Sodium bicarbonate
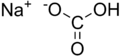
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sodium hydrogen carbonate
| |||
| Other names
Baking soda, bicarbonate of soda, nahcolite
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 4153970 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.122 | ||
| EC Number |
| ||
| E number | E500(ii) (acidity regulators, ...) | ||
| KEGG | |||
| MeSH | Sodium+bicarbonate | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| NaHCO 3 | |||
| Molar mass | 84.0066 g mol−1 | ||
| Appearance | White crystals | ||
| Odor | odorless | ||
| Density | 2.20 g/cm3 as a solid[1]
1.1 to 1.3 as a powder[2] | ||
| Melting point | 50 °C (122 °F; 323 K) (decomposes to sodium carbonate) | ||
| 9 g/100 mL
69 g/L (0 °C)[3] | |||
| Solubility | 0.02 %wt acetone, 2.13 %wt methanol @22 °C.[5] insoluble in ethanol | ||
| log P | -0.82 | ||
| Acidity (pKa) | 10.329[6]
6.351 (carbonic acid)[6] | ||
Refractive index (nD)
|
1.3344 | ||
| Structure | |||
| monoclinic | |||
| Thermochemistry | |||
Heat capacity (C)
|
87.61 J/mol K | ||
Std molar
entropy (S⦵298) |
102 J/mol K | ||
Std enthalpy of
formation (ΔfH⦵298) |
-947.7 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
-851.9 kJ/mol | ||
| Pharmacology | |||
| B05CB04 (WHO) B05XA02 (WHO), QG04BQ01 (WHO) | |||
| Intravenous, oral | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Causes serious eye irritation | ||
| NFPA 704 (fire diamond) | |||
| Flash point | incombustible | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
4220 mg/kg ( rat, oral ) [7] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Other anions
|
Sodium carbonate | ||
Other cations
|
Ammonium bicarbonate | ||
Related compounds
|
Sodium bisulfate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Sodium bicarbonate[note 1] (IUPAC name: sodium hydrogen carbonate) is a chemical compound with the formula NaHCO3. It is a salt composed of sodium ions and bicarbonate ions. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs. It is among the food additives encoded by European Union, identified as E 500. Since it has long been known and is widely used, the salt has many related names such as baking soda, bread soda, cooking soda, and bicarbonate of soda.[note 2] The word saleratus, from Latin sal æratus meaning "aerated salt", was widely used in the 19th century for both sodium bicarbonate and potassium bicarbonate.
History
The ancient Egyptians used natural deposits of natron, a mixture consisting mostly of sodium carbonate decahydrate, and sodium bicarbonate. The natron was ground up, solvated, and used as paint for hieroglyphics.[citation needed]
In 1791, a French chemist, Nicolas Leblanc, produced sodium carbonate, also known as soda ash. In 1846, two New York bakers, John Dwight and Austin Church, established the first factory to develop baking soda from sodium carbonate and carbon dioxide.[8]
This compound, referred to as saleratus, is mentioned in the novel Captains Courageous by Rudyard Kipling as being used extensively in the 1800s in commercial fishing to prevent freshly caught fish from spoiling.[9]
Production
NaHCO3 is mainly prepared by the Solvay process, which is the reaction of sodium chloride, ammonia, and carbon dioxide in water. Calcium carbonate is used as the source of CO2 and the resultant calcium oxide is used to recover the ammonia from the ammonium chloride. The product shows a low purity (75%). Pure product is obtained from sodium carbonate, water, and carbon dioxide as reported in one of the following reactions. It is produced on the scale of about 100,000 tonnes/year (as of 2001).[10]
NaHCO3 may be obtained by the reaction of carbon dioxide with an aqueous solution of sodium hydroxide. The initial reaction produces sodium carbonate:
- CO2 + 2 NaOH → Na2CO3 + H2O
Further addition of carbon dioxide produces sodium bicarbonate, which at sufficiently high concentration will precipitate out of solution:
- Na2CO3 + CO2 + H2O → 2 NaHCO3
Commercial quantities of baking soda are also produced by a similar method: soda ash, mined in the form of the ore trona, is dissolved in water and treated with carbon dioxide. Sodium bicarbonate precipitates as a solid from this method:
- Na2CO3 + CO2 + H2O → 2 NaHCO3
Mining
Naturally occurring deposits of nahcolite (NaHCO3) are found in the Eocene-age (55.8–33.9 Mya) Green River Formation, Piceance Basin in Colorado. Nahcolite was deposited as beds during periods of high evaporation in the basin. It is commercially mined using common underground mining techniques such as Bore, Drum, and longwall mining in a fashion very similar to coal mining. A very small portion is also obtained using in situ leach techniques involving dissolution of the nahcolite by heated water pumped through the nahcolite beds that have been previously been mined using the aforementioned techniques. It is then reconstituted through a natural cooling crystallisation process. Currently only Tronox (formerly FMC) in the Green River WY basin has successfully commercially solution mined the product.
