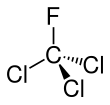
Trichlorofluoromethane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trichloro(fluoro)methane | |||
| Other names
Trichlorofluoromethane
Fluorotrichloromethane Fluorochloroform Freon 11 CFC 11 R 11 Arcton 9 Freon 11A Freon 11B Freon HE Freon MF | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.812 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CCl3F | |||
| Molar mass | 137.36 g·mol−1 | ||
| Appearance | Colorless liquid/gas | ||
| Odor | nearly odorless[1] | ||
| Density | 1.494 g/cm3 | ||
| Melting point | −110.48 °C (−166.86 °F; 162.67 K) | ||
| Boiling point | 23.77 °C (74.79 °F; 296.92 K) | ||
| 1.1 g/L (at 20 °C) | |||
| log P | 2.53 | ||
| Vapor pressure | 89 kPa at 20 °C 131 kPa at 30 °C | ||
| Hazards | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published)
|
26,200 ppm (rat, 4 hr) 100,000 ppm (rat, 20 min) 100,000 ppm (rat, 2 hr)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1000 ppm (5600 mg/m3)[1] | ||
REL (Recommended)
|
C 1000 ppm (5600 mg/m3)[1] | ||
IDLH (Immediate danger)
|
2000 ppm[1] | ||
| Safety data sheet (SDS) | ICSC 0047 | ||
| Supplementary data page | |||
| Trichlorofluoromethane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon. It is a colorless, faint ethereal, and sweetish-odor liquid that boils around room temperature.
Uses
It was the first widely used refrigerant. Because of its high boiling point (compared to most refrigerants), it can be used in systems with a low operating pressure, making the mechanical design of such systems less demanding than that of higher-pressure refrigerants R-12 or R-22.
R-11 is assigned an ozone depletion potential of 1.0, and U.S. production was ended in January 1, 1996.
Trichlorofluoromethane is used as a reference compound for fluorine-19 NMR studies.
Prior to the knowledge of the ozone depletion potential of chlorine in refrigerants and other possible harmful effects on the environment, trichlorofluoromethane was sometimes used as a cleaning/rinsing agent for low-pressure systems.[citation needed]
Trichloromethane was formerly used in the drinking bird novelty, largely because it has a boiling point of 74.79 F. The replacement, dichloromethane, boiling point 103.3 F, requires a higher ambient temperature to work.
Gallery
-
Hemispheric and Global mean concentrations of CFC-11 (NOAA/ESRL)
-
Time-series of atmospheric concentrations of CFC-11 (Walker et al., 2000)
-
"Present day" (1990s) sea surface CFC-11 concentration
-
"Present day" (1990s) CFC-11 oceanic vertical inventory
References
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0290". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Fluorotrichloromethane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
External links
- CFC-11 NOAA/ESRL Global measurements
- Public health goal for trichlorofluoromethane in drinking water
- Names at webbook.nist.gov
- Data sheet at speclab.com
- International Chemical Safety Card 0047
- NIOSH Pocket Guide to Chemical Hazards. "#0290". National Institute for Occupational Safety and Health (NIOSH).
- Phase change data at webbook.nist.gov
- Thermochemistry data at chemnet.ru
- ChemSub Online: Trichlorofluoromethane - CFC-11