Beta-ketoacyl-ACP synthase
| 3-oxoacyl-ACP synthase, mitochondrial | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | OXSM | ||||||
| NCBI gene | 54995 | ||||||
| HGNC | 26063 | ||||||
| OMIM | 610324 | ||||||
| RefSeq | NM_017897 | ||||||
| UniProt | Q9NWU1 | ||||||
| Other data | |||||||
| EC number | 2.3.1.41 | ||||||
| Locus | Chr. 3 p24.2 | ||||||
| |||||||
| Beta-ketoacyl synthase, N-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
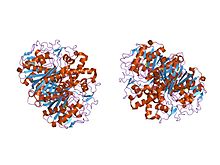 the crystal structure of beta-ketoacyl-[acyl carrier protein] synthase ii from streptococcus pneumoniae, triclinic form | |||||||||
| Identifiers | |||||||||
| Symbol | ketoacyl-synt | ||||||||
| Pfam | PF00109 | ||||||||
| Pfam clan | CL0046 | ||||||||
| InterPro | IPR014030 | ||||||||
| PROSITE | PDOC00529 | ||||||||
| SCOP2 | 1kas / SCOPe / SUPFAM | ||||||||
| |||||||||
| Beta-ketoacyl synthase, C-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 arabidopsis thaliana mitochondrial beta-ketoacyl acp synthase hexanoic acid complex | |||||||||
| Identifiers | |||||||||
| Symbol | Ketoacyl-synt_C | ||||||||
| Pfam | PF02801 | ||||||||
| Pfam clan | CL0046 | ||||||||
| InterPro | IPR014031 | ||||||||
| PROSITE | PDOC00529 | ||||||||
| SCOP2 | 1kas / SCOPe / SUPFAM | ||||||||
| |||||||||
In molecular biology, Beta-ketoacyl-ACP synthase EC 2.3.1.41, is an enzyme involved in fatty acid synthesis. It typically uses malonyl-CoA as a carbon source to elongate ACP-bound acyl species, resulting in the formation of ACP-bound β-ketoacyl species such as acetoacetyl-ACP.[1]
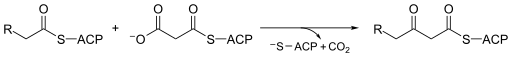
Beta-ketoacyl-ACP synthase is a highly conserved enzyme that is found in almost all life on earth as a domain in fatty acid synthase (FAS). FAS exists in two types, aptly named type I and II. In animals, fungi, and lower eukaryotes, Beta-ketoacyl-ACP synthases make up one of the catalytic domains of larger multifunctional proteins (Type I), whereas in most prokaryotes as well as in plastids and mitochondria, Beta-ketoacyl-ACP synthases are separate protein chains that usually form dimers (Type II).[1][2] Beta-ketoacyl-ACP synthase III, perhaps the most well known of this family of enzymes, catalyzes a Claisen condensation between acetyl CoA and malonyl ACP. The image below reveals how CoA fits in the active site as a substrate of synthase III.

Beta-ketoacyl-ACP synthases I and II only catalyze acyl-ACP reactions with malonyl ACP. Synthases I and II are capable of producing long-chain acyl-ACPs. Both are efficient up to acyl-ACPs with a 14 carbon chain, at which point synthase II is the more efficient choice for further carbon additions. Type I FAS catalyzes all the reactions necessary to create palmitic acid, which is a necessary function in animals for metabolic processes, one of which includes the formation of sphingosines.[1]
Beta-ketoacyl-ACP synthase is found as a component of a number of enzymatic systems, including fatty acid synthetase (FAS); the multi-functional 6-methysalicylic acid synthase (MSAS) from Penicillium patulum,[3] which is involved in the biosynthesis of a polyketide antibiotic; polyketide antibiotic synthase enzyme systems; Emericella nidulans multifunctional protein Wa, which is involved in the biosynthesis of conidial green pigment; Rhizobium nodulation protein nodE, which probably acts as a beta-ketoacyl synthase in the synthesis of the nodulation Nod factor fatty acyl chain; and yeast mitochondrial protein CEM1.