Chemistry
Sodium bicarbonate is an amphoteric compound. Aqueous solutions are very mildly alkaline due to the formation of carbonic acid and hydroxide ion:
- HCO−
3 + H2O → H
2CO
3 + OH−
Sodium bicarbonate can be used as a wash to remove any acidic impurities from a "crude" liquid, producing a purer sample. Reaction of sodium bicarbonate and an acid produce a salt and carbonic acid, which readily decomposes to carbon dioxide and water:
- NaHCO3 + HCl → NaCl + H2CO3
- H2CO3 → H2O + CO2(g)
Sodium bicarbonate reacts with acetic acid (found in vinegar), producing sodium acetate, water, and carbon dioxide:
- NaHCO3 + CH3COOH → CH3COONa + H2O + CO2(g)
Sodium bicarbonate reacts with bases such as sodium hydroxide to form carbonates:
- NaHCO3 + NaOH → Na2CO3 + H2O
Sodium bicarbonate reacts with carboxyl groups in proteins to give a brisk effervescence from the formation of CO
2. This reaction is used to test for the presence of carboxylic groups in protein.[citation needed]
Thermal decomposition
Above 50 °C, sodium bicarbonate gradually decomposes into sodium carbonate, water and carbon dioxide. The conversion is fast at 200 °C:[11]
- 2 NaHCO3 → Na2CO3 + H2O + CO2
Most bicarbonates undergo this dehydration reaction. Further heating converts the carbonate into the oxide (at over 850 °C):[11]
- Na2CO3 → Na2O + CO2
These conversions are relevant to the use of NaHCO3 as a fire-suppression agent ("BC powder") in some dry powder fire extinguishers.
Applications
Sodium bicarbonate has a wide variety of uses.
Cooking
With acids
Sodium bicarbonate, referred to as "baking soda", is primarily used in baking, as a leavening agent. It reacts with acidic components in batters, releasing carbon dioxide, which causes expansion of the batter and forms the characteristic texture and grain in pancakes, cakes, quick breads, soda bread, and other baked and fried foods. Acidic compounds that induce this reaction include phosphates, cream of tartar, lemon juice, yogurt, buttermilk, cocoa and vinegar. Natural acids in sourdough can be leavened with the addition of small amounts as well.[12] Sodium bicarbonate can be substituted for baking powder provided sufficient acid reagent is also added to the recipe.[13] Many forms of baking powder contain sodium bicarbonate combined with calcium acid phosphate, sodium aluminium phosphate [14] or cream of tartar.
Sodium bicarbonate was sometimes used in cooking vegetables, to make them softer, although this has gone out of fashion, as most people now prefer firmer vegetables. However, it is still used in Asian and Latin American cuisine to tenderise meats. Baking soda may react with acids in food, including vitamin C (L-ascorbic acid). It is also used in breading such as for fried foods to enhance crispness and allow passages for steam to escape, so the breading is not blown off during cooking.
