Aciclovir: Difference between revisions
→See also: already linked in the article |
|||
| Line 118: | Line 118: | ||
* Herpes simplex [[blepharitis]] (not to be mistaken with ocular herpes) |
* Herpes simplex [[blepharitis]] (not to be mistaken with ocular herpes) |
||
* Prophylaxis against herpesviruses in immunocompromised patients (such as patients undergoing cancer chemotherapy)<ref name="Cancer">{{cite journal |author=Elad S, Zadik Y, Hewson I, ''et al.'' |title=A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea |journal=Support Care Cancer |volume=18 |issue=8 |pages=993–1006 |year=2010 |month=August |pmid=20544224 |doi=10.1007/s00520-010-0900-3}}</ref> |
* Prophylaxis against herpesviruses in immunocompromised patients (such as patients undergoing cancer chemotherapy)<ref name="Cancer">{{cite journal |author=Elad S, Zadik Y, Hewson I, ''et al.'' |title=A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea |journal=Support Care Cancer |volume=18 |issue=8 |pages=993–1006 |year=2010 |month=August |pmid=20544224 |doi=10.1007/s00520-010-0900-3}}</ref> |
||
* Gardnerella vaginalis |
|||
HIV-1 progression can be delayed by using aciclovir, according to study led by Dr Jairam Lingappa. Effective in 16% of cases, it can delay the [[HAART]] treatment by 1–2 years. University of Washington, Seattle. |
HIV-1 progression can be delayed by using aciclovir, according to study led by Dr Jairam Lingappa. Effective in 16% of cases, it can delay the [[HAART]] treatment by 1–2 years. University of Washington, Seattle. |
||
Revision as of 14:09, 30 January 2013
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zovirax |
| Other names | acycloguanosine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681045 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous, oral, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 10–20% (oral) |
| Protein binding | 9–33% |
| Metabolism | Viral thymidine kinase |
| Elimination half-life | 2.2–20 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.056.059 |
| Chemical and physical data | |
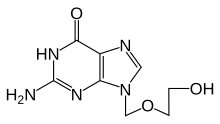
| Formula | C8H11N5O3 |
| Molar mass | 225.21 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 256.5 °C (493.7 °F) |
| |
| |
| (verify) | |

Aciclovir (INN) (/[invalid input: 'icon']eɪˈsaɪkl[invalid input: 'ɵ']vɪər/) or acyclovir (USAN, former BAN), chemical name acycloguanosine, abbreviated as ACV,[1] is a guanosine analogue antiviral drug, marketed under trade names such as Cyclovir, Herpex, Acivir, Acivirax, Zovirax, Zoral, and Xovir. One of the most commonly used antiviral drugs, it is primarily used for the treatment of herpes simplex virus infections, as well as in the treatment of varicella zoster (chickenpox) and herpes zoster (shingles).
History
Aciclovir was seen as the start of a new era in antiviral therapy,[1] as it is extremely selective and low in cytotoxicity. Nucleosides isolated from a Caribbean sponge, Cryptotethya crypta, were the basis for the synthesis of aciclovir.[2][3][4] It was codiscovered by Howard Schaffer following his work with Robert Vince, S. Bittner and S. Gurwara on the adenosine analog acycloadenosine which showed promising antiviral activity.[5] Later, Schaffer joined Burroghs-Wellcome and continued the development of aciclovir with Pharmacologist Gertrude B. Elion.[6] Vince later went on to invent abacavir, the NNRTI drug for HIV patients.[7] Elion was awarded the 1988 Nobel Prize in Medicine, partly for the development of aciclovir. Dr. Richard Whitley, a University of Alabama at Birmingham researcher and pioneer in antiviral therapy, was the first to successfully use the drug in humans.
Pharmacology
Mechanism of action
Aciclovir differs from previous nucleoside analogues in containing only a partial nucleoside structure: the sugar ring is replaced with an open-chain structure. It is selectively converted into acyclo-guanosine monophosphate (acyclo-GMP) by viral thymidine kinase, which is far more effective (3000 times) in phosphorylation than cellular thymidine kinase. Subsequently, the monophosphate form is further phosphorylated into the active triphosphate form, acyclo-guanosine triphosphate (acyclo-GTP), by cellular kinases. Acyclo-GTP has approximately 100 times greater affinity for viral than cellular polymerase. As a substrate, acyclo-GTP is incorporated into viral DNA, resulting in premature chain termination. Although aciclovir resembles a nucleotide, it has no 3' end. Therefore, after its incorporation into a growing DNA strand, no further nucleotides can be added to this strand. It has also been shown that viral enzymes cannot remove acyclo-GTP from the chain, which results in inhibition of further activity of DNA polymerase. Acyclo-GTP is fairly rapidly metabolised within the cell, possibly by cellular phosphatases.
In sum, aciclovir can be considered a prodrug: it is administered in an inactive (or less active) form and is metabolised into a more active species after administration.
