Yoneyama H, Nakae T (2002). "High-level fluoroquinolone resistance in Pseudomonas aeruginosa due to interplay of the MexAB-OprM efflux pump and the DNA gyrase mutation". Microbiol. Immunol. 46 (6): 391–95. PMID 12153116.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Gould, Stephen J. (2002). The Structure of Evolutionary Theory. Belknap Press. ISBN 0-674-00613-5. pp. 1235–36
- ^ Salesa MJ, Antón M, Peigné S, Morales J (2006). "Evidence of a false thumb in a fossil carnivore clarifies the evolution of pandas". Proc. Natl. Acad. Sci. U.S.A. 103 (2): 379–82. PMID 16387860.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Fong D, Kane T, Culver D (1995). "Vestigialization and Loss of Nonfunctional Characters". Ann. Rev. Ecol. Syst. 26: 249–68. doi:10.1146/annurev.es.26.110195.001341.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jeffery WR (2005). "Adaptive evolution of eye degeneration in the Mexican blind cavefish". J. Hered. 96 (3): 185–96. PMID 15653557.
- ^ Maxwell EE, Larsson HC (2007). "Osteology and myology of the wing of the Emu (Dromaius novaehollandiae), and its bearing on the evolution of vestigial structures". J. Morphol. 268 (5): 423–41. PMID 17390336.
- ^ Bejder L, Hall BK (2002). "Limbs in whales and limblessness in other vertebrates: mechanisms of evolutionary and developmental transformation and loss". Evol. Dev. 4 (6): 445–58. PMID 12492145.
- ^ Silvestri AR, Singh I (2003). "The unresolved problem of the third molar: would people be better off without it?". Journal of the American Dental Association (1939). 134 (4): 450–55. PMID 12733778.
- ^ Johnson NA, Porter AH (2001). "Toward a new synthesis: population genetics and evolutionary developmental biology". Genetica. 112–113: 45–58. PMID 11838782.
- ^ Baguñà J, Garcia-Fernàndez J (2003). "Evo-Devo: the long and winding road". Int. J. Dev. Biol. 47 (7–8): 705–13. PMID 14756346.
*Gilbert SF (2003). "The morphogenesis of evolutionary developmental biology". Int. J. Dev. Biol. 47 (7–8): 467–77. PMID 14756322. - ^ Allin EF (1975). "Evolution of the mammalian middle ear". J. Morphol. 147 (4): 403–37. PMID 1202224.
- ^ Harris MP, Hasso SM, Ferguson MW, Fallon JF (2006). "The development of archosaurian first-generation teeth in a chicken mutant". Curr. Biol. 16 (4): 371–77. PMID 16488870.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wade MJ (2007). "The co-evolutionary genetics of ecological communities". Nat. Rev. Genet. 8 (3): 185–95. PMID 17279094.
- ^ Geffeney S, Brodie ED, Ruben PC, Brodie ED (2002). "Mechanisms of adaptation in a predator-prey arms race: TTX-resistant sodium channels". Science. 297 (5585): 1336–9. PMID 12193784.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
*Brodie ED, Ridenhour BJ, Brodie ED (2002). "The evolutionary response of predators to dangerous prey: hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts". Evolution. 56 (10): 2067–82. PMID 12449493.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sachs J (2006). "Cooperation within and among species". J. Evol. Biol. 19 (5): 1415–8, discussion 1426–36. PMID 16910971.
*Nowak M (2006). "Five rules for the evolution of cooperation". Science. 314 (5805): 1560–63. PMID 17158317. - ^ Paszkowski U (2006). "Mutualism and parasitism: the yin and yang of plant symbioses". Curr. Opin. Plant Biol. 9 (4): 364–70. PMID 16713732.
- ^ Hause B, Fester T (2005). "Molecular and cell biology of arbuscular mycorrhizal symbiosis". Planta. 221 (2): 184–96. PMID 15871030.
- ^ Reeve HK, Hölldobler B (2007). "The emergence of a superorganism through intergroup competition". Proc Natl Acad Sci U S A. 104 (23): 9736–40. PMID 17517608.
- ^ Axelrod R, Hamilton W (1981). "The evolution of cooperation". Science. 211 (4489): 1390–96. PMID 7466396.
- ^ Wilson EO, Hölldobler B (2005). "Eusociality: origin and consequences". Proc. Natl. Acad. Sci. U.S.A. 102 (38): 13367–71. PMID 16157878.
- ^ a b Gavrilets S (2003). "Perspective: models of speciation: what have we learned in 40 years?". Evolution. 57 (10): 2197–215. PMID 14628909.
- ^ Jiggins CD, Bridle JR (2004). "Speciation in the apple maggot fly: a blend of vintages?". Trends Ecol. Evol. (Amst.). 19 (3): 111–4. PMID 16701238.
