Adams decarboxylation
Tools
Actions
General
Print/export
In other projects
Appearance
From Wikipedia, the free encyclopedia
This is an old revision of this page, as edited by Y-S.Ko (talk | contribs) at 00:37, 9 July 2022. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
The Adams decarboxylation is a chemical reaction that involved the decarboxylation of coumarins which have carboxylic acid group in the third position. The decarboxylation is achieved by aqueous solution of sodium bisulfite, heat and a concentrated solution of sodium hydroxide.[1][2][3]
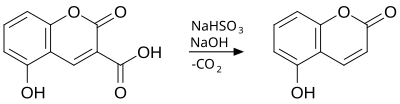
References
- ^ Adams, R.; Mathieu, J. (1948). "A New Synthesis of Atranol (2,6-Dihydroxy-4-methylbenzaldehyde) and the Corresponding Cinnamic Acid". J. Am. Chem. Soc. 70 (6): 2120. doi:10.1021/ja01186a037.
- ^ Adams, R.; Bockstaheler, T.E. (1952). "Preparation and Reactions of o-Hydroxycinnamic Acids and Esters". J. Am. Chem. Soc. 74 (21): 5346. doi:10.1021/ja01141a038.
- ^ Cramer, F.; Windel, H. (1956). "Über Einschlußverbindungen, X. Mitteil.: Die blauen Jodverbindungen der Cumarine und anderer verwandter Verbindungen". Chem. Ber. 89 (2): 354. doi:10.1002/cber.19560890227.
