User:Joflaher/sandbox: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DISPLAYTITLE:α-Ketoglutaric acid}} |
|||
'''2-Oxoglutarate receptor 1''' ('''OXGR1'''), also known as cysteinyl leukotriene receptor E (CysLT<sub>E</sub>) and GPR99,<ref name="pmid23504326">{{cite journal | vauthors = Kanaoka Y, Maekawa A, Austen KF | title = Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand | journal = The Journal of Biological Chemistry | volume = 288 | issue = 16 | pages = 10967–72 | date = Apr 2013 | pmid = 23504326 | pmc = 3630866 | doi = 10.1074/jbc.C113.453704 | doi-access = free }}</ref> is a [[Receptor (biochemistry)|receptor]] located on the [[cell surface membrane|surface membrane]]s of certain cells. It functions by binding one of its [[Ligand (biochemistry)|ligands]] and thereby becoming active in triggering pre-programmed responses in its parent cells. OXGR1 has been shown to bind and become activated by alpha-ketoglutarate,<ref name="pmid28771454">{{cite journal | vauthors = Grimm PR, Welling PA | title = α-Ketoglutarate drives electroneutral NaCl reabsorption in intercalated cells by activating a G-protein coupled receptor, Oxgr1 | journal = Current Opinion in Nephrology and Hypertension | volume = 26 | issue = 5 | pages = 426–433 | date = September 2017 | pmid = 28771454 | doi = 10.1097/MNH.0000000000000353 | url = }}</ref> itaconate,<ref name="pmid36919698">{{cite journal | vauthors = Zeng YR, Song JB, Wang D, Huang ZX, Zhang C, Sun YP, Shu G, Xiong Y, Guan KL, Ye D, Wang P | title = The immunometabolite itaconate stimulates OXGR1 to promote mucociliary clearance during the pulmonary innate immune response | journal = The Journal of Clinical Investigation | volume = 133 | issue = 6 | pages = | date = March 2023 | pmid = 36919698 | pmc = 10014103 | doi = 10.1172/JCI160463 | url = }}</ref> and three different [[Cysteine|cysteiny]]-containing [[leukotrienes]] (abbreviated as CysLTs), leukotriene (i.e,, LT) [[LTE4]], [[LTC4]], and [[LTD4]].<ref name="pmid23504326"/><ref name="pmid31135881">{{cite journal | vauthors = Sasaki F, Yokomizo T | title = The leukotriene receptors as therapeutic targets of inflammatory diseases | journal = International Immunology | volume = 31 | issue = 9 | pages = 607–615 | date = August 2019 | pmid = 31135881 | doi = 10.1093/intimm/dxz044 | url = }}</ref> Alpha-ketoglutarate and itaconate are short-chain [[dicarboxylic acid]]s containing two [[carboxyl group]]s both of which are bound to [[hydrogen]] (notated as -{{chem2|CO2H}}). However, at the [[Base (chemistry)|basic]] [[pH]] levels (i.e., pH>7) in almost all mammalian tissues, [[alpha-ketoglutaric acid]] and [[itaconic acid]] exit almost exclusively as alpha-ketoglutarate and itaconate, i.e., with their carboxy residues being negatively charged because they are not bound to H (notated as -{{chem2|CO2}}<math>^{-}</math>), i.e., they are [[dianion]]s. It is the dianions of alpha-ketoglutaric acid and alpha-ketoglutarate that bind to and activate OXGR1. (Note that alpha-ketoglutaric acid is also termed 2-oxoglutaric acid and alpha-ketoglutarate is also termed 2-oxoglutarate.)<ref name="pmid28771454"/><ref name="pmid36919698"/> |
|||
{{chembox |
|||
| Verifiedfields = changed |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 477319214 |
|||
| Name = α-Ketoglutaric acid |
|||
| Reference = <ref>''Merck Index'', 13th Edition, '''5320'''.</ref> |
|||
| ImageFile = Alpha-ketoglutaric acid.png |
|||
| ImageSize = |
|||
| PIN = 2-Oxopentanedioic acid |
|||
| OtherNames = 2-Ketoglutaric acid<br>alpha-Ketoglutaric acid<br>2-Oxoglutaric acid<br>Oxoglutaric acid |
|||
|Section1={{Chembox Identifiers |
|||
| IUPHAR_ligand = 3636 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 50 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 8ID597Z82X |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| KEGG = C00026 |
|||
| InChI = 1/C5H6O5/c6-3(5(9)10)1-2-4(7)8/h1-2H2,(H,7,8)(H,9,10) |
|||
| InChIKey = KPGXRSRHYNQIFN-UHFFFAOYAN |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C5H6O5/c6-3(5(9)10)1-2-4(7)8/h1-2H2,(H,7,8)(H,9,10) |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = KPGXRSRHYNQIFN-UHFFFAOYSA-N |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| CASNo = 328-50-7 |
|||
| PubChem = 51 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = 30915 |
|||
| DrugBank_Ref = {{drugbankcite|changed|drugbank}} |
|||
| DrugBank = DB02926 |
|||
| SMILES = O=C(O)C(=O)CCC(=O)O |
|||
| MeSHName = alpha-ketoglutaric+acid |
|||
}} |
|||
|Section2={{Chembox Properties |
|||
| C=5 | H=6 | O=5 |
|||
| Appearance = |
|||
| Density = |
|||
| MeltingPtC = 115 |
|||
| MeltingPt_notes = |
|||
| BoilingPt = |
|||
}} |
|||
|Section3={{Chembox Hazards |
|||
| MainHazards = |
|||
| FlashPt = |
|||
| AutoignitionPt = |
|||
}} |
|||
}} |
|||
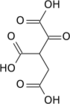
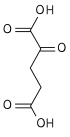
'''α-Ketoglutaric acid''' ( also termed 2-oxoglutaric acid) is a [dicarboxylic acid]], i.e., a short-chain fatty acid containing two [[carboxyl group]]s (carboxy group notated as {{chem2|CO2H}} with C, O, and H standing for [[carbon]], [[oxygen]], and hydrogen, respectively; see adjacent figure}. However, almost all animal [[extracellular fluids]] have a [[pH]] above 7. At these pH levels, α-Ketoglutaric acid exists almost exclusively as its [[conjugate base]], i.e., as a double negatively charged dicarboxylate due to the release of H from its two carboxy groups, {{chem2|RCO2-}} termed '''α-ketoglutarte''' (see [[Conjugate (acid-base theory)|Conjugate acid-base theory)]]. |
|||
== History == |
|||