*Boxhorn, J (1995). "Observed Instances of Speciation". The TalkOrigins Archive. Retrieved 2007-05-10.
*Weinberg JR, Starczak VR, Jorg, D (1992). "Evidence for Rapid Speciation Following a Founder Event in the Laboratory". Evolution. 46 (4): 1214–20. doi:10.2307/2409766.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hoskin CJ, Higgle M, McDonald KR, Moritz C (2005). "Reinforcement drives rapid allopatric speciation". Nature. 437: 1353–356. doi:10.1038/nature04004.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Templeton AR (1980). "The theory of speciation via the founder principle". Genetics. 94 (4): 1011–38. PMID 6777243.
- ^ Antonovics J (2006). "Evolution in closely adjacent plant populations X: long-term persistence of prereproductive isolation at a mine boundary". Heredity. 97 (1): 33–37. PMID 16639420.
- ^ Nosil P, Crespi B, Gries R, Gries G (2007). "Natural selection and divergence in mate preference during speciation". Genetica. 129 (3): 309–27. PMID 16900317.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Savolainen V, Anstett M-C, Lexer C, Hutton I, Clarkson JJ, Norup MV, Powell MP, Springate D, Salamin N, Baker WJr (2006). "Sympatric speciation in palms on an oceanic island". Nature. 441: 210–13. PMID 16467788.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
*Barluenga M, Stölting KN, Salzburger W, Muschick M, Meyer A (2006). "Sympatric speciation in Nicaraguan crater lake cichlid fish". Nature. 439: 719–723. PMID 16467837.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gavrilets S (2006). "The Maynard Smith model of sympatric speciation". J. Theor. Biol. 239 (2): 172–82. PMID 16242727.
- ^ Belderok, Bob & Hans Mesdag & Dingena A. Donner. (2000) Bread-Making Quality of Wheat. Springer. p.3. ISBN 0-7923-6383-3.
*Hancock, James F. (2004) Planti Evolution and the Origin of Crop Species. CABI Publishing. ISBN 0-85199-685-X. - ^ Albertin W, Brabant P, Catrice O, Eber F, Jenczewski E, Chèvre AM, Thiellement H (2005). "Autopolyploidy in cabbage (Brassica oleracea L.) does not alter significantly the proteomes of green tissues". Proteomics. 5 (8): 2131–39. PMID 15852348.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ayala FJ, Escalante AA (1996). "The evolution of human populations: a molecular perspective". Mol. Phylogenet. Evol. 5 (1): 188–201. PMID 8673287.
- ^ Gould SJ (1994). "Tempo and mode in the macroevolutionary reconstruction of Darwinism". Proc. Natl. Acad. Sci. U.S.A. 91 (15): 6764–71. PMID 8041695.
- ^ Benton MJ (1995). "Diversification and extinction in the history of life". Science. 268 (5207): 52–58. PMID 7701342.
- ^ Raup DM (1986). "Biological extinction in earth history". Science. 231: 1528–33. PMID 11542058.
- ^ a b c Raup DM (1994). "The role of extinction in evolution" (PDF). Proc. Natl. Acad. Sci. U.S.A. 91 (15): 6758–63. PMID 8041694.
- ^ Novacek MJ, Cleland EE (2001). "The current biodiversity extinction event: scenarios for mitigation and recovery". Proc. Natl. Acad. Sci. U.S.A. 98 (10): 5466–70. PMID 11344295.
- ^ Pimm S, Raven P, Peterson A, Sekercioglu CH, Ehrlich PR (2006). "Human impacts on the rates of recent, present, and future bird extinctions". Proc. Natl. Acad. Sci. U.S.A. 103 (29): 10941–6. PMID 16829570.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
*Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB (2004). "Assessing the causes of late Pleistocene extinctions on the continents". Science. 306 (5693): 70–05. PMID 15459379.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lewis OT (2006). "Climate change, species-area curves and the extinction crisis" (PDF). Philos. Trans. R. Soc. Lond., B, Biol. Sci. 361 (1465): 163–71. PMID 16553315.
- ^ a b c Kutschera U, Niklas K (2004). "The modern theory of biological evolution: an expanded synthesis". Naturwissenschaften. 91 (6): 255–76. PMID 15241603.
- ^ Isaak, Mark (2005), "Claim CB090: Evolution without abiogenesis", TalkOrigins Archive Accessed 13 May 2007
- ^ Peretó J (2005). "Controversies on the origin of life" (PDF). Int. Microbiol. 8 (1): 23–31. PMID 15906258.