By heating
Heat causes sodium bicarbonate to act as a raising agent by releasing carbon dioxide when used in baking. The carbon dioxide production starts at temperatures above 80 °C.
- 2NaHCO3 → Na2CO3 + H2O + CO2
Since the reaction occurs slowly at room temperature, mixtures (cake batter, etc.) can be allowed to stand without rising until they are heated in the oven.
Pest control
Used to kill cockroaches. Once consumed, it causes internal organs of cockroaches to burst due to gas collection.[15]
Paint and corrosion removal
Sodium bicarbonate is used in a process for removing paint and corrosion called sodablasting; the process is particularly suitable for cleaning aluminium panels which can be distorted by other types of abrasive.
pH balancer
It can be administered to pools, spas, and garden ponds to raise pH levels.[16]
Pyrotechnics
Sodium bicarbonate is one of the main components of the common incendiary "black snake" firework. The effect is caused by the thermal decomposition, which produces carbon dioxide gas to produce a long snake-like ash as a combustion product of the other main component, sucrose.
Mild disinfectant
It has weak disinfectant properties,[17][18] and it may be an effective fungicide against some organisms.[19] Because baking soda will absorb musty smells, it has become a reliable method for used-book sellers when making books less malodorous.[20]
Fire extinguisher
Sodium bicarbonate can be used to extinguish small grease or electrical fires by being thrown over the fire, as heating of sodium bicarbonate releases carbon dioxide.[21] However, it should not be applied to fires in deep fryers; the sudden release of gas may cause the grease to splatter.[21] Sodium bicarbonate is used in BC dry chemical fire extinguishers as an alternative to the more corrosive ammonium phosphate in ABC extinguishers. The alkaline nature of sodium bicarbonate makes it the only dry chemical agent, besides Purple-K, that was used in large-scale fire suppression systems installed in commercial kitchens. Because it can act as an alkali, the agent has a mild saponification effect on hot grease, which forms a smothering, soapy foam.
Neutralisation of acids and bases
Sodium bicarbonate is amphoteric, reacting with acids and bases. It reacts violently with acids, releasing CO2 gas as a reaction product. However, sodium bicarbonate is not recommended for the clean-up of acid spills. The heat produced increases the reactivity of the acid,[citation needed] and the large amount of carbon dioxide produced may increase the area of the spill.
A wide variety of applications follows from its neutralisation properties, including reducing the spread of white phosphorus from incendiary bullets inside an afflicted soldier's wounds.[22][medical citation needed]
Medical uses
Sodium bicarbonate mixed with water can be used as an antacid to treat acid indigestion and heartburn.[23]
Intravenous sodium bicarbonate is an aqueous solution that is sometimes used for cases of acidosis, or when insufficient sodium or bicarbonate ions are in the blood.[24] In cases of respiratory acidosis, the infused bicarbonate ion drives the carbonic acid/bicarbonate buffer of plasma to the left and, thus, raises the pH. It is for this reason that sodium bicarbonate is used in medically supervised cardiopulmonary resuscitation. Infusion of bicarbonate is indicated only when the blood pH is markedly (<7.1–7.0) low.[25]
It is used for treatment of hyperkalemia, as it will drive K+ back into cells during periods of hypochloremic metabolic alkalosis.[26] Since sodium bicarbonate can cause alkalosis, it is sometimes used to treat aspirin overdoses. Aspirin requires an acidic environment for proper absorption, and the basic environment diminishes aspirin absorption in the case of an overdose.[27] Sodium bicarbonate has also been used in the treatment of tricyclic antidepressant overdose.[28] It can also be applied topically as a paste, with three parts baking soda to one part water, to relieve some kinds of insect bites and stings (as well as accompanying swelling).[29]
Adverse reactions to the administration of sodium bicarbonate can include metabolic alkalosis, edema due to sodium overload, congestive heart failure, hyperosmolar syndrome, hypervolemic hypernatremia, and hypertension due to increased sodium. In patients consuming a high-calcium or dairy-rich diet, calcium supplements, or calcium-containing antacids such as calcium carbonate (e.g., Tums), the use of sodium bicarbonate can cause milk-alkali syndrome, which can result in metastatic calcification, kidney stones, and kidney failure.