In 2001, a gene projected to code for a [[G protein-coupled receptor]] (i.e., a receptor that stimulates cells by activating [[G protein]]s) was reported. The gene's apparent [[protein]] product was classified as an [[orphan receptor]] (i.e., a receptor whose activating ligand and function were unknown). The projected [[Protein primary structure|amino acid sequence]] of the protein encoded by its gene bore similarities to the [[purinergic receptor]], [[P2Y1]], and therefore might, like P2Y1, be a receptor for [[purine]]s. This study named the new receptor and its gene GPR80 and ''GPR80'', respectively.<ref name="pmid11574155">{{cite journal | vauthors = Lee DK, Nguyen T, Lynch KR, Cheng R, Vanti WB, Arkhitko O, Lewis T, Evans JF, George SR, O'Dowd BF | title = Discovery and mapping of ten novel G protein-coupled receptor genes | journal = Gene | volume = 275 | issue = 1 | pages = 83–91 | year = 2001 | pmid = 11574155 | doi = 10.1016/s0378-1119(01)00651-5}}</ref> Shortly thereafter, a second study found this same gene, indicated that it coded for a G protein-coupled receptor, had an amino acid sequence similar to two [[purinergic receptor]]s, P2Y1 and [[SUCNR1|GPR91]], and determined that a large series of purine nucleotides, other [[nucleotides]], and derivatives of these compounds did not activate these receptors. The study named this orphan receptor GPR99.<ref name="pmid12098360">{{cite journal | vauthors = Wittenberger T, Hellebrand S, Munck A, Kreienkamp HJ, Schaller HC, Hampe W | title = GPR99, a new G protein-coupled receptor with homology to a new subgroup of nucleotide receptors | journal = BMC Genomics | volume = 3 | issue = | pages = 17 | date = July 2002 | pmid = 12098360 | pmc = 117779 | doi = 10.1186/1471-2164-3-17 | url = }}</ref> A third study published in 2004 reported an "orphan" G protein-coupled receptor with an amino acid sequence similar to the [[P2Y receptor]] family of nucleotides was activated by to two purines, [[adenosine]] and [[adenosine monophosphate]]. The study nominated this receptor as a true purinergic receptor and named it the P2Y15 receptor.<ref name="pmid15001573">{{cite journal | vauthors = Inbe H, Watanabe S, Miyawaki M, Tanabe E, Encinas JA | title = Identification and characterization of a cell-surface receptor, P2Y15, for AMP and adenosine | journal = The Journal of Biological Chemistry | volume = 279 | issue = 19 | pages = 19790–9 | year = 2004 | pmid = 15001573 | doi = 10.1074/jbc.M400360200 | doi-access = free }}</ref> However, a review of these studies in the same year by members of the [[International Union of Pharmacology]] (IUPHAR) Subcommittee for P2Y Receptor Nomenclature and Classification decided that GPR80/GPR99 is not a receptor for adenosine, adenosine monophosphate, or any other nucleotide.<ref name="pmid15629198">{{cite journal | vauthors = Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA | title = The recently deorphanized GPR80 (GPR99) proposed to be the P2Y15 receptor is not a genuine P2Y receptor | journal = Trends in Pharmacological Sciences | volume = 26 | issue = 1 | pages = 8–9 | year = 2005 | pmid = 15629198 | doi = 10.1016/j.tips.2004.10.010 | pmc = 6905457 }}</ref> A fourth study, also published in 2004, found that GPR80/GPR99 -bearing cells responded to alpha-ketoglutarate.<ref name="pmid15141213">{{cite journal | vauthors = He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L | title = Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors | journal = Nature | volume = 429 | issue = 6988 | pages = 188–93 | date = May 2004 | pmid = 15141213 | doi = 10.1038/nature02488 | url = }}</ref> In 2013, IUPHAR accepted this report and the names OXGR1 and ''OXGR1'' for the alpha-ketoglutarate responsive receptor and its gene, respectively.<ref name="pmid23686350">{{cite journal | vauthors = Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, Liew WC, Mpamhanga CP, Bonner TI, Neubig RR, Pin JP, Spedding M, Harmar AJ | title = International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands | journal = Pharmacological Reviews | volume = 65 | issue = 3 | pages = 967–86 | year = 2013 | pmid = 23686350 | pmc = 3698937 | doi = 10.1124/pr.112.007179 }}</ref>. In 2013, a fifth study found that CysLTs LTE, LTC4, and LTD4 bound to and activated OXGR1.<ref name="pmid23504326"/> Finally, a 2023 study provided evidence that [[itaconate]] activated the OXGR1 receptor.<ref name="pmid36919698"/> The latter findings, while of potential clinical importance, have not yet lead to a renaming of the ''OXGR1'' gene or its OXGR1 receptor protein product. |
|||
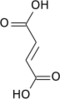
Its [[carboxylate]], '''α-ketoglutarate''' (also called '''2-oxoglutarate'''), is an important biological compound. It is produced by [[deamination]] of [[glutamate]], and is an intermediate in the [[Krebs cycle]]. |
|||
== Gene and product == |
|||
The ''OXGR1'' gene is localized to human [[chromosome 13]] at position 13q32.2; that is, the gene resides at position 32.2 in the [[q arm]]s (also termed long arm ) of chromosome 13 ''OXGR1'' codes for a cellular G protein coupled receptor that is primarily linked to and activates G protein heterotrimers containing the [[Gq alpha subunit]]; i.e., when bound to any of its activating ligands, OXGR1 stimulates Gq alpha subunit-regulated cellular pathways (see [[Gq alpha subunit#Function|Function of the Gq alpha]]) that trigger the cellular responses they are programmed to regulate.<ref>{{Cite web|url=http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=162|title = Oxoglutarate receptor | Oxoglutarate receptor | IUPHAR/BPS Guide to PHARMACOLOGY}}</ref><ref name="pmid24588652">{{cite journal | vauthors = Bäck M, Powell WS, Dahlén SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE | title = Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7 | journal = British Journal of Pharmacology | volume = 171 | issue = 15 | pages = 3551–74 | year = 2014 | pmid = 24588652 | pmc = 4128057 | doi = 10.1111/bph.12665 }}</ref> |