Microbiology
Aciclovir is active against most species in the herpesvirus family. In descending order of activity:[8]
- Herpes simplex virus type I (HSV-1)
- Herpes simplex virus type II (HSV-2)
- Varicella zoster virus (VZV)
- Epstein-Barr virus (EBV)
- Cytomegalovirus (CMV) – least activity
Activity is predominantly against HSV, and to a lesser extent VZV. It is only of limited efficacy against EBV and CMV. It is inactive against latent viruses in nerve ganglia.
Resistance
Resistance to aciclovir is rare, but is more common in patients on chronic antiviral prophylaxis (transplant recipients, people with acquired immunodeficiency syndrome due to HIV infection). Mechanisms of resistance in HSV include deficient viral thymidine kinase; and mutations to viral thymidine kinase and/or DNA polymerase, altering substrate sensitivity.[9] Aciclovir has also shown cross-resistance with valaciclovir and famciclovir.
Pharmacokinetics
Aciclovir is poorly water soluble and has poor oral bioavailability (15–30%), hence intravenous administration is necessary if high concentrations are required. When orally administered, peak plasma concentration occurs after 1–2 hours. Aciclovir has a high distribution rate; protein binding is reported to range from 9 to 33%.[10] The elimination half-life of aciclovir is approximately 3 hours. It is renally excreted, partly by glomerular filtration and partly by tubular secretion.
The poor oral bioavailability may also be improved by administering valaciclovir, which has an oral bioavailability of about 55%. Valaciclovir is then converted to aciclovir by esterases via hepatic first-pass metabolism.
Clinical use


Indications
Aciclovir is indicated for the treatment of HSV and VZV infections, including:[11]
- Genital herpes simplex (treatment and prophylaxis)
- Herpes simplex labialis (cold sores)
- Herpes zoster (shingles)
- Acute chickenpox in immunocompromised patients
- Herpes simplex encephalitis
- Acute mucocutaneous HSV infections in immunocompromised patients
- Herpes simplex keratitis (ocular herpes)
- Herpes simplex blepharitis (not to be mistaken with ocular herpes)
- Prophylaxis against herpesviruses in immunocompromised patients (such as patients undergoing cancer chemotherapy)[12]
- Gardnerella vaginalis
HIV-1 progression can be delayed by using aciclovir, according to study led by Dr Jairam Lingappa. Effective in 16% of cases, it can delay the HAART treatment by 1–2 years. University of Washington, Seattle. During a 2 year trial, 284 people progressed with the HIV-1, versus 324 who had not been treated with aciclovir.[13] It has been claimed that the evidence for the effectiveness of topically applied cream for recurrent labial outbreaks is weak.[14] An earlier review of scientific literature showed there is some effect in reducing the number and duration of lesions if aciclovir is applied at an early stage of an outbreak.[15] However, oral therapy for episodes was found to be inappropriate for most nonimmunocompromised patients based on costs and benefits, presumably in countries where aciclovir is only available on prescription. There is evidence for an oral prophylactic role in preventing recurrences.
Dosage forms
Aciclovir is commonly marketed as tablets (200 mg, 400 mg, 800 mg and 1 gram), topical cream (5%), intravenous injection (25 mg/mL) and ophthalmic ointment (3%). Cream preparations are used primarily for labial herpes simplex. The intravenous injection is used when high concentrations of aciclovir are required. The ophthalmic ointment preparation is only used for herpes simplex keratitis.
Adverse effects
Systemic therapy
Common adverse drug reactions (≥1% of patients) associated with systemic aciclovir therapy (oral or IV) include: nausea, vomiting, diarrhea and/or headache. In high doses, hallucinations have been reported. Infrequent adverse effects (0.1–1% of patients) include: agitation, vertigo, confusion, dizziness, oedema, arthralgia, sore throat, constipation, abdominal pain, hair loss, rash and/or weakness. Rare adverse effects (<0.1% of patients) include: coma, seizures, neutropenia, leukopenia, crystalluria, anorexia, fatigue, hepatitis, Stevens–Johnson syndrome, toxic epidermal necrolysis and/or anaphylaxis.[11]
Additional common adverse effects, when aciclovir is administered IV, include encephalopathy (1% of patients) and injection site reactions. The injection formulation is alkaline (pH 11), and extravasation may cause local tissue pain and irritation.[11] Renal impairment has been reported when aciclovir is given in large, fast doses intravenously, due to the crystallisation of aciclovir in the kidneys.[16][17]
Topical therapy
Aciclovir topical cream is commonly associated (≥1% of patients) with: dry or flaking skin or transient stinging/burning sensations. Infrequent adverse effects include erythema or itch.[11] When applied to the eye, aciclovir is commonly associated (≥1% of patients) with transient mild stinging. Infrequently (0.1–1% of patients), ophthalmic aciclovir is associated with superficial punctate keratitis or allergic reactions.[11]
Toxicity
Since cellular DNA can incorporate aciclovir into itself, the drug acts as a chromosome mutagen; therefore it "...should not be used during pregnancy unless the potential benefit justifies the potential risk to the foetus..."[18] However, it has not been shown to have any teratogenic or carcinogenic effects and is frequently prescribed for pregnant women, to prevent transmission of HSV to the neonate.[citation needed] The acute toxicity (LD50) of aciclovir when given orally is greater than 1 g/kg, due to its low oral bioavailability.[citation needed] Patients with renal impairment often exhibit elimination half-lives for the drug that are five to six times longer than in those with normal renal function, leading to accumulation of aciclovir in the plasma and the likelihood of development of toxic reactions, such as lethargy, confusion and myoclonus.[19]
Cotard delusion has also been the result of adverse drug reactions to aciclovir. The symptoms were associated with high serum concentrations of 9-carboxymethoxymethylguanine (CMMG), the principal metabolite of aciclovir.