Sodium bicarbonate can be used to treat an allergic reaction to plants such as poison ivy, poison oak, or poison sumac to relieve some of the associated itching.[30]
Bicarbonate of soda can also be useful in removing splinters from the skin.[31]
Some alternative practitioners, such as Tullio Simoncini, have promoted baking soda as a cancer cure, which the American Cancer Society has warned against due to both its unproven effectiveness and potential danger in use.[32]
Sodium bicarbonate can be added to local anaesthetics, to speed up the onset of their effects and make their injection less painful.[33] It is also a component of Moffett's solution, used in nasal surgery.
Personal hygiene
Toothpaste containing sodium bicarbonate has in several studies been shown to have a better whitening[34][34][35][36] and plaque removal effect[37][38] than toothpastes without it.
Sodium bicarbonate is also used as an ingredient in some mouthwashes. It has anticaries and abrasive properties.[39] It works as a mechanical cleanser on the teeth and gums, neutralises the production of acid in the mouth, and also acts as an antiseptic to help prevent infections.[40][41]
Sodium bicarbonate in combination with other ingredients can be used to make a dry or wet deodorant.[42][43] It may also be used as a shampoo.[44]
Sodium bicarbonate may be used as a buffering agent, combined with table salt, when creating a solution for nasal irrigation.[45]
It is used in eye hygiene to treat blepharitis. This is done by addition of a tablespoon of sodium bicarbonate to cool water that was recently boiled, followed by gentle scrubbing of the eyelash base with a cotton swab dipped in the solution.[46]
In sports
Small amounts of sodium bicarbonate have been shown to be useful as a supplement for athletes in speed-based events, such as middle-distance running, lasting from about one to seven minutes.[47][48] However, overdose is a serious risk because sodium bicarbonate is slightly toxic;[49] and gastrointestinal irritation is of particular concern.[48] Additionally, this practice causes a significant increase in dietary sodium.
As a cleaning agent
A paste from baking soda can be very effective when used in cleaning and scrubbing.[50] For cleaning aluminium objects, the use of sodium bicarbonate is discouraged, as it attacks the thin unreactive protective oxide layer of this otherwise very reactive metal.[citation needed] A solution in warm water will remove the tarnish from silver when the silver is in contact with a piece of aluminium foil.[51][52] A paste of sodium bicarbonate and water is useful in removing surface rust as the rust forms a water-soluble compound when in a concentrated alkaline solution.[53] Cold water should be used, as hot water solutions can corrode steel.[54]
Baking soda is commonly added to washing machines as a replacement for softener and to remove odors from clothes. Sodium bicarbonate is also effective in removing heavy tea and coffee stains from cups when diluted with warm water.
During the Manhattan Project to develop the nuclear bomb in the early 1940s, many scientists investigated the toxic properties of uranium. They found that uranium oxides stick very well to cotton cloth, but did not wash out with soap or laundry detergent. The uranium would wash out with a 2% solution of sodium bicarbonate (baking soda). Clothing can become contaminated with depleted uranium (DU) dust, and then normal laundering will not remove it. Those at risk of DU dust exposure should have their clothing washed with about 6 ounces (170 g) of baking soda in 2 gallons (7.5 l) of water.[55]
As a biopesticide
Sodium bicarbonate can be an effective way of controlling fungal growth,[56] and in the United States is registered by the Environmental Protection Agency as a biopesticide.[57]
Cattle feed supplements
Sodium bicarbonate is sold as a cattle feed supplement, in particular as a buffering agent for the rumen.[58]
In popular culture
Film
Sodium bicarbonate, as "bicarbonate of soda", was a frequent source of punch lines for Groucho Marx in Marx brothers movies. In Duck Soup, Marx plays the leader of a nation at war. In one scene, he receives a message from the battlefield that his general is reporting a gas attack, and Groucho tells his aide: "Tell him to take a teaspoonful of bicarbonate of soda and a half a glass of water."[59] In A Night at the Opera, Groucho's character addresses the opening night crowd at an opera by saying of the lead tenor: "Signor Lassparri comes from a very famous family. His mother was a well-known bass singer. His father was the first man to stuff spaghetti with bicarbonate of soda, thus causing and curing indigestion at the same time."[60]
Difference between baking soda and baking powder
Quite simply, baking powder contains baking soda, as well as a powdered acid and cornstarch. In scientific terms, baking soda is a pure substance; baking powder is a mixture.