|||
The term "[[ketoglutaric acid]]", when not further qualified, almost always refers to the alpha variant. [[Acetonedicarboxylic acid|β-Ketoglutaric acid]] varies only by the position of the [[ketone]] [[functional group]], and is much less common. |
|||
== Activating ligands == |
|||
OXGR1 is the receptor for alpha-ketoglutarate, LTE4, LTC4, LTD4, and itaconate. These ligands have the following relative potencies in stimulating responses in OXGR1-bearing cells: |
|||
==Functions== |
|||
:::LTE4 >> LTC4 = LTD4 > alpha-ketoglutarate = itaconate |
|||
===Alanine transaminase=== |
|||
LTE4 is able to stimulate responses in at least some of its target cells at concentrations as low as a few [[picomole]]s/liter<ref name="pmid23504326"/><ref name="pmid36919698"/> whereas itaconate requires far higher levels to elicit responses in its target cells, e.g., in the 50–300 [[micromole]]s/liter range.<ref name="pmid38448252">{{cite journal | vauthors = Ye D, Wang P, Chen LL, Guan KL, Xiong Y | title = Itaconate in host inflammation and defense | journal = Trends in Endocrinology and Metabolism: TEM | volume = | issue = | pages = | date = March 2024 | pmid = 38448252 | doi = 10.1016/j.tem.2024.02.004 | url = }}</ref> |
|||
The enzyme [[alanine transaminase]] (also termed alanine aminotransferase and glutamate pyruvate transaminase) [[catalyzes]], i.e. converts, '''1)''' α-ketoglutarate to [[Glutamate|<small>L</small>-glutamate]] and '''2)''' [[Alanine|<small>L</small>-alanine]] to [[pyruvate]]. Both of these catalyzed reactions are fully [[reversible reaction|reversible]], i.e., run in both the forward and reverse directions.<ref name="pmid19085960">{{cite journal | vauthors = Yang RZ, Park S, Reagan WJ, Goldstein R, Zhong S, Lawton M, Rajamohan F, Qian K, Liu L, Gong DW | title = Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity | journal = Hepatology (Baltimore, Md.) | volume = 49 | issue = 2 | pages = 598–607 | date = February 2009 | pmid = 19085960 | pmc = 2917112 | doi = 10.1002/hep.22657 | url = }}</ref> |
|||
===Krebs cycle=== |
|||
== Inhibiting ligands == |
|||
α-Ketoglutarate is a key intermediate in the [[Krebs cycle]], coming after [[isocitrate]] and before [[succinyl CoA]]. [[Anaplerotic reactions]] can replenish the cycle at this juncture by synthesizing α-ketoglutarate from transamination of glutamate, or through action of [[glutamate dehydrogenase]] on glutamate.{{citation needed|date=November 2019}} |
|||
OXGR1 is inhibited by [[montelukast]], a well-known and clinically useful inhibitor of [[cysteinyl leukotriene receptor 1]] (CysLTR1); this drug binds to but does not activate CysLTR1. In consequence, montelukast blocks the binding and and thereby the actions of LTD4, LTC4, and LTE4. It is presumed to act similarly to block the actions of alpha-ketoglutarate and itaconates on OXGR1.<ref name="pmid23504326"/><ref name="pmid34179130">{{cite journal | vauthors = Guerrero A, Visniauskas B, Cárdenas P, Figueroa SM, Vivanco J, Salinas-Parra N, Araos P, Nguyen QM, Kassan M, Amador CA, Prieto MC, Gonzalez AA | title = α-Ketoglutarate Upregulates Collecting Duct (Pro)renin Receptor Expression, Tubular Angiotensin II Formation, and Na+ Reabsorption During High Glucose Conditions | journal = Frontiers in Cardiovascular Medicine | volume = 8 | issue = | pages = 644797 | date = 2021 | pmid = 34179130 | pmc = 8220822 | doi = 10.3389/fcvm.2021.644797 | url = }}</ref> It is not known if other CysLTR1 inhibitors (see [[Cysteinyl leukotriene receptor 1#Clinical significance]]) can mimic montelukast in blocking OXGR1. |
|||
===Formation of amino acids=== |
|||
== Expression == |
|||
[[Glutamine]] is synthesized from glutamate by [[glutamine synthetase]], which utilizes adenosine triphosphate to form glutamyl phosphate; this intermediate is attacked by ammonia as a nucleophile giving glutamine and inorganic phosphate. [[Proline]], [[arginine]], and lysine (in some organisms) are other amino acids synthesized as well.<ref>{{Cite journal|last1=Ledwidge|first1=Richard|last2=Blanchard|first2=John S.|title=The Dual Biosynthetic Capability of N-Acetylornithine Aminotransferase in Arginine and Lysine Biosynthesis†|journal=Biochemistry|language=en|volume=38|issue=10|pages=3019–3024|doi=10.1021/bi982574a|pmid=10074354|year=1999}}</ref> These three amino acids derive from glutamate with the addition of further steps or enzymes to facilitate reactions.<ref>{{Cite book |last1=Schousboe |first1=Arne |last2=Scafidi |first2=Susanna |last3=Bak |first3=Lasse K. |last4=Waagepetersen |first4=Helle S. |last5=McKenna |first5=Mary C. |chapter=Glutamate Metabolism in the Brain Focusing on Astrocytes |series=Advances in Neurobiology |date=2014 |title=Glutamate and ATP at the Interface of Metabolism and Signaling in the Brain |volume=11 |pages=13–30 |doi=10.1007/978-3-319-08894-5_2 |issn=2190-5215 |pmc=4667713 |pmid=25236722|isbn=978-3-319-08893-8 }}</ref> |
|||
Based on their content of OXGR1 [[mRNA]], OXGR1 is expressed in human kidney, placenta, [[fetal]] brain, and tissues involved in allergic and hypersensitivity reactions such as the lung trachea, salivary glands, [[eosinophils]], [[mast cells]] derived from [[umbilical cord blood]], and nasal [[mucosa]], particularly the vascular smooth muscle in the latter tissue.<ref name="pmid23504326"/><ref name="pmid24768603">{{cite journal | vauthors = Steinke JW, Negri J, Payne SC, Borish L | title = Biological effects of leukotriene E4 on eosinophils | journal = Prostaglandins, Leukotrienes, and Essential Fatty Acids | volume = 91 | issue = 3 | pages = 105–10 | year = 2014 | pmid = 24768603 | pmc = 4127125 | doi = 10.1016/j.plefa.2014.02.006 }}</ref><ref name="pmid27324180">{{cite journal | vauthors = Shirasaki H, Kanaizumi E, Himi T | title = Expression and localization of OXGR1 in human nasal mucosa | journal = Auris, Nasus, Larynx | year = 2016 | volume = 44 | issue = 2 | pages = 162–167 | pmid = 27324180 | doi = 10.1016/j.anl.2016.05.010 }}</ref> In mice, Oxgr1 [[mRNA]] is expressed in kidneys, testes, and smooth muscle.<ref name="pmid23504326"/> |