Detection in biological fluids
Aciclovir may be quantitated in plasma or serum to monitor for drug accumulation in patients with renal dysfunction or to confirm a diagnosis of poisoning in acute overdose victims.[19]
See also
References
- ^ a b de Clercq, Erik; Field, Hugh J (5 October 2005). "Antiviral prodrugs – the development of successful prodrug strategies for antiviral chemotherapy". British Journal of Pharmacology. Vol. 147, no. 1. Wiley-Blackwell (published January 2006). pp. 1–11. doi:10.1038/sj.bjp.0706446. PMC 1615839. PMID 16284630.
- ^ Garrison, Tom (1999). Oceanography: An Invitation to Marine Science, 3rd ed. Belmont, CA: Wadsworth Publishing Company. p. 471.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1081/TXR-100100318, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1081/TXR-100100318instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19149592, please use {{cite journal}} with
|pmid=19149592instead. - ^ Schaffer, Howard (1971). "Novel substrate of adenosine deaminase". Journal of Medicinal Chemistry. 14 (4): 367–369. doi:10.1021/jm00286a024. PMID 5553754.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Elion, Gertrude (1977). "Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine". Proc Natl Acad Sci USA. 74 (12): 5716–5720. doi:10.1073/pnas.74.12.5716. PMC 431864. PMID 202961.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vince, R. "A brief history of the development of Ziagen" Chemtracts 2008, 21, 127–134.
- ^ O'Brien JJ, Campoli-Richards DM. (1989). "Aciclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy". Drugs. 37 (3): 233–309. PMID 2653790.
- ^ Sweetman S, editor. Martindale: The complete drug reference. 34th ed. London: Pharmaceutical Press; 2004. ISBN 0-85369-550-4
- ^ Aciclovir Tablets BP 400mg - Summary of Product Characteristics (SPC) - (eMC)
- ^ a b c d e Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ^ Elad S, Zadik Y, Hewson I; et al. (2010). "A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea". Support Care Cancer. 18 (8): 993–1006. doi:10.1007/s00520-010-0900-3. PMID 20544224.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "HIV illness 'delayed by' herpes drug aciclovir". BBC News Onine. 15 February 2010. Retrieved 20. May 2010.
{{cite news}}: Check date values in:|accessdate=(help) - ^ Graham Worrall (1996). "Evidence for efficacy of topical acyclovir in recurrent herpes labialis is weak". BMJ. 313 (7048): 46. doi:10.1136/bmj.313.7048.46a. PMC 2351426. PMID 8664786.
{{cite journal}}: Unknown parameter|month=ignored (help) – Letter - ^ Graham Worrall (1996). "Acyclovir in recurrent herpes labialis". BMJ. 312 (7022): 6. doi:10.1136/bmj.312.7022.6. PMC 2349724. PMID 8555890.
{{cite journal}}: Unknown parameter|month=ignored (help) – Editorial - ^ Brigden D, Rosling AE, Woods NC (1982). "Renal function after acyclovir intravenous injection". The American Journal of Medicine. 73 (1A): 182–5. doi:10.1016/0002-9343(82)90087-0. PMID 6285711.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sawyer MH, Webb DE, Balow JE, Straus SE (1988). "Acyclovir-induced renal failure. Clinical course and histology". The American Journal of Medicine. 84 (6): 1067–71. doi:10.1016/0002-9343(88)90313-0. PMID 3376977.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ GLOBAL ACYCLOVIR, Medsafe Website
- ^ a b R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 29–31.
Further reading
- Harvey Stewart C. in Remington’s Pharmaceutical Sciences 18th edition: (ed. Gennard, Alfonso R.) Mack Publishing Company, 1990. ISBN 0-912734-04-3.
- Huovinen P., Valtonen V. in Kliininen Farmakologia (ed. Neuvonen et al.). Kandidaattikustannus Oy, 1994. ISBN 951-8951-09-8.
- Périgaud C., Gosselin G., Imbach J. -L.: Nucleoside analogues as chemotherapeutic agents: a review. Nucleosides and nucleotides 1992; 11(2–4)
- Rang H.P., Dale M.M., Ritter J.M.: Pharmacology, 3rd edition. Pearson Professional Ltd, 1995. 2003 (5th) edition ISBN 0-443-07145-4; 2001 (4th) edition ISBN 0-443-06574-8; 1990 edition ISBN 0-443-03407-9.
