Methoxyethane
Appearance
This article needs additional citations for verification. (May 2010) |
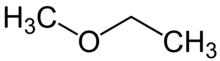
| |

| |
| Names | |
|---|---|
| IUPAC name
Methoxyethane
| |
| Other names
Methyl ethyl ether
Ethyl methyl ether | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.128.000 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H8O | |
| Molar mass | 60.096 g·mol−1 |
| Appearance | Colorless gas[1] |
| Density | 0.7251 g cm−3 (at 0 °C)[1] |
| Melting point | −113 °C (−171 °F; 160 K) |
| Boiling point | 7.4 °C (45.3 °F; 280.5 K) |
Refractive index (nD)
|
1.3420 (at 4 °C)[1] |
| Viscosity | 0.224 cP at 25 °C |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Extremely Flammable (F+), Liquefied gas |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related Ethers
|
Dimethyl ether Diethyl ether Methoxypropane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methoxyethane, also known as ethyl methyl ether, is an ethyl group with a bonded methoxy. Methoxyethane is a colorless gaseous ether with a medicine-like odor. It is extremely flammable, and its inhalation may cause asphyxiation or dizziness. As a Lewis base, it can react with Lewis acids to form salts and reacts violently with oxidizing agents.
References
- ^ a b c Haynes, William M. (2010). Handbook of Chemistry and Physics (91 ed.). Boca Raton, Florida, USA: CRC Press. p. 3-248. ISBN 978-1-43982077-3.