|||
===Nitrogen transporter=== |
|||
== Function == |
|||
Another function is to combine with [[nitrogen]] released in cells, therefore preventing nitrogen overload.<ref>{{Cite journal |last=Katayama |first=Kazuhiro |date=2004-12-01 |title=Ammonia metabolism and hepatic encephalopathy |url=https://www.sciencedirect.com/science/article/pii/S138663460400227X |journal=Hepatology Research |volume=30 |pages=73–80 |doi=10.1016/j.hepres.2004.08.013 |pmid=15607143 |issn=1386-6346}}</ref> |
|||
OXGR1 binds and is activated by LTE4 at concentrations far lower than the other major CysLT receptors, [[Cysteinyl leukotriene receptor 1]] (CysLTR1) and [[Cysteinyl leukotriene receptor 2]] (CysLTR2), both of which appear to be physiological receptors for LTD4 and LTC4 but not LTF4 (see [[Cysteinyl leukotriene receptor 1#Function]]). This suggests that the actions of LTE4 are mediated, at least to a large extent, by OXGR1. Several findings support this notion: '''a)''' pretreatment of guinea pig trachea and human bronchial smooth muscle with LTE4 but not LTC4 or LTD4 enhances their contraction responses to [[histamine]]; '''b)''' LTE4 is as potent as LTC4 and LTD4 in eliciting vascular leakage when injected into the skin of guinea pigs and humans; '''c)''' inhalation of LTE4 but not LTD4 by asthmatic subjects caused the accumulation of [[eosinophil]]s and [[basophil]]s in their bronchial mucosa; '''d)''' mice engineered to lack Cysltr1 and Cysltr2 receptors exhibited edema responses to the intradermal injection of LTC4, LTD4, and LTE4 but only LTE4 was more potent (by a factor of 64-fold) in these mice compared to in [[wild type]] mice; and '''e)''' mice engineered to lack all three Cysltr1, Cysltr2, and OXGR1 receptors showed no dermal edema responses to the injection of LTC4, LTD4, or LTE4.<ref name="pmid23504326"/> |
|||
α-Ketoglutarate is one of the most important nitrogen transporters in metabolic pathways. The amino groups of amino acids are attached to it (by [[transamination]]) and carried to the liver where the [[urea cycle]] takes place.<ref>{{Cite journal |last1=Li |first1=Tao |last2=Kootstra |first2=Anna B. |last3=Fotheringham |first3=Ian G. |date=2002-07-01 |title=Nonproteinogenic α-Amino Acid Preparation Using Equilibrium Shifted Transamination |url=https://pubs.acs.org/doi/10.1021/op025518x |journal=Organic Process Research & Development |language=en |volume=6 |issue=4 |pages=533–538 |doi=10.1021/op025518x |issn=1083-6160}}</ref> |
|||
Mice deficient in OXGPR1 (i.e., ''Oxgr1'' [[gene knockout]] mice) develop (82% [[penetrance]]) spontaneous [[Otitis media]] with many characteristics of the human disease; while the underlying cause of this development, the ''Oxgr1-/-'' mouse is proposed to be a good model to study and relate to human ear pathology.<ref name="pmid23200873">{{cite journal | vauthors = Kerschner JE, Hong W, Taylor SR, Kerschner JA, Khampang P, Wrege KC, North PE | title = A novel model of spontaneous otitis media with effusion (OME) in the Oxgr1 knock-out mouse | journal = International Journal of Pediatric Otorhinolaryngology | volume = 77 | issue = 1 | pages = 79–84 | year = 2013 | pmid = 23200873 | pmc = 3535456 | doi = 10.1016/j.ijporl.2012.09.037 }}</ref> |
|||
α-Ketoglutarate is transaminated, along with glutamine, to form the excitatory [[neurotransmitter]] [[glutamate]]. Glutamate can then be [[Carboxylation|decarboxylated]] (requiring [[Vitamin B6|vitamin B<sub>6</sub>]]) into the inhibitory neurotransmitter [[gamma-aminobutyric acid]].<ref>{{Cite journal |last1=Kaneko |first1=T. |last2=Mizuno |first2=N. |date=August 1994 |title=Glutamate-synthesizing enzymes in GABAergic neurons of the neocortex: a double immunofluorescence study in the rat |url=https://pubmed.ncbi.nlm.nih.gov/7838383/ |journal=Neuroscience |volume=61 |issue=4 |pages=839–849 |doi=10.1016/0306-4522(94)90407-3 |issn=0306-4522 |pmid=7838383|s2cid=19178915 }}</ref> |
|||
OXGR1 also appears to be involved in the adaptive regulation of [[bicarbonate]] ({{chem2|HCO3-}}) secretion and salt ([[NaCl]]) reabsorption in the mouse kidneys undergoing acid-base stress: the kidneys of OXGR1 [[gene knockout]] mice did not respond to alpha-Ketoglutaric acid by upregulating bicarbonate/NaCl exchange and exhibited a reduced ability to maintain acid-base balance.<ref name="pmid23934124">{{cite journal | vauthors = Tokonami N, Morla L, Centeno G, Mordasini D, Ramakrishnan SK, Nikolaeva S, Wagner CA, Bonny O, Houillier P, Doucet A, Firsov D | title = α-Ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism | journal = The Journal of Clinical Investigation | volume = 123 | issue = 7 | pages = 3166–71 | year = 2013 | pmid = 23934124 | pmc = 3696567 | doi = 10.1172/JCI67562 | url = https://serval.unil.ch/resource/serval:BIB_93F2D96819D4.P001/REF}}</ref> |
|||
It is reported that high [[ammonia]] and/or high nitrogen levels may occur with high [[protein]] intake, excessive aluminium exposure, [[Reye's syndrome]], [[cirrhosis]], and [[urea cycle disorder]].<ref>{{Cite web |title=Hyperammonemia: What It Is, Causes, Symptoms & Treatment |url=https://my.clevelandclinic.org/health/diseases/24065-hyperammonemia |access-date=2023-11-03 |website=Cleveland Clinic |language=en}}</ref> |
