Alkylbenzene

An alkylbenzene is a chemical compound that contains a monocyclic aromatic ring attaching to one or more saturated hydrocarbon chains.[1] Alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by alkyl groups. The simplest member, toluene (or methylbenzene), has the hydrogen atom of the benzene ring replaced by a methyl group. The chemical formula of alkylbenzenes is CnH2n-6.[2]

Alkylbenzenes are a very important class of hydrocarbons, especially in the synthetic production industry. It is the raw material in the production of synthetic sulfonate detergents, which are found in a variety of household products such as soap, shampoo, toothpaste, laundry detergent, etc. Linear alkylbenzenes (LAB) and branched alkylbenzenes (BAB) are families of alkylbenzene used to prepare synthetic sulfonates. However, LABs are more industrially favoured since the discovery of its extensive biodegradable yield over BAB-based sulfonates in the 1960s.[3]
Examples of alkylbenzenes
[edit]| Parent compound: | Alkylbenzenes | ||
|---|---|---|---|
 Toluene Toluene
| |||
| Xylenes | |||
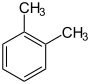 o-xylene o-xylene
|
 m-xylene m-xylene
|
||
| Trimethylbenzenes | |||
 hemimellitene hemimellitene
|
 pseudocumene pseudocumene
|
 mesitylene mesitylene
| |
| Tetramethylbenzenes | |||
 prehnitene prehnitene
|
 isodurene isodurene
|
 durene durene
| |
| Miscellaneous | |||
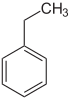 Ethylbenzene Ethylbenzene
|
 Cumene Cumene
|
 p-Cymene p-Cymene
| |

Reactions
[edit]- Friedel-Crafts alkylation: alkylbenzenes can be synthesized from olefins or alkyl halides with aromatic compounds in the presence of a catalyst such as AlCl3, HF, or H2SO4.[4]
- Gattermann-Koch reaction: named after German chemists Ludwig Gattermann and Julius Arnold Koch, the Gattermann-Koch reaction is a catalyzed formylation of alkylbenzenes with carbon monoxide and hydrochloric acid.[5]
- Alkylbenzene sulfonation reaction: electrophilic addition of a sulfonic acid group onto the aromatic ring.[4]
Spectroscopy
[edit]Alkylbenzene isomers can be differentiated by observing the position of alkyl substituents on the benzene ring using chemical ionization-proton exchange mass spectrometry. Conventional GC-MS yields limited results because the isomers have identical molecular weight and substituents.[6]
Production
[edit]Some alkylbenzenes such as toluene, trimethylbenzenes, and tetramethylbenzenes occur naturally in coal tar oil and as byproducts of the crude oil refinery process. Others can be prepared by Friedel-Crafts alkylation.[3]
Alkylbenzenes used to be synthesized from tetrapropylene, however, the reaction is now rarely used because of the low biodegradable alkylbenzene sulfonates it yields.[3]
Safety hazards
[edit]Alkylbenzenes are flammable. Most of them are eye and skin irritants and pose an acute health hazard when ingested. Alkylbenzenes are toxic to aquatic life with long-lasting effects.
Application
[edit]Alkylbenzenes are the primary raw material in making synthetic alkylbenzene sulfonates. Synthetic sulfonates are the most widely used detergents, as industrial oil, emulsifiers, demulsifiers, rust inhibitors, dispersants, surfactants for enhanced oil recovery, ore-floatation agents, and wetting agents, among others. LABs such as alkylbenzene, dialkylbenzene, and alkyltoluene are most commonly used to prepare sulfonate detergents.[4]
Solvent use
[edit]Some less substituted alkylbenzenes such as toluene and xylene are commonly used as solvents industrially.
Literature
[edit]- Allinger, Cava, de Jongh, Johnson, Lebel, Stevens: Organische Chemie, 1. Auflage, Walter de Gruyter, Berlin 1980, ISBN 3-11-004594-X, pp. 367–368, 560–562.
- Streitwieser / Heathcock: Organische Chemie, 1. Auflage, Verlag Chemie, Weinheim 1980, ISBN 3-527-25810-8, pp. 1051, 1073–1080.
- Beyer / Walter: Lehrbuch der Organischen Chemie, 19. Auflage, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2, pp. 442–444.
- Morrison / Boyd: Lehrbuch der Organischen Chemie, 3. Auflage, Verlag Chemie, Weinheim 1986, ISBN 3-527-26067-6, pp. 707–728.
References
[edit]- ^ Francis, Alfred W. (1948-02-01). "Properties of Alkylbenzenes". Chemical Reviews. 42 (1): 107–162. doi:10.1021/cr60131a003. ISSN 0009-2665. PMID 18904921.
- ^ Sazhin, S. S. (2022). Droplets and sprays : simple models of complex processes. Cham, Switzerland: Springer. ISBN 978-3-030-99746-5. OCLC 1333919856.
- ^ a b c Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (2013-09-16), "Hydrocarbons", in Wiley-VCH Verlag GmbH & Co. KGaA (ed.), Ullmann's Encyclopedia of Industrial Chemistry, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, pp. 1–61, doi:10.1002/14356007.a13_227.pub2, ISBN 978-3-527-30673-2, S2CID 242394133, retrieved 2023-04-12
- ^ a b c Lubricant additives : chemistry and applications. Leslie R. Rudnick. [Place of publication not identified]. 2017. ISBN 978-1-351-64696-3. OCLC 1003859957.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ De, Surya K. (2021-01-26). Applied Organic Chemistry: Reaction Mechanisms and Experimental Procedures in Medicinal Chemistry (1 ed.). Wiley. doi:10.1002/9783527828166.ch5. ISBN 978-3-527-34785-8. S2CID 242435919.
- ^ Hawthorne, Steven B.; Miller, David J. (1985-03-01). "Identifying alkylbenzene isomers with chemical ionization-proton exchange mass spectrometry". Analytical Chemistry. 57 (3): 694–698. doi:10.1021/ac00280a027. ISSN 0003-2700.