- ^ Luisi PL, Ferri F, Stano P (2006). "Approaches to semi-synthetic minimal cells: a review". Naturwissenschaften. 93 (1): 1–13. PMID 16292523.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Trevors JT, Abel DL (2004). "Chance and necessity do not explain the origin of life". Cell Biol. Int. 28 (11): 729–39. PMID 15563395. Forterre P, Benachenhou-Lahfa N, Confalonieri F, Duguet M, Elie C, Labedan B (1992). "The nature of the last universal ancestor and the root of the tree of life, still open questions". BioSystems. 28 (1–3): 15–32. PMID 1337989.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Joyce GF (2002). "The antiquity of RNA-based evolution". Nature. 418 (6894): 214–21. PMID 12110897.
- ^ Trevors JT, Psenner R (2001). "From self-assembly of life to present-day bacteria: a possible role for nanocells". FEMS Microbiol. Rev. 25 (5): 573–82. PMID 11742692.
- ^ Penny D, Poole A (1999). "The nature of the last universal common ancestor". Curr. Opin. Genet. Dev. 9 (6): 672–77. PMID 10607605.
- ^ Bapteste E, Walsh DA (2005). "Does the 'Ring of Life' ring true?". Trends Microbiol. 13 (6): 256–61. PMID 15936656.
- ^ Jablonski D (1999). "The future of the fossil record". Science. 284 (5423): 2114–16. PMID 10381868.
- ^ Mason SF (1984). "Origins of biomolecular handedness". Nature. 311 (5981): 19–23. PMID 6472461.
- ^ Wolf YI, Rogozin IB, Grishin NV, Koonin EV (2002). "Genome trees and the tree of life". Trends Genet. 18 (9): 472–79. PMID 12175808.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Varki A, Altheide TK (2005). "Comparing the human and chimpanzee genomes: searching for needles in a haystack". Genome Res. 15 (12): 1746–58. PMID 16339373.
- ^ Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P (2006). "Toward automatic reconstruction of a highly resolved tree of life". Science. 311 (5765): 1283–87. PMID 16513982.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Cavalier-Smith T (2006). "Cell evolution and Earth history: stasis and revolution" (PDF). Philos Trans R Soc Lond B Biol Sci. 361 (1470): 969–1006. PMID 16754610.
- ^ Schopf J (2006). "Fossil evidence of Archaean life" (PDF). Philos Trans R Soc Lond B Biol Sci. 361 (1470): 869–85. PMID 16754604.
*Altermann W, Kazmierczak J (2003). "Archean microfossils: a reappraisal of early life on Earth". Res Microbiol. 154 (9): 611–17. PMID 14596897. - ^ Schopf J (1994). "Disparate rates, differing fates: tempo and mode of evolution changed from the Precambrian to the Phanerozoic". Proc Natl Acad Sci U S A. 91 (15): 6735–42. PMID 8041691.
- ^ Poole A, Penny D (2007). "Evaluating hypotheses for the origin of eukaryotes". Bioessays. 29 (1): 74–84. PMID 17187354.
- ^ Dyall S, Brown M, Johnson P (2004). "Ancient invasions: from endosymbionts to organelles". Science. 304 (5668): 253–57. PMID 15073369.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Martin W (2005). "The missing link between hydrogenosomes and mitochondria". Trends Microbiol. 13 (10): 457–59. PMID 16109488.
- ^ Lang B, Gray M, Burger G (1999). "Mitochondrial genome evolution and the origin of eukaryotes". Annu Rev Genet. 33: 351–97. PMID 10690412.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
*McFadden G (1999). "Endosymbiosis and evolution of the plant cell". Curr Opin Plant Biol. 2 (6): 513–19. PMID 10607659. - ^ DeLong E, Pace N (2001). "Environmental diversity of bacteria and archaea". Syst Biol. 50 (4): 470–8. PMID 12116647.
- ^ Kaiser D (2001). "Building a multicellular organism". Annu. Rev. Genet. 35: 103–23. PMID 11700279.
- ^ Valentine JW, Jablonski D, Erwin DH (1999). "Fossils, molecules and embryos: new perspectives on the Cambrian explosion". Development. 126 (5): 851–9. PMID 9927587.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ohno S (1997). "The reason for as well as the consequence of the Cambrian explosion in animal evolution". J. Mol. Evol. 44 Suppl 1: S23–7. PMID 9071008.
*Valentine J, Jablonski D (2003). "Morphological and developmental macroevolution: a paleontological perspective". Int. J. Dev. Biol. 47 (7–8): 517–22. PMID 14756327. - ^ Waters ER (2003). "Molecular adaptation and the origin of land plants". Mol. Phylogenet. Evol. 29 (3): 456–63. PMID 14615186.
- ^ Orme CD, Isaac NJ, Purvis A (2002). "Are most species small? Not within species-level phylogenies". Proc. Biol. Sci. 269 (1497): 1279–87. PMID 12065045.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wright, S (1984). Evolution and the Genetics of Populations, Volume 1: Genetic and Biometric Foundations. The University of Chicago Press. ISBN 0-226-91038-5.
