Wikipedia:Reference desk/Archives/Science/2017 May 3
| Science desk | ||
|---|---|---|
| < May 2 | << Apr | May | Jun >> | May 4 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
May 3
[edit]What's the smallest feature of 14 and 10nm chips in the horizontal direction?
[edit]Is it the fin width? (8nm in 14nm chips I think) Controlling the vertical thicknesses of features to that accuracy is much easier right? (by controlling how long the UV is on) Sagittarian Milky Way (talk) 00:54, 3 May 2017 (UTC)
- This is a truly messy subject. Durng the heyday of Moore's law, the process width was defined as the half-pitch of the DRAM memory cell size (i.e., the center if a memory cell was twice this length away from the neighboring cell) and the same (or a very similar process that was used for DRAM was also used for other devices, so they inherited the same number. As it became harder to achieve a factor-of-two reduction every two years or so, first Intel and then other manufacturers began to cheat by redefining exactly what they meant by a particular number. -Arch dude (talk) 01:59, 3 May 2017 (UTC)
- All written in 14_nanometer#Comparison of 16nm and 14nm process nodes. --Kharon (talk) 00:35, 4 May 2017 (UTC)
Nicotine parent
[edit]What do you call a nicotine molecule without the methyl-group?32ieww (talk) —Preceding undated comment added 00:56, 3 May 2017 (UTC)
Is it addictive and bad for you the way nicotine is? 32ieww (talk) 02:21, 5 May 2017 (UTC)
What is the reason to remove jewlarys in case of burn?
[edit]I saw "Initial First Aid Treatment for Minor Burns" of American Burn Association which says: "Remove all jewelry, watches, rings and clothing around the burned area as soon as possible". There is no there a rational explanation for that. what is the reason? 93.126.88.30 (talk) 02:15, 3 May 2017 (UTC)
- According to [1], removal of any tight-fitting clothing or jewelry is related to the risk of swelling after a burn. I presume to prevent the item from becoming irremovable after swelling begins, and possibly restricting blood supply. Someguy1221 (talk) 02:18, 3 May 2017 (UTC)
- Also:
- 1) Bacteria could be present on the jewelry that will infect the skin, which has lost it's protective outer layer.
- 2) Scabs could adhere the skin to the jewelry, such that the scabs will be broken when the jewelry is removed or just by the normal motion of the jewelry as the patient moves.
- 3) Even without scabs, the normal movement of the jewelry could still aggravate the burned area.
- An interesting Q is what to do with medical bracelets, like this one that announces a penicillin allergy: [2]. If the other wrist is unburned, I suppose it can be relocated there. StuRat (talk) 15:29, 3 May 2017 (UTC)
- Although StuRat's points have some merits, the primary reason by a long chalk is to avoid constriction when the burn inevitably swells, unless it's very superficial. [3] The accident department (ER) is equipped with cutting equipment to remove rings, should the first-aider not be quick enough. Alansplodge (talk) 13:19, 4 May 2017 (UTC)
Temperature change of air due to pressure drop
[edit]I got curious about water rockets recently, and found a site with pressure specs on various plastic soda bottles, and I was surprised that a 2L soda bottle can take 11 atmospheres of pressure.
So I got to wondering, if I pumped 11 atmospheres into a soda bottle and let it out all at once, what's the temperature of the air left in the bottle once the internal pressure equalizes at 1 atmosphere?
The ideal gas law wouldn't help me because with P×V=constant, it assumes constant temperature. Additionally, I'm not dealing with a change in volume in a closed container, I've got more of a free expansion. So I need a formula for temperature change purely as a function of pressure change.
Failing to find anything that would help me quickly on Wikipedia (I checked free expansion and Joule expansion, among other articles), I found this NASA document about isentropic compression (for which Wikipedia doesn't have an article). According to NASA:
where for air, according to our article on heat capacity ratio. γ looks reasonably constant over a wide range of temperatures around a comfortable-for-humans 300K.
