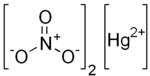
Mercury(II) nitrate
| |
| Names | |
|---|---|
| IUPAC names
Mercury dinitrate
Mercury(II) nitrate | |
| Other names
Mercuric nitrate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.126 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UN number | 1625 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Hg(NO3)2 | |
| Molar mass | 324.60 g/mol (anhydrous) |
| Appearance | colorless crystals or white powder |
| Odor | sharp |
| Density | 4.3 g/cm3 (monohydrate) |
| Melting point | 79 °C (174 °F; 352 K) (monohydrate) |
| soluble | |
| Solubility | soluble in nitric acid, acetone, ammonia insoluble in alcohol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 0980 |
| Related compounds | |
Other anions
|
Mercury(II) sulfate Mercury(II) chloride |
Other cations
|
Zinc nitrate Cadmium nitrate |
Related compounds
|
Mercury(I) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mercury(II) nitrate is a toxic colorless or white soluble crystalline mercury(II) salt of nitric acid. It was also used to treat fur to make felt in a process called 'carroting'. The phrase 'mad as a hatter' is associated with psychological illness brought on by excessive exposure to mercury(II) nitrate. The practice continued in the United States until it was banned in December 1941 by the United States Public Health Service. Although this sounds beneficial to health, the ban actually freed mercury(II)nitrate to be used as a detonator in the then ongoing war.[1]
Production
Mercury(II) nitrate is made by reacting hot concentrated nitric acid with mercury metal, under these conditions, the nitric acid is an oxidizing agent. Dilute nitric acid would produce mercury(I) nitrate.
Uses
Mercuric acid is used in mercuration reactions. In particular, it is used in reactions involving ketones. One of the chemicals which it is most effective with is acetone. This reaction uses mercuric nitrate, mercuric oxide, and calcium sulfate to change acetone, which is CH3C(O)CH3, into CH3C(O)CH2HgI. Acetone is a compound for which most other methods of mercuration prove ineffective.[2] The mercuric nitrate compound works because it is a strong oxidizing agent.[3] In addition, when mercury is dissolved in nitric acid the acid form of mercuric nitrate is formed.[4] The acidic form is capable of inverting molecules of sucrose.[5]
Health Information
Mercury nitrate tends to impact the body as Hg2+, which is considered a form of inorganic mercury. Forms of inorganic mercury can be found in various contexts including in skin lightening cream. If the inorganic mercury is ingested it can change the structure of important proteins within the body. If it gets into the soil it can then be absorbed and remain to be taken up by plants.[6] Those suffering from poisoning tend to experience vomiting and diarrhea as their earliest symptoms.[3]
Reactivity
Although mercuric nitrate is not flammable it can speed up flames since it acts as an oxidizer. In addition, it can form explosive compounds when combined with alcohols.[7]
See also
References
- ^ "The Not-So-Mad Hatter: Occupational Hazards of Mercury".
- ^ "Mercuration of Ketones and Some Other Compounds with Mercuric Nitrate".
- ^ a b "Mercuric Nitrate".
- ^ "Foods:Their Compostition and Analysis".
- ^ "The Inversion of Sucrosse by Acid Mercuric Nitrate".
- ^ "Elemental Mercury and Inorganic Mercury Compounds:Human Health Aspects" (PDF).
- ^ "Mercuric Nitrate".
External links
- ATSDR - Toxic Substances Portal - Mercury (11/14/2013)
- ATSDR - Public Health Statement: Mercury (11/14/2013)
- ATSDR - ALERT! Patterns of Metallic Mercury Exposure, 6/26/97 (link not traceable 11/14/2013)
- ATSDR - Medical Management Guidelines for Mercury (11/14/2013)
- ATSDR - Toxicological Profile: Mercury (11/14/2013)
- Safety data (MSDS) (link not traceable 11/14/2013)
- Mercuric Nitrate (ICSC)
- Mercury
- Mercury Information Packages
- How to Make Good Mercury Electrical Connections, Popular Science monthly, February 1919, Unnumbered page, Scanned by Google Books: http://books.google.com/books?id=7igDAAAAMBAJ&pg=PT14