Structure

Beta-ketoacyl synthase contains two protein domains. The active site is located between the N- and C-terminal domains. The N-terminal domain contains most of the structures involved in dimer formation and also the active site cysteine. Residues from both domains contribute to substrate binding and catalysis[4]
In animals and in prokaryotes, beta-ketoacyl-ACP synthase is a domain on type I FAS, which is a large enzyme complex that has multiple domains to catalyze multiple different reactions. Analogously, beta-ketoacyl-ACP synthase in plants is found in type II FAS; note that synthases in plants have been documented to have a range of substrate specificities.[1] The presence of similar ketoacyl synthases present in all living organisms point to a common ancestor.[5] Further examination of beta-ketoacyl-ACP synthases I and II of E. coli revealed that both are homodimeric, but synthase II is slightly larger. However, even though they are both involved in fatty acid metabolism, they also have highly divergent primary structure.[6] In synthase II, each subunit consists of a five-stranded beta pleated sheet surrounded by multiple alpha helices, shown in the image on the left. The active sites are relatively close, only about 25 angstroms apart, and consist of a mostly hydrophobic pocket.[4] Certain experiments have also suggested the presence of “fatty acid transport tunnels” within the beta-ketoacyl-ACP synthase domain that lead to one of many “fatty acid cavities”, which essentially acts as the active site.[7]
Mechanism
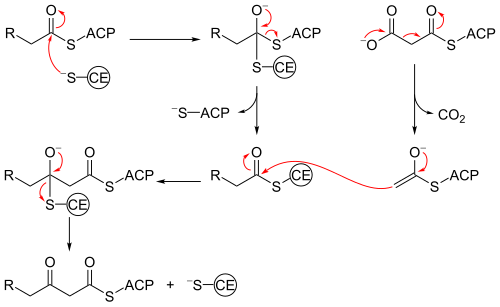
Beta-ketoacyl-synthase’s mechanism is a topic of debate among chemists. Many agree that Cys171 of the active site attacks acetyl ACP's carbonyl, and, like most enzymes, stabilizes the intermediate with other residues in the active site. ACP is subsequently eliminated, and it deprotonates His311 in the process. A thioester is then regenerated with the cysteine in the active site. Decarboxylation of a malonyl CoA that is also in the active site initially creates an enolate, which is stabilized by His311 and His345. The enolate tautomerizes to a carbanion that attacks the thioester of the acetyl-enzyme complex.[8] Some sources speculate that an activated water molecule also resides in the active site as a means of hydrating the released CO2 or of attacking C3 of malonyl CoA. Another proposed mechanism considers the creation of a tetrahedral transition state.[1] The driving force of the reaction comes from the decarboxylation of malonyl ACP; the energy captured in that bond technically comes from ATP, which is what is initially used to carboxylate acetyl CoA to malonyl CoA.[9]
Biological function
The main function of beta-ketoacyl-ACP synthase is to produce fatty acids of various lengths for use by the organism. These uses include energy storage and creation of cell membranes. Fatty acids can also be used to synthesize prostaglandins, phospholipids, and vitamins, among many other things. Further, palmitic acid, which is created by the beta-ketoacyl-synthases on type I FAS, is used in a number of biological capacities. It is a precursor of both stearic and palmitoleic acids. Palmitoleic can subsequently be used to create a number of other fatty acids.[10] Palmitic acid is also used to synthesize sphingosines, which play a role in cell membranes.[1]
Clinical significance
The different types of beta-ketoacyl-ACP synthases in type II FAS are called FabB, FabF, and FabH synthases. FabH catalyzes the quintessential ketoacyl synthase reaction with malonyl ACP and acetyl CoA. FabB and FabF catalyze other related reactions. Given that their function is necessary for proper biological function surrounding lipoprotein, phospholipid, and lipopolysaccharide synthesis, they have become a target in antibacterial drug development. In order to adapt to their environment, bacteria alter the phospholipid composition of their membranes. Inhibiting this pathway may thus be a leverage point in disrupting bacterial proliferation.[11] By studying Yersinia pestis, which causes bubonic, pneumonic, and septicaemic plagues, researchers have shown that FabB, FabF, and FabH can theoretically all be inhibited by the same drug due to similarities in their binding sites. However, such a drug has not yet been developed.[12] Cerulenin, a molecule that appears to inhibit by mimicking the “condensation transition state” can only inhibit B or F, but not H. Another molecule, thiolactomycin, which mimics malonyl ACP in the active site, can ony inhibit FabB.[13] Lastly, platensimycin also has possible antibiotic use due to its inhibition of FabF.[14]
