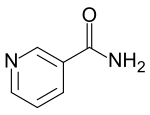
Nicotinamide
| |
| Names | |
|---|---|
| IUPAC name
pyridine-3-carboxamide
| |
| Other names
3-pyridinecarboxamide
niacinamide nicotinamide nicotinic acid amide Vitamin PP | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.002.467 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H6N2O | |
| Molar mass | 122.12 |
| Melting point | 128-131 C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nicotinamide, also known as niacinamide and nicotinic acid amide, is the amide of nicotinic acid (vitamin B3 / niacin). Nicotinamide is a water-soluble vitamin and is part of the vitamin B group. Nicotinic acid, also known as niacin, is converted to nicotinamide in vivo, and though the two are identical in their vitamin functions, nicotinamide does not have the same pharmacologic and toxic effects of niacin, which occur incidental to niacin's conversion. Thus nicotinamide does not reduce cholesterol or cause flushing,[1] although nicotinamide may be toxic to the liver at doses exceeding 3 g/day for adults.[2] In cells, niacin is incorporated into nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), although the pathways for nicotinamide and nicotinic acid are very similar. NAD+ and NADP+ are coenzymes in a wide variety of enzymatic oxidation-reduction reactions.[3]
Use in medicine
Nicotinamide has demonstrated anti-inflammatory actions which may be of benefit in patients with inflammatory skin conditions.[4] These conditions include acne vulgaris, and the compound can suppress antigen induced-lymphocytic transformation and inhibit of 3'-5' cyclic AMP phosphodiesterase. Nicotinamide has demonstrated the ability to block the inflammatory actions of iodides known to precipitate or exacerbate inflammatory acne.
Nicomide (take note the naming similarity), is an acne medication, and in its vitamin supplement form, the most predominant ingredient is 750 mg of nicotinamide, based on this area of research. Alternatively it is used topically as a 4% or 5% gel or cream - as effective as topical 1% clindamycin (8-week double-blind trial ) performed at the New York University College of Medicine.
Animal studies show that nicotinamide has anti-anxiety (anxiolytic) properties. It may work in a way similar to benzodiazepines.[5]
Nicotinamide lacks the vasodilator, gastrointestinal, hepatic, and hypolipidemic actions of nicotinic acid. As such, nicotinamide has not been shown to produce the flushing, itching and burning sensations of the skin as is commonly seen when large doses of nicotinic acid are administered orally. However, nicotinamide can produce liver toxicity at doses above 3 grams per day.[2] In overall, it rarely causes side effects, and is considered generally safe as a food additive, and as a component in cosmetics and medication.[6]
Nicotinamide is produced by the aqueous ammonolysis of 3-cyanopyridine (nicotinonitrile) and subsequent crystallisation.
Nicotinamide is an inhibitor of sirtuins and has been reported to restore cognition in Alzheimer's disease transgenic mice.[7] A safety study of niacinamide for the treatment of Alzheimer's disease is currently underway at the University of California, Irvine.[8]
Nicotinamide has been reported to increase the endurance of mice.[9]
Nicotinamide, or Vitamin B3, prevents immunosuppression caused by UVA and UVB radiation, and could be added to sunscreen.[10]
Nicotinamide has been reported to be an effective skin whitener in topical application.[11]
Niacinamide acts as a chemo- and radio-sensitizing agent by enhancing tumor blood flow, thereby reducing tumor hypoxia. Niacinamide also inhibits poly(ADP-ribose) polymerases (PARP-1), enzymes involved in the rejoining of DNA strand breaks induced by radiation or chemotherapy.[2]. PARP-1 appears to be an important target for Triple Negative Breast Cancers, because the cells are sensitive to inhibition of PARP-1.[3] Niacinamide is also used by some patients in combination with intravenous vitamin C therapy for cancer.[4]
See also
References
- ^ Jacenollo, P. (1992). Niacin versus niacinamide
- ^ a b Knip M, Douek IF, Moore WP; et al. (2000). "Safety of high-dose nicotinamide: a review". Diabetologia. 43 (11): 1337–45. doi:10.1007/s001250051536. PMID 11126400.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Belenky P (2007). "NAD+ metabolism in health and disease" (PDF). Trends Biochem. Sci. 32 (1): 12–9. doi:10.1016/j.tibs.2006.11.006. PMID 17161604. Retrieved 2007-12-23.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Niren NM (2006). "Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: a review". Cutis. 77 (1 Suppl): 11–6. PMID 16871774.
- ^ Tallman JF, Paul SM, Skolnick P, Gallager DW (1980). "Receptors for the age of anxiety: pharmacology of the benzodiazepines". Science. 207 (4428): 274–81. doi:10.1126/science.6101294. PMID 6101294.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Final report of the safety assessment of niacinamide and niacin". Int. J. Toxicol. 24 Suppl 5: 1–31. 2005. PMID 16596767.
- ^ Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, LaFerla FM (2008-11-05). "Journal of Neuroscience - Nicotinamide Restores Cognition in Alzheimer's Disease Transgenic Mice via a Mechanism Involving Sirtuin Inhibition and Selective Reduction of Thr231-Phosphotau". Retrieved 2008-11-05.
{{cite web}}: CS1 maint: multiple names: authors list (link). - ^ [1]
- ^ Fukuwatari T, Shibata K, Ishihara K, Fushiki T, Sugimoto E (2001). "Elevation of blood NAD level after moderate exercise in young women and mice". J. Nutr. Sci. Vitaminol. 47 (2): 177–9. PMID 11508711.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM (2008). "UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide". J. Invest. Dermatol. 128 (2): 447–54. doi:10.1038/sj.jid.5701058. PMID 17882270.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hakozaki T, Minwalla L, Zhuang J; et al. (2002). "The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer". Br. J. Dermatol. 147 (1): 20–31. doi:10.1046/j.1365-2133.2002.04834.x. PMID 12100180.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