|||
The activation of OXGR1 by itaconate inhibits a wide-range of potentially deleterious inflammatory reactions<ref name="pmid36459716">{{cite journal | vauthors = Shi X, Zhou H, Wei J, Mo W, Li Q, Lv X | title = The signaling pathways and therapeutic potential of itaconate to alleviate inflammation and oxidative stress in inflammatory diseases | journal = Redox Biology | volume = 58 | issue = | pages = 102553 | date = December 2022 | pmid = 36459716 | pmc = 9713374 | doi = 10.1016/j.redox.2022.102553 | url = }}</ref> and suppresses the growth of certain cancers (See [[Itaconic acid#Actions of itaconate and its analogs|Actions of itaconate and its analogs]]).<ref name="pmid38042791">{{cite journal | vauthors = Yang W, Wang Y, Tao K, Li R | title = Metabolite itaconate in host immunoregulation and defense | journal = Cellular & Molecular Biology Letters | volume = 28 | issue = 1 | pages = 100 | date = December 2023 | pmid = 38042791 | pmc = 10693715 | doi = 10.1186/s11658-023-00503-3 | doi-access = free | url = }}</ref> |
|||
It plays a role in [[detoxification]] of ammonia in brain.<ref>{{Cite web |url=http://www.autismstudies.info/AutismStudies.info/Fever_glutamine.html |title=Does infectious fever relieve autistic behavior by releasing glutamine from skeletal muscles as provisional fuel? |access-date=2014-05-19 |archive-url=https://web.archive.org/web/20140519184046/http://www.autismstudies.info/AutismStudies.info/Fever_glutamine.html |archive-date=2014-05-19 |url-status=dead }}</ref><ref>{{cite journal|last=Ott|first=P|author2=Clemmesen, O |author3=Larsen, FS |title=Cerebral metabolic disturbances in the brain during acute liver failure: from hyperammonemia to energy failure and proteolysis.|journal=Neurochemistry International|date=Jul 2005|volume=47|issue=1–2|pages=13–8|pmid=15921824|doi=10.1016/j.neuint.2005.04.002|s2cid=916739}}</ref><ref>{{cite journal|last=Hares|first=P|author2=James, IM |author3=Pearson, RM |title=Effect of ornithine alpha ketoglutarate (OAKG) on the response of brain metabolism to hypoxia in the dog.|journal=Stroke: A Journal of Cerebral Circulation|date=May–Jun 1978|volume=9|issue=3|pages=222–4|doi=10.1161/01.STR.9.3.222|pmid=644619|doi-access=free}}</ref> |
|||
== Clinical significance == |
|||
Montelukast is in use to treat various conditions including [[asthma]], [[exercise-induced bronchoconstriction]], [[allergic rhinitis]], primary dysmenorrhea (i.e. dysmenorrhea not associated with known causes; see [[dysmenorrhea#Causes]]), and [[urticaria]]. It has been presumed that this drug's beneficial effects in these diseases is due to its well-known ability to act as a [[receptor antagonist]] for the cysteinyl leukotriene receptor 1 (CysLTR1), i.e. it binds to but does not activate this receptor thereby interfering with LTD4, LTC4, and LTE4 provocative actions by blocking their binding to CysLTR1 (the drug does not block the [[cysteinyl leukotriene receptor 2]]) (see [[cysteinyl leukotriene receptor 1#Clinical significance]]). The more recently discovered ability of this drug to block the ability of LTE4 and LTD4 to stimulate OXGR1 in OXGR1-bearing cells<ref name="pmid23504326"/> allows that montelukast’s beneficial effects on these conditions might reflect its ability to block not only CysLTR1 but also OXGR1. |
|||
===Relationship to molecular oxygen=== |
|||
== References == |
|||
Acting as a co-substrate for [[alpha-ketoglutarate-dependent hydroxylase|α-ketoglutarate-dependent hydroxylase]], it also plays important function in oxidation reactions involving molecular oxygen.{{citation needed|date=November 2019}} |
|||
{{reflist}} |
|||
Molecular oxygen (O<sub>2</sub>) directly [[oxidize]]s many compounds to produce useful products in an organism, such as [[antibiotic]]s, in reactions catalyzed by [[oxygenase]]s. In many oxygenases, α-ketoglutarate helps the reaction by being oxidized with the main [[Substrate (chemistry)|substrate]]. [[EGLN1]], one of the α-ketoglutarate-dependent oxygenases, is an O<sub>2</sub> sensor, informing the organism of the oxygen level in its environment.{{clarify|The meaning of this sentence is unclear|date=November 2019}} |
|||
== Further reading == |
|||
{{refbegin | 2}} |
|||
* {{cite journal | vauthors = Lee DK, Nguyen T, Lynch KR, Cheng R, Vanti WB, Arkhitko O, Lewis T, Evans JF, George SR, O'Dowd BF | title = Discovery and mapping of ten novel G protein-coupled receptor genes | journal = Gene | volume = 275 | issue = 1 | pages = 83–91 | date = Sep 2001 | pmid = 11574155 | doi = 10.1016/S0378-1119(01)00651-5 }} |
|||
* {{cite journal | vauthors = Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S | title = Identification of G protein-coupled receptor genes from the human genome sequence | journal = FEBS Letters | volume = 520 | issue = 1–3 | pages = 97–101 | date = Jun 2002 | pmid = 12044878 | doi = 10.1016/S0014-5793(02)02775-8 | doi-access = free }} |
|||
* {{cite journal | vauthors = Inbe H, Watanabe S, Miyawaki M, Tanabe E, Encinas JA | title = Identification and characterization of a cell-surface receptor, P2Y15, for AMP and adenosine | journal = The Journal of Biological Chemistry | volume = 279 | issue = 19 | pages = 19790–9 | date = May 2004 | pmid = 15001573 | doi = 10.1074/jbc.M400360200 | doi-access = free }} |
|||
{{refend}} |
|||
In combination with molecular oxygen, alpha-ketoglutarate is one of the requirements for the hydroxylation of proline to [[hydroxyproline]] in the production of many [[collagens]].{{citation needed|date=November 2019}} |
|||
{{NLM content}} |
|||
{{G protein-coupled receptors}} |
|||
===Antioxidant=== |
|||
[[Category:G protein-coupled receptors]] |
|||