Baking soda is alkaline, so acid is used in baking powder to avoid a metallic taste when the chemical change during baking creates sodium carbonate. However, to avoid the over-flavouring of acidic taste, non-acid ingredients such as whole milk or Dutch-processed cocoa must be added.[61]
See also
- Carbonic acid
- Soda bread
- List of ineffective cancer treatments
- List of minerals
- Nahcolite
- Natron
- Natrona (disambiguation)
- Trona
- Washing soda
Notes
- ^ The prefix "bi" in "bicarbonate" comes from an outdated naming system and is based on the observation that there is two times as much carbonate (CO3) in sodium bicarbonate (NaHCO3) and other bicarbonates as in sodium carbonate (Na2CO3) and other carbonates.
- ^ In colloquial usage, the names sodium bicarbonate and bicarbonate of soda are often truncated. Forms such as sodium bicarb, bicarb soda, bicarbonate, bicarb, or even bica are common.
References
- ^ "Physical Constants of Inorganic Compounds". CRC Handbook, p. 4-85.
- ^ "Densities of some Common Materials". Retrieved 3 March 2016.
- ^ a b "Aqueous solubility of inorganic compounds at various temperatures". CRC Handbook, p. 8-116.
- ^ a b "Sodium Bicarbonate" (PDF). UNEP Publications.
- ^ J. L. Ellingboe, J. H. Runnels (1966). "Solubilities of Sodium Carbonate and Sodium Bicarbonate in Acetone-Water and Methanol-Water Mixtures". J. Chem. Eng. Data. 11 (3): 323–324. doi:10.1021/je60030a009.
- ^ a b Goldberg, Robert N.; Kishore, Nand; Lennen, Rebecca M. "Thermodynamic quantities for the ionisation reactions of buffers in water". CRC Handbook. pp. 7–13.
- ^ Michael Chambers. "ChemIDplus - 144-55-8 - UIIMBOGNXHQVGW-UHFFFAOYSA-M - Sodium bicarbonate [USP:JAN] - Similar structures search, synonyms, formulas, resource links, and other chemical information". Retrieved 4 August 2015.
- ^ "Company History". Church & Dwight Co.
- ^ Rudyard Kipling. "Captains Courageous".
{{cite journal}}: Cite journal requires|journal=(help) p. 25 - ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ a b "Decomposition of Carbonates". General Chemistry Online.
- ^ "Sourdough Pancakes Recipe". whatscookingamerica.com.
- ^ Radiation Cookery Book 45th Edition, Radiation Group Sales Ltd 1954
- ^ "Glossary Ingredients". Cooking.com.
- ^ "Best Home Remedies To Kill And Control Cockroaches". HRT.whw1.com. Retrieved 2015-06-20.
- ^ "Arm & Hammer Baking Soda – Basics – The Magic Of Arm & Hammer Baking Soda". Armhammer.com. Retrieved 2009-07-30.