OK, supposing I pump 11 atmospheres into the bottle, let it sit for a while so that the inside temperature equals the outside temperature 300K, then release the cap to let the air out (I did a numerical calculation of mass flow rate, taking into account choked flow, that indicated the pressure would equalize in less than 0.3 sec).
The formula above says that the final temperature is or −122°C!
That's pretty damn cold! That's well below the freezing point of CO2 at −78.5°C. I'd definitely expect to see ice form on the bottle.
Does that final temperature make sense? It looks unrealistically cold to me. ~Anachronist (talk) 05:02, 3 May 2017 (UTC)
- We have a fairly substantial adiabatic process article that gives that same T–P formula. DMacks (talk) 05:13, 3 May 2017 (UTC)
- Ah, I see it now, thanks. My question remains though: Do the seemingly extreme results I get have any bearing on real-world experience? ~Anachronist (talk) 05:22, 3 May 2017 (UTC)
- It occurs to me that the 2 liters of air remaining in the bottle weighs only a few grams (at room temperature it'd be under 2.5 grams); I weighed an empty, uncapped 2-liter bottle just now and it was 47 grams. I don't know the heat capacity of the plastic, but it doesn't seem as though the bottle would cool all that much even if the air did. --76.71.6.254 (talk) 05:41, 3 May 2017 (UTC)
- Oh, and another point. If its temperature drops that much, then the air will not immediately expand to 11 times its compressed volume (which in turn means that the temperature won't exactly drop that much). I don't know the correct way to allow for this, though. --76.71.6.254 (talk) 05:44, 3 May 2017 (UTC)
- That formula is supposed to account for this already, I thought from reading the NASA document. It isn't the same as the ideal gas law, which doesn't account for temperature drops. ~Anachronist (talk) 06:37, 3 May 2017 (UTC)
- Oh, and another point. If its temperature drops that much, then the air will not immediately expand to 11 times its compressed volume (which in turn means that the temperature won't exactly drop that much). I don't know the correct way to allow for this, though. --76.71.6.254 (talk) 05:44, 3 May 2017 (UTC)
- It occurs to me that the 2 liters of air remaining in the bottle weighs only a few grams (at room temperature it'd be under 2.5 grams); I weighed an empty, uncapped 2-liter bottle just now and it was 47 grams. I don't know the heat capacity of the plastic, but it doesn't seem as though the bottle would cool all that much even if the air did. --76.71.6.254 (talk) 05:41, 3 May 2017 (UTC)
- Ah, I see it now, thanks. My question remains though: Do the seemingly extreme results I get have any bearing on real-world experience? ~Anachronist (talk) 05:22, 3 May 2017 (UTC)
The equation quoted above, and attributed to NASA, is only applicable in a reversible, adiabatic process. Free expansion of a compressed gas is irreversible and so leads to an increase in entropy. The increase in entropy will manifest as a higher temperature than T2 calculated from the equation.
I believe the process described in the original question can be analysed using a version of the First Law of Thermodynamics called the uniform-state, uniform-flow process. I can suggest text books by van Wylen and Sonntag as suitable sources of information on the uniform-state, uniform-flow process. Dolphin (t) 12:19, 3 May 2017 (UTC)
- @Dolphin51: Well, this isn't exactly free expansion because it is reversible; you can always pump air back in. The concept of free expansion includes an assumption that no work is done, which isn't the case here. Work is being done. Mass is being accelerated, whether air mass ejected upward, or bottle mass if you turn it on its side. You may be onto something with uniform-state, uniform-flow processes; I've found some tutorials online that have been helpful. A typical problem presented is, what is the final temperature inside a tank that initially contains only a vacuum and ambient air is allowed to flow in? It's counterintuitive to me that the final temperature is higher than ambient. Mine is the opposite problem: what is the final temperature inside a pressurized tank from which air is released? ~Anachronist (talk) 17:26, 3 May 2017 (UTC)