α-Ketoglutarate, which is released by several cell types, decreases the levels of [[hydrogen peroxide]], and the α-ketoglutarate was depleted and converted to [[succinate]] in cell culture media.<ref>{{cite journal|last=Long|first=L|author2=Halliwell, B |title=Artefacts in cell culture: α-Ketoglutarate can scavenge hydrogen peroxide generated by ascorbate and epigallocatechin gallate in cell culture media.|journal=Biochemical and Biophysical Research Communications |date=2011 |volume=406 |issue=1 |pages=20–24 |doi=10.1016/j.bbrc.2011.01.091 |pmid=21281600}}</ref> |
|||
== Supplementation == |
|||
===Longevity=== |
|||
Studies have linked α-ketoglutarate with increased lifespan in [[nematode]] worms<ref |
|||
name="doi:10.1038/nature13264">{{cite journal|doi=10.1038/nature13264 | title=The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR | journal=Nature| pmc=4263271| pmid=24828042| volume=510| issue=7505 | year=2014| pages=397–401 | last1 = Chin | first1 = RM | last2 = Fu | first2 = X | last3 = Pai | first3 = MY | last4 = Vergnes | first4 = L | last5 = Hwang | first5 = H | last6 = Deng | first6 = G | last7 = Diep | first7 = S | last8 = Lomenick | first8 = B | last9 = Meli | first9 = VS | last10 = Monsalve | first10 = GC | last11 = Hu | first11 = E | last12 = Whelan | first12 = SA | last13 = Wang | first13 = JX | last14 = Jung | first14 = G | last15 = Solis | first15 = GM | last16 = Fazlollahi | first16 = F | last17 = Kaweeteerawat | first17 = C | last18 = Quach | first18 = A | last19 = Nili | first19 = M | last20 = Krall | first20 = AS | last21 = Godwin | first21 = HA | last22 = Chang | first22 = HR | last23 = Faull | first23 = KF | last24 = Guo | first24 = F | last25 = Jiang | first25 = M | last26 = Trauger | first26 = SA | last27 = Saghatelian | first27 = A | last28 = Braas | first28 = D | last29 = Christofk | first29 = HR | last30 = Clarke | first30 = CF | last31 = Teitell | first31 = MA | last32 = Petrascheck | first32 = M | last33 = Reue | first33 = K | last34 = Jung | first34 = ME | last35 = Frand | first35 = AR | last36 = Huang | first36 = J| bibcode=2014Natur.510..397C }}</ref> and increased healthspan/lifespan in mice.<ref>{{Cite web|last=Kaiser 1|first=Jocelyn|date=2020-09-01|title=Bodybuilding supplement promotes healthy aging and extends life span, at least in mice|url=https://www.science.org/content/article/bodybuilding-supplement-promotes-healthy-aging-and-extends-life-span-least-mice|access-date=2020-09-01|website=Science {{!}} AAAS|language=en}}</ref><ref>{{Cite web|title=A metabolite produced by the body increases lifespan and dramatically compresses late-life morbidity in mice|url=https://www.buckinstitute.org/news/a-metabolite-produced-by-the-body-increases-lifespan-and-dramatically-compresses-late-life-morbidity-in-mice/|access-date=2020-09-01|website=BUCK|language=en-US}}</ref><ref>{{Cite web|last1=Shahmirzadi|first1=Azar Asadi|last2=Edgar|first2=Daniel|last3=Liao|first3=Chen-Yu|date=2020-09-01|title=Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice|url=https://www.cell.com/cell-metabolism/fulltext/S1550-4131(20)30417-4|access-date=2020-09-20|website=Cell Metabolism|language=en-US}}</ref> |
|||
===Immune regulation=== |
|||
A study showed that in glutamine deprived conditions, α-ketoglutarate promotes [[naïve T cell|naïve]] CD4+ T cell differentiation into TH1 whilst inhibiting their differentiation into anti-inflammatory [[Treg cell]]s.<ref name="DOI:10.1126/scisignal.aab2610">{{cite journal |last1=Klysz |first1=Dorota |last2=Tai |first2=Xuguang |date=29 September 2015 |title=Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation |journal=Science Signaling |volume=8 |issue=396 |pages=ra97 |doi=10.1126/scisignal.aab2610 |pmid=26420908 |doi-access=free }}</ref> |
|||
=== Enzyme cofactor === |
|||
α-Ketoglutarate has been shown to be a cofactor for [[demethylase]]s that contain the Jumonji C (JmjC) domain.<ref>{{Cite journal|last1=Tsukada|first1=Yu-ichi|last2=Fang|first2=Jia|last3=Erdjument-Bromage|first3=Hediye|last4=Warren|first4=Maria E.|last5=Borchers|first5=Christoph H.|last6=Tempst|first6=Paul|last7=Zhang|first7=Yi|date=February 2006|title=Histone demethylation by a family of JmjC domain-containing proteins|url=https://www.nature.com/articles/nature04433|journal=Nature|language=en|volume=439|issue=7078|pages=811–816|doi=10.1038/nature04433|pmid=16362057 |s2cid=4415889 |issn=1476-4687}}</ref><ref>{{Cite journal|last1=Yamane|first1=Kenichi|last2=Toumazou|first2=Charalambos|last3=Tsukada|first3=Yu-ichi|last4=Erdjument-Bromage|first4=Hediye|last5=Tempst|first5=Paul|last6=Wong|first6=Jiemin|last7=Zhang|first7=Yi|date=2006-05-05|title=JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor|journal=Cell|volume=125|issue=3|pages=483–495|doi=10.1016/j.cell.2006.03.027|issn=0092-8674|pmid=16603237|doi-access=free}}</ref> |
|||
==Production== |
|||
α-Ketoglutarate can be produced by: |
|||
* [[Oxidative decarboxylation]] of [[isocitrate]] by [[isocitrate dehydrogenase]] |
|||
* [[Oxidative deamination]] of [[glutamate]] by [[glutamate dehydrogenase]] |
|||
* From [[galacturonic acid]] by the organism ''[[Agrobacterium tumefaciens]]''<ref name="RichardHilditch2009">{{cite journal|last1=Richard|first1=Peter|last2=Hilditch|first2=Satu|title=d-Galacturonic acid catabolism in microorganisms and its biotechnological relevance|journal=Applied Microbiology and Biotechnology|volume=82|issue=4|year=2009|pages=597–604|issn=0175-7598|doi=10.1007/s00253-009-1870-6|pmid=19159926|s2cid=22671282 }}</ref> |
|||
Alpha-ketoglutarate can be used to produce: |
|||
*[[Creatine-alpha ketoglutarate]] |
|||
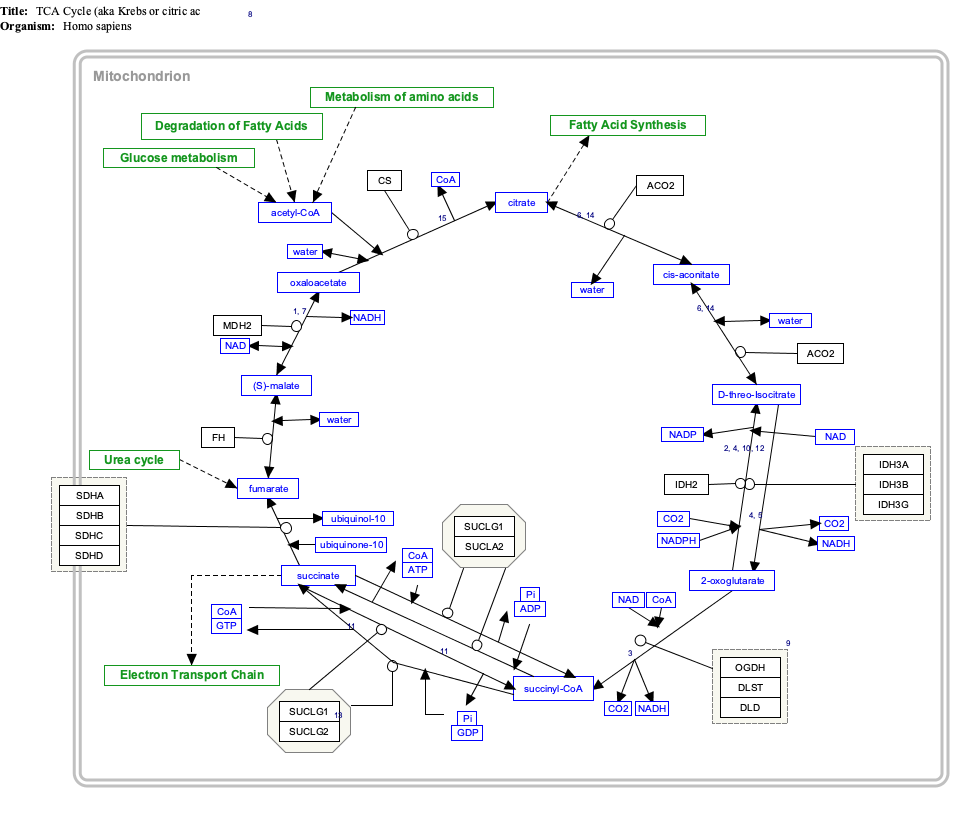
== Interactive pathway map == |
|||
{{TCACycle_WP78|highlight=Alpha-Ketoglutaric_acid}} |