- ^ Zirkle C (1941). "Natural Selection before the "Origin of Species"". Proceedings of the American Philosophical Society. 84 (1): 71–123.
- ^ Terrall, M (2002). The Man Who Flattened the Earth: Maupertuis and the Sciences in the Enlightenment. The University of Chicago Press. ISBN 978-0226793610.
- ^ Wallace, A (1858). "On the Tendency of Species to form Varieties, and on the Perpetuation of Varieties and Species by Natural Means of Selection". Journal of the Proceedings of the Linnean Society of London. Zoology. 3: 53–62. Retrieved 2007-05-13.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Stafleu F (1971). "Lamarck: The birth of biology". Taxon. 20: 397–442. PMID 11636092.
- ^ Magner, LN (2002). A History of the Life Sciences, Third Edition, Revised and Expanded. CRC. ISBN 978-0824708245.
- ^ Weiling F (1991). "Historical study: Johann Gregor Mendel 1822–1884". Am. J. Med. Genet. 40 (1): 1–25, discussion 26. PMID 1887835.
- ^ Bowler, Peter J. (1989). The Mendelian Revolution: The Emergence of Hereditarian Concepts in Modern Science and Society. Baltimore: Johns Hopkins University Press. ISBN 978-0801838880.
- ^ For an overview of the philosophical, religious, and cosmological controversies, see: Dennett, D (1995). Darwin's Dangerous Idea: Evolution and the Meanings of Life. Simon & Schuster. ISBN 978-0684824710.
*For the scientific and social reception of evolution in the 19th and early 20th centuries, see: Johnston, Ian C. "History of Science: Origins of Evolutionary Theory". And Still We Evolve. Liberal Studies Department, Malaspina University College. Retrieved 2007-05-24.
*Bowler, PJ (2003). Evolution: The History of an Idea, Third Edition, Completely Revised and Expanded. University of California Press. ISBN 978-0520236936.
*Zuckerkandl E (2006). "Intelligent design and biological complexity". Gene. 385: 2–18. PMID 17011142. - ^ Sarfati, J. "Evolution & creation, science & religion, facts & bias". Answers in Genesis. Retrieved 2007-04-16.
{{cite web}}: External link in|publisher= - ^ Miller JD, Scott EC, Okamoto S (2006). "Science communication. Public acceptance of evolution". Science. 313 (5788): 765–66. PMID 16902112.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Spergel, D. N. (2003). "First-Year Wilkinson Microwave Anisotropy Probe (WMAP) Observations: Determination of Cosmological Parameters". The Astrophysical Journal Supplement Series. 148: 175–94. doi:10.1086/377226.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Wilde SA, Valley JW, Peck WH, Graham CM (2001). "Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago". Nature. 409 (6817): 175–78. PMID 11196637.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kevles DJ (1999). "Eugenics and human rights". BMJ. 319 (7207): 435–8. PMID 10445929.
- ^ On the history of eugenics and evolution, see Kevles, D (1998). In the Name of Eugenics: Genetics and the Uses of Human Heredity. Harvard University Press. ISBN 978-0674445574.
- ^ Darwin strongly disagreed with attempts by Herbert Spencer and others to extrapolate evolutionary ideas to all possible subjects; see Midgley, M (2004). The Myths we Live By. Routledge. p. 62. ISBN 978-0415340779.
*Allhoff F (2003). "Evolutionary ethics from Darwin to Moore". History and philosophy of the life sciences. 25 (1): 51–79. PMID 15293515. - ^ Doebley JF, Gaut BS, Smith BD (2006). "The molecular genetics of crop domestication". Cell. 127 (7): 1309–21. PMID 17190597.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fraser AS (1958). "Monte Carlo analyses of genetic models". Nature. 181 (4603): 208–9. PMID 13504138.
- ^ Rechenberg, Ingo (1973). Evolutionsstrategie - Optimierung technischer Systeme nach Prinzipien der biologischen Evolution (PhD thesis) (in German). Fromman-Holzboog.
- ^ Holland, John H. (1975). Adaptation in Natural and Artificial Systems. University of Michigan Press. ISBN 0262581116.
- ^ Jamshidi M (2003). "Tools for intelligent control: fuzzy controllers, neural networks and genetic algorithms". Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 361 (1809): 1781–808. PMID 12952685.
External links
General information
- Understanding Evolution from University of California, Berkeley
- National Academies Evolution Resources
- Everything you wanted to know about evolution by New Scientist
- Howstuffworks.com — How Evolution Works
- Synthetic Theory Of Evolution: An Introduction to Modern Evolutionary Concepts and Theories
- Evolution News by the Genome News Network
History of evolutionary thought
- The Complete Work of Charles Darwin Online
- Understanding Evolution: History, Theory, Evidence, and Implications