These types of drugs are highly relevant. For example, Y. pestis was the main agent in the Justinian Plague, Black Death, and the modern plague. Even within the last five years, China, Peru, and Madagascar all experienced an outbreak of infection by Y. pestis. If it is not treated within 24 hours, it normally results in death. Furthermore, there is worry that it can now be used as a possible biological warfare weapon.[12]
Unfortunately, many drugs that target prokaryotic beta-ketoacyl-synthases carry many side effects. Given the similarities between prokaryotic ketoacyl synthases and mitochondrial ones, these types of drugs tend to unintentionally also act upon mitochondrial synthases, leading to many biological consequences for humans.[2]
Industrial applications
Recent efforts in bioengineering include engineering of FAS proteins, which includes beta-ketoacyl-ACP synthase domains, in order to favor the synthesis of branched carbon chains as a renewable energy source. Branched carbon chains contain more energy and can be used in colder temperatures because of their lower freezing point. Using E. coli as the organism of choice, engineers have replaced the endogenous FabH domain on FAS, which favors unbranched chains, with FabH versions that favor branching due to their high substrate specificity for branched acyl-ACPs.[15]
See also
- Beta-ketoacyl-ACP synthase I
- Beta-ketoacyl-ACP synthase II
- Beta-ketoacyl-ACP synthase III
- 3-oxoacyl-(acyl-carrier-protein) reductase
References
- ^ a b c d e f Witkowski, Andrzej; Joshi, Anil K.; Smith, Stuart (2002). "Mechanism of the β-Ketoacyl Synthase Reaction Catalyzed by the Animal Fatty Acid Synthase †". Biochemistry. 41 (35): 10877–10887. doi:10.1021/bi0259047. PMID 12196027.
- ^ a b Christensen, Caspar Elo; Kragelund, Birthe B.; von Wettstein-Knowles, Penny; Henriksen, Anette (2007-02-01). "Structure of the human β-ketoacyl [ACP] synthase from the mitochondrial type II fatty acid synthase". Protein Science. 16 (2): 261–272. doi:10.1110/ps.062473707. ISSN 0961-8368. PMC 2203288. PMID 17242430.
- ^ Beck J, Ripka S, Siegner A, Schiltz E, Schweizer E (Sep 1990). "The multifunctional 6-methylsalicylic acid synthase gene of Penicillium patulum. Its gene structure relative to that of other polyketide synthases". European Journal of Biochemistry / FEBS. 192 (2): 487–98. doi:10.1111/j.1432-1033.1990.tb19252.x. PMID 2209605.
- ^ a b Huang W, Jia J, Edwards P, Dehesh K, Schneider G, Lindqvist Y (Mar 1998). "Crystal structure of beta-ketoacyl-acyl carrier protein synthase II from E.coli reveals the molecular architecture of condensing enzymes". The EMBO Journal. 17 (5): 1183–91. doi:10.1093/emboj/17.5.1183. PMC 1170466. PMID 9482715.
- ^ Beld, Joris; Blatti, Jillian L.; Behnke, Craig; Mendez, Michael; Burkart, Michael D. (2014-08-01). "Evolution of acyl-ACP-thioesterases and β-ketoacyl-ACP-synthases revealed by protein-protein interactions". Journal of Applied Phycology. 26 (4): 1619–1629. doi:10.1007/s10811-013-0203-4. ISSN 0921-8971. PMC 4125210. PMID 25110394.
- ^ Garwin, J. L.; Klages, A. L.; Cronan, J. E. (1980-12-25). "Structural, enzymatic, and genetic studies of beta-ketoacyl-acyl carrier protein synthases I and II of Escherichia coli". Journal of Biological Chemistry. 255 (24): 11949–11956. ISSN 0021-9258. PMID 7002930.
- ^ Cui, Wei; Liang, Yan; Tian, Weixi; Ji, Mingjuan; Ma, Xiaofeng (2016-03-01). "Regulating effect of β-ketoacyl synthase domain of fatty acid synthase on fatty acyl chain length in de novo fatty acid synthesis". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1861 (3): 149–155. doi:10.1016/j.bbalip.2015.12.002. PMID 26680361.