- ^ Malik, Ys; Goyal, Sm (May 2006). "Virucidal efficacy of sodium bicarbonate on a food contact surface against feline calicivirus, a norovirus surrogate". International Journal of Food Microbiology. 109 (1–2): 160–3. doi:10.1016/j.ijfoodmicro.2005.08.033. ISSN 0168-1605. PMID 16540196.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ William A. Rutala, Susan L. Barbee, Newman C. Aguiar, Mark D. Sobsey, David J. Weber, (2000). "Antimicrobial Activity of Home Disinfectants and Natural Products Against Potential Human Pathogens". Infection Control and Hospital Epidemiology. 21 (1). The University of Chicago Press on behalf of The Society for Healthcare Epidemiology of America: 33–38. doi:10.1086/501694. PMID 10656352.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Zamani, M; Sharifi, Tehrani, A; Ali, Abadi, Aa (2007). "Evaluation of antifungal activity of carbonate and bicarbonate salts alone or in combination with biocontrol agents in control of citrus green mold". Communications in agricultural and applied biological sciences. 72 (4): 773–7. PMID 18396809.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gail Altman (2006-05-22). "Book Repair for BookThinkers: How To Remove Odors From Books". The BookThinker (69).
- ^ a b "Arm & Hammer Baking Soda – Basics – The Magic Of Arm & Hammer Baking Soda". Armhammer.com. Retrieved 2009-07-30.
- ^ "White Phosphorus". GlobalSecurity.org. Retrieved 2007-09-26.
- ^ "Sodium Bicarbonate". Jackson Siegelbaum Gastroenterology. 1998.
- ^ "Sodium Bicarbonate Intravenous Infusion" (PDF). Consumer Medicine Information. Better Health Channel. 2004-07-13.
- ^ "Respiratory Acidosis: Treatment & Medication". emedicine.
- ^ Medical Toxicology, Richard C. Dart.
- ^ Cloth Diapers, Leah Leverich Ph.D.
- ^ [needs update]Knudsen, K; Abrahamsson, J (Apr 1997). "Epinephrine and sodium bicarbonate independently and additively increase survival in experimental amitriptyline poisoning". Critical Care Medicine. 25 (4): 669–74. doi:10.1097/00003246-199704000-00019. ISSN 0090-3493. PMID 9142034.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Insect bites and stings: First aid". Mayo Clinic. 2008-01-15.
- ^ [unreliable medical source?]What is Sodium Bicarbonate Used For?. Virtuowl.com. Retrieved on 2010-09-24.
- ^ "How to Remove a Splinter with Baking Soda". wikiHow. Retrieved 4 August 2015.
- ^ "Sodium Bicarbonate". American Cancer Society. 28 November 2008. Retrieved 19 February 2013.
- ^ http://www.frca.co.uk/article.aspx?articleid=100505
- ^ a b Kleber, CJ; Moore, MH; Nelson, BJ (1998). "Laboratory assessment of tooth whitening by sodium bicarbonate dentifrices". The Journal of clinical dentistry. 9 (3): 72–5. PMID 10518866.
- ^ Koertge, TE; Brooks, CN; Sarbin, AG; Powers, D; Gunsolley, JC (1998). "A longitudinal comparison of tooth whitening resulting from dentifrice use". The Journal of clinical dentistry. 9 (3): 67–71. PMID 10518865.
- ^ Yankell, SL; Emling, RC; Petrone, ME; Rustogi, K; Volpe, AR; DeVizio, W; Chaknis, P; Proskin, HM (1999). "A six-week clinical efficacy study of four commercially available dentifrices for the removal of extrinsic tooth stain". The Journal of clinical dentistry. 10 (3 Spec No): 115–8. PMID 10825858.
- ^ Mankodi, S; Berkowitz, H; Durbin, K; Nelson, B (1998). "Evaluation of the effects of brushing on the removal of dental plaque". The Journal of clinical dentistry. 9 (3): 57–60. PMID 10518862.
- ^ Putt, MS; Milleman, KR; Ghassemi, A; Vorwerk, LM; Hooper, WJ; Soparkar, PM; Winston, AE; Proskin, HM (2008). "Enhancement of plaque removal efficacy by tooth brushing with baking soda dentifrices: results of five clinical studies". The Journal of clinical dentistry. 19 (4): 111–9. PMID 19278079.
