Copper(II) nitrate
| |

| |
| Names | |
|---|---|
| IUPAC name
Copper(II) nitrate
| |
| Other names
Cupric nitrate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.019.853 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cu(NO3)2 | |
| Molar mass | 187.5558 g/mol (anhydrous) 241.60 g/mol (trihydrate) 232.591 g/mol (hemipentahydrate) |
| Appearance | blue crystals hygroscopic |
| Density | 3.05 g/cm3 (anhydrous) 2.32 g/cm3 (trihydrate) 2.07 g/cm3 (hexahydrate) |
| Melting point | 256 °C (493 °F; 529 K) (anhydrous, decomposes) 114.5 °C (trihydrate) 26.4 °C (hexahydrate, decomposes) |
| Boiling point | 170 °C (338 °F; 443 K) (trihydrate, decomposes) |
| trihydrate:[1] 381 g/100 mL (40 °C) 666 g/100 mL (80 °C) hexahydrate:[1] 243.7 g/100 mL (80 °C) | |
| Solubility | hydrates very soluble in ethanol, ammonia, water; insoluble in ethyl acetate |
| Structure | |
| orthorhombic (anhydrous) rhombohedral (hydrates) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant, Oxidizer |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3 (as Cu)[2] |
REL (Recommended)
|
TWA 1 mg/m3 (as Cu)[2] |
IDLH (Immediate danger)
|
TWA 100 mg/m3 (as Cu)[2] |
| Safety data sheet (SDS) | Cu(NO3)2·3H2O |
| Related compounds | |
Other anions
|
Copper(II) sulfate Copper(II) chloride |
Other cations
|
Nickel(II) nitrate Zinc nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Copper(II) nitrate, Cu(NO3)2, is an inorganic compound that forms a blue crystalline solid. Anhydrous copper nitrate forms deep blue-green crystals and sublimes in a vacuum at 150-200 °C.[3] Copper nitrate also occurs as five different hydrates, the most common ones being the trihydrate and hexahydrate. These materials are more commonly encountered in commerce than in the laboratory.
Synthesis and reactions

Hydrated copper nitrate can be prepared by hydration of the anhydrous material or by treating copper metal with an aqueous solution of silver nitrate or concentrated nitric acid:[4]
- Cu + 4 HNO3 → Cu(NO3)2 + 2 H2O + 2 NO2
Anhydrous Cu(NO3)2 forms when copper metal is treated with N2O4:
- Cu + 2 N2O4 → Cu(NO3)2 + 2 NO
Attempted dehydration of any of the hydrated copper(II) nitrates by heating instead affords the oxides, not Cu(NO3)2. At 80 °C, the hydrates convert to "basic copper nitrate" (Cu2(NO3)(OH)3), which converts to CuO at 180 °C.[4] Exploiting this reactivity, copper nitrate can be used to generate nitric acid by heating it until decomposition and passing the fumes directly into water. This method is similar to the last step in the Ostwald process. The equations are as follows:
- 2 Cu(NO3)2 → 2 CuO + 4 NO2 + O2
- 3NO2 + H2O → 2HNO3 + NO
Natural basic copper nitrates include the rare minerals gerhardtite and rouaite, both being polymorphs of Cu2(NO3)(OH)3 substance.[5]
Structure
Anhydrous copper(II) nitrate
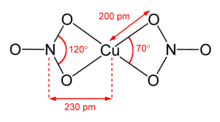
Anhydrous copper(II) nitrate has been crystallized in two solvate-free polymorphs.[6][7] α- and β-Cu(NO3)2 are fully 3D coordination polymer networks. The alpha form has only one Cu environment, with [4+1] coordination, but the beta form has two different copper centers, one with [4+1] and one that is square planar. The nitromethane solvate also features "[4+ 1] coordination", with four short Cu-O bonds of approximately 200 pm and one longer bond at 240 pm.[8] They are coordination polymers, with infinite chains of copper(II) centers and nitrate groups. In the gas phase, copper(II) nitrate features two bidentate nitrate ligands (see image at upper right).[9] Thus, evaporation of the solid entails "cracking" to give the copper(II) nitrate molecule.
