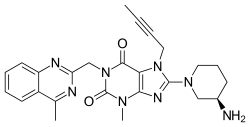
Linagliptin
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌlɪnəˈɡlɪptɪn/ LIN-ə-GLIP-tin |
| Trade names | Tradjenta, Trajenta |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611036 |
| License data |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~30% (Tmax = 1.5 hours) |
| Protein binding | 75–99% (concentration-dependent) |
| Metabolism | Minimal (~10% metabolized) |
| Metabolites | Pharmacologically inactive |
| Elimination half-life | ~12 hours |
| Excretion | Feces (80%), urine (5%)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H28N8O2 |
| Molar mass | 472.54 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Linagliptin (INN,[3] previously known as BI-1356, marketed under trade names Tradjenta (U.S.) and Trajenta (worldwide)) is a dipeptidyl peptidase-4 inhibitor developed by Boehringer Ingelheim for treatment of diabetes mellitus type 2.
Once-daily linagliptin was approved by the U.S. Food and Drug Administration (FDA) on 2 May 2011 for treatment of type 2 diabetes.[4] It is being marketed by Boehringer Ingelheim and Lilly.
Medical uses
Results in 2010 from a Phase III clinical trial of linagliptin showed that the drug can effectively reduce blood sugar.[5]
Side effects
Linagliptin may cause severe joint pain.[2][6]
Mechanism of action
Linagliptin belongs to a class of drugs called DPP-4 inhibitors. DPP-4 inhibitors prevent the hormone incretin from being degraded, allowing insulin to be released from the pancreatic beta cells. While incretin remains in the blood stream, the pancreas is stimulated to produce more insulin. Meanwhile, glucagon release from the pancreas is staggered, preventing glucose level increase. In other words, linagliptin, along with diet and exercise, can help the body produce more insulin and lower blood glucose. Managing blood sugar can mean a lower HbA1c, an index for glycemia control that theoretically correlates with glucose level in the blood. However, the use of HbA1c to predict diabetes in patients can sometimes be limited due to other external factors, such as blood transfusion, acute blood loss, or drug interference. [7]
See also
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b "Tradjenta (linagliptin) Tablets. Full Prescribing Information" (PDF). Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA. Retrieved 10 November 2016.
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary names: List 61" (PDF). World Health Organization. p. 66. Retrieved 10 November 2016.
- ^ "FDA Approves Type 2 Diabetes Drug from Boehringer Ingelheim and Lilly". 3 May 2011.
- ^ "Four Phase III Trials Confirm Benefits of BI's Oral, Once-Daily Type 2 Diabetes Therapy". Genetic Engineering & Biotechnology News. 28 June 2010.
- ^ "DPP-4 Inhibitors for Type 2 Diabetes: Drug Safety Communication - May Cause Severe Joint Pain". FDA. 2015-08-28. Retrieved 1 September 2015.
- ^ "Drugs affecting HbA1c levels". Indian J Endocrinol Metab. 2012.
{{cite journal}}: Cite journal requires|journal=(help)
- H. Spreitzer (September 1, 2008). "Neue Wirkstoffe - BI-1356". Österreichische Apothekerzeitung (in German) (18/2008): 918.
- Wang, Y, Serradell, N, Rosa, E, Castaner, R (2008). "BI-1356". Drugs of the Future. 33 (6): 473–477. doi:10.1358/dof.2008.033.06.1215244.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
