Beryllium nitrate
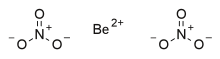
| |
| Names | |
|---|---|
| Systematic IUPAC name
Beryllium nitrate | |
| Other names
Beryllium dinitrate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.678 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| UN number | 2464 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Be(NO3)2 | |
| Molar mass | 133.021982 g/mol |
| Appearance | white to yellow solid |
| Odor | odorless |
| Density | 1.56 g/cm3 |
| Melting point | 60.5 °C (140.9 °F; 333.6 K) |
| Boiling point | 142 °C (288 °F; 415 K) (decomposes) |
| 166 g/100 mL | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-700.4 kJ/mol |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[1] |
REL (Recommended)
|
Ca C 0.0005 mg/m3 (as Be)[1] |
IDLH (Immediate danger)
|
Ca [4 mg/m3 (as Be)][1] |
| Related compounds | |
Other cations
|
Magnesium nitrate Calcium nitrate Strontium nitrate Barium nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Beryllium nitrate, also known as beryllium dinitrate, is an ionic beryllium salt of nitric acid with the chemical formula Be(NO3)2.[2] Each formula unit is composed of one Be2+ cation and two NO3− anions.
Hazards
Beryllium nitrate is a toxic chemical,[2] like all other beryllium compounds. It is also an irritant in small doses. When burned, it gives off irritating or toxic fumes. However, when massive short-term exposure occurs, acute pneumonitis can set in, but symptoms do not manifest themselves for 3 days.[2]
Preparation
Beryllium nitrate can be prepared by reacting beryllium hydroxide in nitric acid.[3]
- Be(OH)2 + 2 HNO3 → Be(NO3)2 + 2 H2O
References
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c "Beryllium Nitrate (ICSC)". IPCS INCHEM. Retrieved 13 September 2010.
- ^ Walsh, Kenneth (2009). Beryllium chemistry and processing. ASM International. pp. 121–122. ISBN 978-0-87170-721-5. Retrieved 3 January 2011.