Hydrated copper(II) nitrate
Five hydrates have been reported: the monohydrate (Cu(NO3)2·H2O),[7] the sesquihydrate (Cu(NO3)2·1.5H2O),[10] the hemipentahydrate (Cu(NO3)2·2.5H2O),[11] a trihydrate (Cu(NO3)2·3H2O),[12] and a hexahydrate (Cu(H2O)6](NO3)2).[13] The hexahydrate is interesting because the Cu-O distances are all equal, not revealing the usual effect of Jahn-Teller distortion that is otherwise characteristic of octahedral Cu(II) complexes. This non-effect is attributed to the strong hydrogen bonding that limits the elasticity of the Cu-O bonds.
Applications
Copper(II) nitrate finds a variety of applications, the main one being its conversion to copper(II) oxide, which is used as catalyst for a variety of processes in organic chemistry. Its solutions are used in textiles and polishing agents for other metals. Copper nitrates are found in some pyrotechnics.[4] It is often used in school laboratories to demonstrate chemical voltaic cell reactions.
Organic Synthesis
Copper nitrate, in combination with acetic anhydride, is an effective reagent for nitration of aromatic compounds, under what are known as "Menke conditions", in honor of the Dutch chemist who discovered that metal nitrates are effective reagents for nitration.[14] Hydrated copper nitrate adsorbed onto clay affords a reagent called "Claycop". The resulting blue-colored clay is used as a slurry, for example for the oxidation of thiols to disulfides. Claycop is also used to convert dithioacetals to carbonyls.[15] A related reagent based on montmorillonite has proven useful for the nitration of aromatic compounds.[16]
References
- ^ a b Perrys' Chem Eng Handbook, 7th Ed
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
- ^ Pass and Sutcliffe (1968). Practical Inorganic Chemistry. London: Chapman and Hall.
- ^ a b c H.Wayne Richardson "Copper Compounds" Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a07 567.
- ^ Mindat, http://www.mindat.org/min-10588.html
- ^ α-Cu(NO3)2S. C. Wallwork, W. E. Addison, "The crystal structures of anhydrous nitrates and their complexes. Part I. The α form of copper(II) nitrate" J. Chem. Soc. (1965) 2925-2933. doi:10.1039/JR9650002925.
- ^ a b β-Cu(NO3)2: S. I. Troyanov, I. V. Morozov, K. O. Znamenkov, Yu. M. Korenev, "Synthesis and X-Ray Structure of New Copper(II) Nitrates: Cu(NO3)2·H2O and β-modification of Cu(NO3)2" Z. Anorg. Allg. Chem. (1995) vol. 621, pp. 1261–1265. doi:10.1002/zaac.19956210727
- ^ B. Duffin and S. C. Wallwork "The crystal structure of anhydrous nitrates and their complexes. II. The 1:1 copper(II) nitrate-nitromethane complex" Acta Crystallographica 1966. volume 20, pp. 210-213. doi:10.1107/S0365110X66000434
- ^ R. E. LaVilla, S. H. Bauer "The Structure of Gaseous Copper(II) Nitrate as Determined by Electron Diffraction" J. Am. Chem. Soc., 1963, volume 85, pp 3597–3600. doi:10.1021/ja00905a015
- ^ K. Dornberger-Schiff, J. Leciejewicz, "Zur Struktur des Kupfernitrates Cu(NO3)2.1.5H2O" Acta Crystallogr. 1958, volume 11, pp. 825–826. doi:10.1107/S0365110X58002322
- ^ B. Morosin, "The crystal structure of Cu(NO3)2.2.5H2O" Acta Crystallogr. 1970, volume B26, pp. 1203–1208. doi:10.1107/S0567740870003898
- ^ J. Garaj, Sbornik Prac. Chem.-Technol. Fak. Svst., Cskosl. 1966, pp. 35–39.
- ^ R. Zibaseresht, R. M. Hartshorn,"Hexaaquacopper(II) dinitrate: absence of Jahn-Teller distortion" Acta Crystallogr. 2006, volume E62, pp. i19–i22. doi:10.1107/S1600536805041851
- ^ Menke J.B. (1925). "Nitration with nitrates". Recueil des Travaux Chimiques des Pays-Bas. 44: 141. doi:10.1002/recl.19250440209.
- ^ Balogh, M. "Copper(II) Nitrate–K10 Bentonite Clay" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
- ^ Collet, C.; Delville, A.; Laszlo, P. "Clays Direct Aromatic Nitration" Angewandte Chemie International Edition in English, 2003, Volume 29, Issue 5 , Pages 535–536. doi:10.1002/anie.199005351.

