Potassium hexachloroplatinate
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.037.239 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| K2PtCl6 | |
| Molar mass | 485.99 g/mol |
| Appearance | orange to yellow solid |
| Density | 3.344 g/cm3 |
| Melting point | 250 °C (482 °F; 523 K) (decomposes) |
| 0.89 g/100ml H2O (at 25 °C) [1] | |
| Hazards | |
| Flash point | 250 °C (482 °F; 523 K) |
| Safety data sheet (SDS) | Oxford MSDS |
| Related compounds | |
Other anions
|
Potassium tetrachloroplatinate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
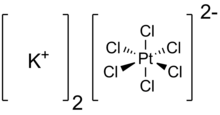
Potassium hexachloroplatinate is the inorganic compound with the formula K2PtCl6. It is a yellow solid that is an example of a comparatively insoluble potassium salt. The salt features the hexachloroplatinate(IV) dianion, which has octahedral coordination geometry.
The precipitation of this compound from solutions of hexachloroplatinic acid was formerly used for the determination of potassium by gravimetric analysis.[2] It is also useful as an intermediate in the recovery of platinum from wastes.[3]
Reactions
Using salt metathesis reactions, potassium hexachloroplatinate is converted to a variety of quaternary ammonium and related lipophilic salts. These include tetrabutylammonium salt (NBu4)2PtCl6, known as Lukevics catalyst.[4]
Reduction of potassium hexachloroplatinate with hydrazine dihydrochloride gives the corresponding tetrachloroplatinate salt.[5][6]
References
- ^ Grinberg, A. A.; Sibirskaya, V. V. (1967). "Solubility of hexammine and hexahalo platinum(IV) complexes". Zhurnal Neorganicheskoi Khimii. 12: 2069–2071.
- ^ G. F. Smith; J. L. Gring (1933). "The Separation and Determination of the Alkali Metals Using Perchloric Acid. V. Perchloric Acid and Chloroplatinic Acid in the Determination of Small Amounts of Potassium in the Presence of Large Amounts of Sodium". J. Am. Chem. Soc. 55 (10): 3957–3961. doi:10.1021/ja01337a007.
- ^ George B. Kauffman, Larry A. Teter "Recovery of Platinum from Laboratory Residues" Inorganic Syntheses, 1963, volume 7, pp. 232-236. doi:10.1002/9780470132388.ch61
- ^ "Quaternary Onium Hexachloroplatinates: Novel Hydrosilylation Catalysts". Organometallics. 6 (7): 1410–1413. 1987. doi:10.1021/om00150a007.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Cis - and trans -Dichlorodiammineplatinum(II)". cis- and trans-Dichlorodiammine Platinum(II). Inorganic Syntheses. Vol. 7. 1963. pp. 239–245. doi:10.1002/9780470132388.ch63. ISBN 9780470132388.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Keller, R. N.; Moeller, T. (1963). "Potassium Tetrachloroplatinate(II)". Inorg. Synth. 7: 247–250. doi:10.1002/9780470132333.ch79.