- Of course the compressed air can't come out "all at once". Outflow begins as an accelerating jet through the open neck of the bottle. The expanding air leaves the bottle in a turbulence where it mixes immediately with the ambient air. Its kinetic energy is dissipated as heat (see Mechanical equivalent of heat) and noise, so one will not observe much cooling outside the bottle. When the pressure in the bottle has decayed to 1 bar (atmospheric pressure), the outflow continues due to inertia of the air column in the neck. This is the start of an acoustic Resonance in the bottle where the internal pressure swings alternately below and above 1 bar, the neck air flows alternately out and in, and one may hear a characteristic decaying tone like when one blows across the open bottle. The inflows continually import small amounts of the air at ambient temperature so that it is difficult to measure the cooling of the original air. Any moisture in the air will make it difficult (require extreme expansion) to cool it below 0 °C because of water's significant Enthalpy of fusion. Blooteuth (talk) 14:50, 3 May 2017 (UTC)
- @Blooteuth: The effects you describe aren't really applicable for this thought experiment. The air can come out effectively all at once if the neck is as wide as the bottle. A 2-L bottle with a standard 22mm neck pumped to 11 atm will release the air in about 250 milliseconds. But it shouldn't really matter how fast the air comes out if we assume no heat transfer through the wall of the bottle. And I'm not referring to cooling outside the bottle, but inside; if the formula above is correct, then I'd expect any moisture remaining to condense into ice inside the bottle. Also, the oscillation you describe is mitigated by the fact that the bottle isn't made from a rigid material, but a flexible polymer, although we can assume it's rigid for the purpose of ignoring any work associated with the walls. And for the sake of argument let's assume no oscillation, say we have a one-way valve at the neck (say, a ping-pong ball in a cage that falls onto the neck before air can flow back in). As for freezing, an extreme case would be a CO2 fire extinguisher, which causes condensation of ice around the nozzle after just a brief use. I'd expect something less from the bottle (it's just air with some humidity, not dry CO2, and the pressure is much less) but I was wondering if it would get below freezing — especially −122°C as indicated by the calculation. Would it be safe to assume that any moisture in the air (let's say the bottle has a bit of water at the bottom when we pump it up, guaranteeing moisture) will effectively throttle the temperature reduction so it is unlikley to go below 0°C? ~Anachronist (talk) 17:26, 3 May 2017 (UTC)
- @Anachronist: You say "this isn't exactly free expansion because it is reversible; you can always pump air back in." Yes, you can always pump air back in but that is not the meaning of reversible in thermodynamics. Thermodynamically speaking, the process you have described is definitely irreversible. There is some very good information available at Irreversible process#Examples of irreversible processes.
- The simplest way to analyse your process is to consider the soda bottle and all the air initially inside it as a thermodynamic system; everything else is part of the surroundings. The system does no work on the surroundings so we say no work is done. Similarly, heat exchanged with the surroundings is zero. Dolphin (t) 12:08, 4 May 2017 (UTC)
- @Dolphin51:. This isn't a free expansion because work is being done. Mass is being accelerated, a force is being applied to move a mass a distance. If you turn the bottle on its side, the outrushing air provides a thrust that moves the bottle. Work is force multiplied by distance. Any astronaut will tell you that a ruptured air tank does indeed perform work. It is reversible. Imagine that the bottle is perfectly rigid and insulating (a reasonable assumption given the short time interval) with a perfectly-insulating bag attached to the neck of the bottle, which inflates with no back-pressure when the air is released from the bottle. You can squeeze the bag to push the same air right back in.
The same amount of work done to eject the mass of air is required to push it back.Addendum: OK, I think I get from irreversible process that this may not be reversible, but I am not sure why.
- @Dolphin51:. This isn't a free expansion because work is being done. Mass is being accelerated, a force is being applied to move a mass a distance. If you turn the bottle on its side, the outrushing air provides a thrust that moves the bottle. Work is force multiplied by distance. Any astronaut will tell you that a ruptured air tank does indeed perform work. It is reversible. Imagine that the bottle is perfectly rigid and insulating (a reasonable assumption given the short time interval) with a perfectly-insulating bag attached to the neck of the bottle, which inflates with no back-pressure when the air is released from the bottle. You can squeeze the bag to push the same air right back in.