- ^ Silje Storehagen, Nanna Ose og Shilpi Midha. "Dentifrices and mouthwashes ingredients and their use" (PDF). Institutt for klinisk odontologi. Universitetet i Oslo.
- ^ Malik, Y; Goyal, S (2006). "Virucidal efficacy of sodium bicarbonate on a food contact surface against feline calicivirus, a norovirus surrogate". International Journal of Food Microbiology. 109 (1–2): 160–3. doi:10.1016/j.ijfoodmicro.2005.08.033. PMID 16540196.
- ^ Zamani, M; Sharifi Tehrani, A; Ali Abadi, AA (2007). "Evaluation of antifungal activity of carbonate and bicarbonate salts alone or in combination with biocontrol agents in control of citrus green mold". Communications in agricultural and applied biological sciences. 72 (4): 773–7. PMID 18396809.
- ^ Lamb, John Henderson (31 May 1946). "Sodium Bicarbonate: An Excellent Deodorant". The Journal of Investigative Dermatology. 7 (3): 131–133. doi:10.1038/jid.1946.13.
- ^ "Bicarb soda: natural body deodorant". Retrieved 5 May 2012.
- ^ Bouchard, Mallory (2010-05-04). "A Green and Healthy Beauty Secret: Going Shampoo-Free". Four Green Steps.
- ^ Ralph B. Metson, M.D., The Harvard Medical School Guide to Healing Your Sinues (McGraw Hill 2005), at p. 68.
- ^ {cite web|author= Mary Harding, MRCGP|title= Blepharitis|url=http://www.patient.co.uk/health/blepharitis-leaflet
- ^ Bee, Peta (2008-08-16). "Is bicarbonate of soda a performance enhancing drug". The Times. London. Retrieved 2010-05-23.
- ^ a b Ergogenic Aids. U. Retrieved on 2011-09-11.
- ^ Baking soda overdose – All Information. Umm.edu (2009-10-19). Retrieved on 2010-09-24.
- ^ "Arm & Hammer Baking Soda – Basics – The Magic Of Arm & Hammer Baking Soda". Armhammer.com. Retrieved 2009-07-30.
- ^ Eco Silver Polishing. instructables.com (2006-12-20). Retrieved on 2011-10-07.
- ^ "Put a Shine on It". scifun.chem.wisc.edu. Retrieved 2011-03-06.
- ^ Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 22: d-block metal chemistry: the first row elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 716. ISBN 978-0-13-175553-6.
- ^ .http://www.sciencelab.com/msds.php?msdsId=9927263
- ^ Orcutt, JA. "Scientist". Pharmacology and Toxicology of Uranium Compounds. McGraw-Hill. Retrieved 21 March 2012.
- ^ Potassium bicarbonate (073508) and Sodium bicarbonate (073505) Fact Sheet United States Environmental Protection Agency. Updated 17 February 2011. Retrieved 25 November 2011.
- ^ Registered Biopesticides 04/29/02 United States Environmental Protection Agency. Updated 29 March 2002. Retrieved 25 November 2011.
- ^ "Acidosis Health Warning for Livestock Farmers". Retrieved 5 May 2012.
- ^ "Duck Soup (1933)". IMDb. Retrieved 4 August 2015.
- ^ "A Night at the Opera (1935)". IMDb. Retrieved 4 August 2015.
- ^ "Baking 101: The Difference Between Baking Soda and Baking Powder". Joy the Baker. Retrieved 4 August 2015.
Further reading
- Bishop, D; Edge, J; Davis, C; Goodman, C (May 2004). "Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability". Medicine and science in sports and exercise. 36 (5): 807–13. doi:10.1249/01.MSS.0000126392.20025.17. ISSN 0195-9131. PMID 15126714.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - David R. Lide, ed. (2003). CRC Handbook of Chemistry and Physics (84th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0484-9.