- ^ Lee, Wook; Engels, Bernd (2014). "The Protonation State of Catalytic Residues in the Resting State of KasA Revisited: Detailed Mechanism for the Activation of KasA by Its Own Substrate". Biochemistry. 53 (5): 919–931. doi:10.1021/bi401308j. PMID 24479625.
- ^ Tymoczko, John; Berg; Stryer (2013). Biochemistry A Short Course. United States of America: W.H. Freeman and Company. ISBN 978-1-4292-8360-1.
- ^ "Palmitic acid, a saturated fatty acid, in Cell Culture". Sigma-Aldrich. Retrieved 2016-02-29.
- ^ Zhang, Yong-Mei; Rock, Charles O. (2008-03-01). "Membrane lipid homeostasis in bacteria". Nature Reviews Microbiology. 6 (3): 222–233. doi:10.1038/nrmicro1839. ISSN 1740-1526. PMID 18264115. S2CID 7888484.
- ^ a b Nanson, Jeffrey D.; Himiari, Zainab; Swarbrick, Crystall M. D.; Forwood, Jade K. (2015-10-15). "Structural Characterisation of the Beta-Ketoacyl-Acyl Carrier Protein Synthases, FabF and FabH, of Yersinia pestis". Scientific Reports. 5: 14797. Bibcode:2015NatSR...514797N. doi:10.1038/srep14797. PMC 4606726. PMID 26469877.
- ^ Price, Allen C.; Choi, Keum-Hwa; Heath, Richard J.; Li, Zhenmei; White, Stephen W.; Rock, Charles O. (2001-03-02). "Inhibition of β-Ketoacyl-Acyl Carrier Protein Synthases by Thiolactomycin and Cerulenin STRUCTURE AND MECHANISM". Journal of Biological Chemistry. 276 (9): 6551–6559. doi:10.1074/jbc.M007101200. ISSN 0021-9258. PMID 11050088.
- ^ Wright, H Tonie; Reynolds, Kevin A (2007-10-01). "Antibacterial targets in fatty acid biosynthesis". Current Opinion in Microbiology. Antimicrobials/Genomics. 10 (5): 447–453. doi:10.1016/j.mib.2007.07.001. PMC 2271077. PMID 17707686.
- ^ Jiang, Wen; Jiang, Yanfang; Bentley, Gayle J.; Liu, Di; Xiao, Yi; Zhang, Fuzhong (2015-08-01). "Enhanced production of branched-chain fatty acids by replacing β-ketoacyl-(acyl-carrier-protein) synthase III (FabH)". Biotechnology and Bioengineering. 112 (8): 1613–1622. doi:10.1002/bit.25583. ISSN 1097-0290. PMID 25788017. S2CID 35469786.
External links
- beta+Ketoacyl+ACP+Synthase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
Further reading
- Jiang W, Jiang Y, Bentley GJ, Liu D, Xiao Y, Zhang F (Aug 2015). "Enhanced production of branched-chain fatty acids by replacing β-ketoacyl-(acyl-carrier-protein) synthase III (FabH)". Biotechnology and Bioengineering. 112 (8): 1613–22. doi:10.1002/bit.25583. PMID 25788017. S2CID 35469786.
- Witkowski A, Joshi AK, Smith S (Sep 2002). "Mechanism of the beta-ketoacyl synthase reaction catalyzed by the animal fatty acid synthase". Biochemistry. 41 (35): 10877–87. doi:10.1021/bi0259047. PMID 12196027.
- Christensen CE, Kragelund BB, von Wettstein-Knowles P, Henriksen A (Feb 2007). "Structure of the human beta-ketoacyl [ACP] synthase from the mitochondrial type II fatty acid synthase". Protein Science. 16 (2): 261–72. doi:10.1110/ps.062473707. PMC 2203288. PMID 17242430.
- Lee W, Engels B (Feb 2014). "The protonation state of catalytic residues in the resting state of KasA revisited: detailed mechanism for the activation of KasA by its own substrate". Biochemistry. 53 (5): 919–31. doi:10.1021/bi401308j. PMID 24479625.
