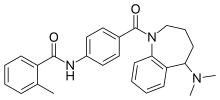
Mozavaptan
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H29N3O2 |
| Molar mass | 427.53 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mozavaptan (INN) is a vasopressin receptor antagonist marketed by Otsuka. In Japan, it was approved in October 2006 for hyponatremia (low blood sodium levels) caused by syndrome of inappropriate antidiuretic hormone (SIADH) due to ADH producing tumors.
References
- H. Spreitzer (November 20, 2006). "Neue Wirkstoffe - Conivaptan". Österreichische Apothekerzeitung (in German) (24/2006).
- Prous Science: Molecule of the Month November 2006