|||
==See also== |
|||
* [[2-oxoglutarate (2OG)-dependent dioxygenases|2OG-dependent dioxygenases]] |
|||
==References== |
|||
{{Reflist}} |
|||
{{Citric acid cycle}} |
|||
{{Amino acid metabolism intermediates}} |
|||
{{DEFAULTSORT:Ketoglutaric Acid, Alpha-}} |
|||
[[Category:Dicarboxylic acids]] |
|||
[[Category:Alpha-keto acids]] |
|||
[[Category:Citric acid cycle compounds]] |
|||
Revision as of 16:24, 25 April 2024

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Oxopentanedioic acid | |
| Other names
2-Ketoglutaric acid
alpha-Ketoglutaric acid 2-Oxoglutaric acid Oxoglutaric acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| MeSH | alpha-ketoglutaric+acid |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H6O5 | |
| Molar mass | 146.098 g·mol−1 |
| Melting point | 115 °C (239 °F; 388 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
α-Ketoglutaric acid ( also termed 2-oxoglutaric acid) is a [dicarboxylic acid]], i.e., a short-chain fatty acid containing two carboxyl groups (carboxy group notated as CO2H with C, O, and H standing for carbon, oxygen, and hydrogen, respectively; see adjacent figure}. However, almost all animal extracellular fluids have a pH above 7. At these pH levels, α-Ketoglutaric acid exists almost exclusively as its conjugate base, i.e., as a double negatively charged dicarboxylate due to the release of H from its two carboxy groups, RCO−2 termed α-ketoglutarte (see Conjugate acid-base theory).
Its carboxylate, α-ketoglutarate (also called 2-oxoglutarate), is an important biological compound. It is produced by deamination of glutamate, and is an intermediate in the Krebs cycle.
The term "ketoglutaric acid", when not further qualified, almost always refers to the alpha variant. β-Ketoglutaric acid varies only by the position of the ketone functional group, and is much less common.
Functions
Alanine transaminase
The enzyme alanine transaminase (also termed alanine aminotransferase and glutamate pyruvate transaminase) catalyzes, i.e. converts, 1) α-ketoglutarate to L-glutamate and 2) L-alanine to pyruvate. Both of these catalyzed reactions are fully reversible, i.e., run in both the forward and reverse directions.[2]
Krebs cycle
α-Ketoglutarate is a key intermediate in the Krebs cycle, coming after isocitrate and before succinyl CoA. Anaplerotic reactions can replenish the cycle at this juncture by synthesizing α-ketoglutarate from transamination of glutamate, or through action of glutamate dehydrogenase on glutamate.[citation needed]
Formation of amino acids
Glutamine is synthesized from glutamate by glutamine synthetase, which utilizes adenosine triphosphate to form glutamyl phosphate; this intermediate is attacked by ammonia as a nucleophile giving glutamine and inorganic phosphate. Proline, arginine, and lysine (in some organisms) are other amino acids synthesized as well.[3] These three amino acids derive from glutamate with the addition of further steps or enzymes to facilitate reactions.[4]
Nitrogen transporter
Another function is to combine with nitrogen released in cells, therefore preventing nitrogen overload.[5]
α-Ketoglutarate is one of the most important nitrogen transporters in metabolic pathways. The amino groups of amino acids are attached to it (by transamination) and carried to the liver where the urea cycle takes place.[6]
α-Ketoglutarate is transaminated, along with glutamine, to form the excitatory neurotransmitter glutamate. Glutamate can then be decarboxylated (requiring vitamin B6) into the inhibitory neurotransmitter gamma-aminobutyric acid.[7]
It is reported that high ammonia and/or high nitrogen levels may occur with high protein intake, excessive aluminium exposure, Reye's syndrome, cirrhosis, and urea cycle disorder.[8]
It plays a role in detoxification of ammonia in brain.[9][10][11]
Relationship to molecular oxygen
Acting as a co-substrate for α-ketoglutarate-dependent hydroxylase, it also plays important function in oxidation reactions involving molecular oxygen.[citation needed]
Molecular oxygen (O2) directly oxidizes many compounds to produce useful products in an organism, such as antibiotics, in reactions catalyzed by oxygenases. In many oxygenases, α-ketoglutarate helps the reaction by being oxidized with the main substrate. EGLN1, one of the α-ketoglutarate-dependent oxygenases, is an O2 sensor, informing the organism of the oxygen level in its environment.[clarification needed]
In combination with molecular oxygen, alpha-ketoglutarate is one of the requirements for the hydroxylation of proline to hydroxyproline in the production of many collagens.[citation needed]
Antioxidant
α-Ketoglutarate, which is released by several cell types, decreases the levels of hydrogen peroxide, and the α-ketoglutarate was depleted and converted to succinate in cell culture media.[12]
Supplementation
Longevity
Studies have linked α-ketoglutarate with increased lifespan in nematode worms[13] and increased healthspan/lifespan in mice.[14][15][16]
Immune regulation
A study showed that in glutamine deprived conditions, α-ketoglutarate promotes naïve CD4+ T cell differentiation into TH1 whilst inhibiting their differentiation into anti-inflammatory Treg cells.[17]
Enzyme cofactor
α-Ketoglutarate has been shown to be a cofactor for demethylases that contain the Jumonji C (JmjC) domain.[18][19]
Production
α-Ketoglutarate can be produced by:
- Oxidative decarboxylation of isocitrate by isocitrate dehydrogenase
- Oxidative deamination of glutamate by glutamate dehydrogenase
- From galacturonic acid by the organism Agrobacterium tumefaciens[20]
Alpha-ketoglutarate can be used to produce:
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78".
