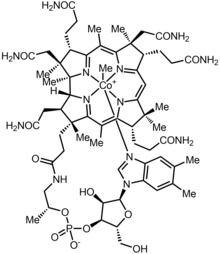
Methylcobalamin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cobolmin |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth, sublingual, injection. |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.200 |
| Chemical and physical data | |
| Formula | C63H91CoN13O14P |
| Molar mass | 1344.405 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Methylcobalamin (mecobalamin, MeCbl, or MeB12) is a cobalamin, a form of vitamin B12. It differs from cyanocobalamin in that the cyano group at the cobalt is replaced with a methyl group.[1] Methylcobalamin features an octahedral cobalt(III) centre and can be obtained as bright red crystals.[2] From the perspective of coordination chemistry, methylcobalamin is notable as a rare example of a compound that contains metal–alkyl bonds. Nickel–methyl intermediates have been proposed for the final step of methanogenesis.
Methylcobalamin is equivalent physiologically to vitamin B12,[3] and can be used to prevent or treat pathology arising from a lack of vitamin B12 intake (vitamin B12 deficiency).
Methylcobalamin is also used in the treatment of peripheral neuropathy, diabetic neuropathy, and as a preliminary treatment for amyotrophic lateral sclerosis.[4]
Methylcobalamin that is ingested is not used directly as a cofactor, but is first converted by MMACHC into cob(II)alamin. Cob(II)alamin is then later converted into the other two forms, adenosylcobalamin and methylcobalamin for use as cofactors. That is, methylcobalamin is first dealkylated and then regenerated.[5][6][7]
According to one author, it is important to treat vitamin B12 deficiency with hydroxocobalamin or cyanocobalamin or a combination of adenosylcobalamin and methylcobalamin, and not methylcobalamin alone.[8]
Production

Methylcobalamin can be produced in the laboratory by reducing cyanocobalamin with sodium borohydride in alkaline solution, followed by the addition of methyl iodide.[2]
Functions
This vitamer, along with adenosylcobalamin, is one of two active coenzymes used by vitamin B12-dependent enzymes and is the specific vitamin B12 form used by 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR), also known as methionine synthase.[citation needed]
Methylcobalamin participates in the Wood-Ljungdahl pathway, which is a pathway by which some organisms utilize carbon dioxide as their source of organic compounds. In this pathway, methylcobalamin provides the methyl group that couples to carbon monoxide (derived from CO2) to afford acetyl-CoA. Acetyl-CoA is a derivative of acetic acid that is converted to more complex molecules as required by the organism.[9]
Methylcobalamin is produced by some bacteria.[citation needed] It plays an important role in the environment. In the environment, it is responsible for the biomethylation of certain heavy metals. For example, the highly toxic methylmercury is produced by the action of methylcobalamin.[10] In this role, methylcobalamin serves as a source of "CH3+".
A lack of cobalamin can lead to megaloblastic anemia and subacute combined degeneration of the spinal cord.[11]
See also
References
- ^ McDowell LR (2000-10-11). Vitamins in animal and human nutrition. ISBN 9780813826301. Retrieved 28 January 2018.
{{cite book}}:|website=ignored (help) - ^ a b David, Dophin (1971). D.B. McCormick and L.D. Wright (ed.). "Preparation of the Reduced Forms of Vitamin B12 and of Some Analogs of the Vitamin B12 Coenzyme Containing a Cobalt-Carbon Bond". XVIII: 34-54. doi:10.1016/S0076-6879(71)18006-8.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Sil A, Kumar H, Mondal RD, Anand SS, Ghosal A, Datta A, Sawant SV, Kapatkar V, Kadhe G, Rao S (July 2018). "A randomized, open labeled study comparing the serum levels of cobalamin after three doses of 500 mcg vs. a single dose methylcobalamin of 1500 mcg in patients with peripheral neuropathy". The Korean Journal of Pain. 31 (3): 183–190. doi:10.3344/kjp.2018.31.3.183. PMC 6037815. PMID 30013732.
- ^ "Eisai Submits New Drug Application for Mecobalamin Ultra-High Dose Preparation as Treatment for Amyotrophic Lateral Sclerosis in Japan" (PDF). Eisai.com. Retrieved 28 January 2018.
- ^ Kim J, Hannibal L, Gherasim C, Jacobsen DW, Banerjee R (November 2009). "A human vitamin B12 trafficking protein uses glutathione transferase activity for processing alkylcobalamins". The Journal of Biological Chemistry. 284 (48): 33418–24. doi:10.1074/jbc.M109.057877. PMC 2785186. PMID 19801555.
- ^ Hannibal L, Kim J, Brasch NE, Wang S, Rosenblatt DS, Banerjee R, Jacobsen DW (August 2009). "Processing of alkylcobalamins in mammalian cells: A role for the MMACHC (cblC) gene product". Molecular Genetics and Metabolism. 97 (4): 260–6. doi:10.1016/j.ymgme.2009.04.005. PMC 2709701. PMID 19447654.
- ^ Froese DS, Gravel RA (November 2010). "Genetic disorders of vitamin B₁₂ metabolism: eight complementation groups--eight genes". Expert Reviews in Molecular Medicine. 12: e37. doi:10.1017/S1462399410001651. PMC 2995210. PMID 21114891.
- ^ Thakkar K, Billa G (January 2015). "Treatment of vitamin B12 deficiency-methylcobalamine? Cyancobalamine? Hydroxocobalamin?-clearing the confusion". European Journal of Clinical Nutrition. 69 (1): 1–2. doi:10.1038/ejcn.2014.165. PMID 25117994.
- ^ Fontecilla-Camps JC, Amara P, Cavazza C, Nicolet Y, Volbeda A (August 2009). "Structure-function relationships of anaerobic gas-processing metalloenzymes". Nature. 460 (7257): 814–22. Bibcode:2009Natur.460..814F. doi:10.1038/nature08299. PMID 19675641. S2CID 4421420.
- ^ Schneider Z, Stroiński A (1987), Comprehensive B12: Chemistry, Biochemistry, Nutrition, Ecology, Medicine, ISBN 9783110082395
- ^ Bémeur C, et al. (2011). Blass JP (ed.). Neurochemical Mechanisms in Disease. Springer. pp. 112–3.