See also
References
- ^ Merck Index, 13th Edition, 5320.
- ^ Yang RZ, Park S, Reagan WJ, Goldstein R, Zhong S, Lawton M, Rajamohan F, Qian K, Liu L, Gong DW (February 2009). "Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity". Hepatology (Baltimore, Md.). 49 (2): 598–607. doi:10.1002/hep.22657. PMC 2917112. PMID 19085960.
- ^ Ledwidge, Richard; Blanchard, John S. (1999). "The Dual Biosynthetic Capability of N-Acetylornithine Aminotransferase in Arginine and Lysine Biosynthesis†". Biochemistry. 38 (10): 3019–3024. doi:10.1021/bi982574a. PMID 10074354.
- ^ Schousboe, Arne; Scafidi, Susanna; Bak, Lasse K.; Waagepetersen, Helle S.; McKenna, Mary C. (2014). "Glutamate Metabolism in the Brain Focusing on Astrocytes". Glutamate and ATP at the Interface of Metabolism and Signaling in the Brain. Advances in Neurobiology. Vol. 11. pp. 13–30. doi:10.1007/978-3-319-08894-5_2. ISBN 978-3-319-08893-8. ISSN 2190-5215. PMC 4667713. PMID 25236722.
- ^ Katayama, Kazuhiro (2004-12-01). "Ammonia metabolism and hepatic encephalopathy". Hepatology Research. 30: 73–80. doi:10.1016/j.hepres.2004.08.013. ISSN 1386-6346. PMID 15607143.
- ^ Li, Tao; Kootstra, Anna B.; Fotheringham, Ian G. (2002-07-01). "Nonproteinogenic α-Amino Acid Preparation Using Equilibrium Shifted Transamination". Organic Process Research & Development. 6 (4): 533–538. doi:10.1021/op025518x. ISSN 1083-6160.
- ^ Kaneko, T.; Mizuno, N. (August 1994). "Glutamate-synthesizing enzymes in GABAergic neurons of the neocortex: a double immunofluorescence study in the rat". Neuroscience. 61 (4): 839–849. doi:10.1016/0306-4522(94)90407-3. ISSN 0306-4522. PMID 7838383. S2CID 19178915.
- ^ "Hyperammonemia: What It Is, Causes, Symptoms & Treatment". Cleveland Clinic. Retrieved 2023-11-03.
- ^ "Does infectious fever relieve autistic behavior by releasing glutamine from skeletal muscles as provisional fuel?". Archived from the original on 2014-05-19. Retrieved 2014-05-19.
- ^ Ott, P; Clemmesen, O; Larsen, FS (Jul 2005). "Cerebral metabolic disturbances in the brain during acute liver failure: from hyperammonemia to energy failure and proteolysis". Neurochemistry International. 47 (1–2): 13–8. doi:10.1016/j.neuint.2005.04.002. PMID 15921824. S2CID 916739.
- ^ Hares, P; James, IM; Pearson, RM (May–Jun 1978). "Effect of ornithine alpha ketoglutarate (OAKG) on the response of brain metabolism to hypoxia in the dog". Stroke: A Journal of Cerebral Circulation. 9 (3): 222–4. doi:10.1161/01.STR.9.3.222. PMID 644619.
- ^ Long, L; Halliwell, B (2011). "Artefacts in cell culture: α-Ketoglutarate can scavenge hydrogen peroxide generated by ascorbate and epigallocatechin gallate in cell culture media". Biochemical and Biophysical Research Communications. 406 (1): 20–24. doi:10.1016/j.bbrc.2011.01.091. PMID 21281600.
- ^ Chin, RM; Fu, X; Pai, MY; Vergnes, L; Hwang, H; Deng, G; Diep, S; Lomenick, B; Meli, VS; Monsalve, GC; Hu, E; Whelan, SA; Wang, JX; Jung, G; Solis, GM; Fazlollahi, F; Kaweeteerawat, C; Quach, A; Nili, M; Krall, AS; Godwin, HA; Chang, HR; Faull, KF; Guo, F; Jiang, M; Trauger, SA; Saghatelian, A; Braas, D; Christofk, HR; Clarke, CF; Teitell, MA; Petrascheck, M; Reue, K; Jung, ME; Frand, AR; Huang, J (2014). "The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR". Nature. 510 (7505): 397–401. Bibcode:2014Natur.510..397C. doi:10.1038/nature13264. PMC 4263271. PMID 24828042.
- ^ Kaiser 1, Jocelyn (2020-09-01). "Bodybuilding supplement promotes healthy aging and extends life span, at least in mice". Science | AAAS. Retrieved 2020-09-01.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ "A metabolite produced by the body increases lifespan and dramatically compresses late-life morbidity in mice". BUCK. Retrieved 2020-09-01.
- ^ Shahmirzadi, Azar Asadi; Edgar, Daniel; Liao, Chen-Yu (2020-09-01). "Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice". Cell Metabolism. Retrieved 2020-09-20.
- ^ Klysz, Dorota; Tai, Xuguang (29 September 2015). "Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation". Science Signaling. 8 (396): ra97. doi:10.1126/scisignal.aab2610. PMID 26420908.
- ^ Tsukada, Yu-ichi; Fang, Jia; Erdjument-Bromage, Hediye; Warren, Maria E.; Borchers, Christoph H.; Tempst, Paul; Zhang, Yi (February 2006). "Histone demethylation by a family of JmjC domain-containing proteins". Nature. 439 (7078): 811–816. doi:10.1038/nature04433. ISSN 1476-4687. PMID 16362057. S2CID 4415889.
- ^ Yamane, Kenichi; Toumazou, Charalambos; Tsukada, Yu-ichi; Erdjument-Bromage, Hediye; Tempst, Paul; Wong, Jiemin; Zhang, Yi (2006-05-05). "JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor". Cell. 125 (3): 483–495. doi:10.1016/j.cell.2006.03.027. ISSN 0092-8674. PMID 16603237.
- ^ Richard, Peter; Hilditch, Satu (2009). "d-Galacturonic acid catabolism in microorganisms and its biotechnological relevance". Applied Microbiology and Biotechnology. 82 (4): 597–604. doi:10.1007/s00253-009-1870-6. ISSN 0175-7598. PMID 19159926. S2CID 22671282.