- @Blooteuth: The effects you describe aren't really applicable for this thought experiment. The air can come out effectively all at once if the neck is as wide as the bottle. A 2-L bottle with a standard 22mm neck pumped to 11 atm will release the air in about 250 milliseconds. But it shouldn't really matter how fast the air comes out if we assume no heat transfer through the wall of the bottle. And I'm not referring to cooling outside the bottle, but inside; if the formula above is correct, then I'd expect any moisture remaining to condense into ice inside the bottle. Also, the oscillation you describe is mitigated by the fact that the bottle isn't made from a rigid material, but a flexible polymer, although we can assume it's rigid for the purpose of ignoring any work associated with the walls. And for the sake of argument let's assume no oscillation, say we have a one-way valve at the neck (say, a ping-pong ball in a cage that falls onto the neck before air can flow back in). As for freezing, an extreme case would be a CO2 fire extinguisher, which causes condensation of ice around the nozzle after just a brief use. I'd expect something less from the bottle (it's just air with some humidity, not dry CO2, and the pressure is much less) but I was wondering if it would get below freezing — especially −122°C as indicated by the calculation. Would it be safe to assume that any moisture in the air (let's say the bottle has a bit of water at the bottom when we pump it up, guaranteeing moisture) will effectively throttle the temperature reduction so it is unlikley to go below 0°C? ~Anachronist (talk) 17:26, 3 May 2017 (UTC)
- Here's an equivalent system. Imagine a perfectly insulating rigid tube floating in the vacuum of space, capped at one end, open at the other, with temperature and pressure sensors in the cap. A piston in the tube compresses a volume of humid air against the cap at pressure P1 and temperature T1. The piston is released, and it moves toward the open end of the tube. Before it reaches the open end, it encounters an obstruction and stops. The mass of the piston is irrelevant, work equals force times distance, all that matters is a force applied to the piston (pressure times area) moved the piston a distance. The distance moved is unknown. The final volume is unknown. All we know is initial and final pressure, and initial temperature. The question is, given that we know P1, T1, and P2, what is the final temperature T2?
This is reversible. The work done to move the piston the distance can be applied to move it right back.- Hmm, now that I've explored some more (thanks), this is looking something like a Joule expansion.
- The original location of the piston, and the original volume of air, are analogous to the neck of the bottle and the volume of the bottle, respectively. P1 is analogous to the original pressure in the bottle. T1 is analogous to ambient temperature, and P2 is analogous to ambient atmospheric pressure. The obstruction in the tube is analogous to ambient atmospheric pressure stopping the inflation of the bag attached to the bottle. The answer to the question "what is T2?" is not the adiabatic expansion formula given above. I'll wager that the actual final temperature doesn't get anywhere near the final temperature predicted by that formula. ~Anachronist (talk) 19:09, 4 May 2017 (UTC)
- Well, from the previous answers, you can tell that is a fairly complicated process.
- If we make the following assumptions, most of which are unrealistic:
- After pumping 11atm of pressure, you wait until temperature inside the bottle is the same as outside (and no gas escapes)
- Releasing the gas is fast enough that no thermal exchange with the outside takes place, and slow enough that it is an adiabatic process, and there is no mixing by turbulence inside the bottle
- ...then yes, the gas will end up at a low temperature.
- However, it does not follow that you will see CO2-ice on the walls. I can see at least two plausible effects that mitigate the raw conclusion from the adiabatic law:
- You will have some thermal exchange with the walls. One of the reasons that exchanges are neglected in such cases is that convection is a rather slow mode of heat transfer, but if you assume CO2 ice starts to form on the walls (technical term: nucleate) then heat conduction kicks in. Although walls have a small volume, they have a large mass compared to the air enclosed; the bottle is about 50g going by [4], and the air is about 0.003g (2L * density of air at 1atm, -25°C). As a consequence they have a much larger thermal mass (the massic heat capacity does not vary much across all materials).
- Heat capacity vs. enthalpy of sublimation effects. Let's say the heat capacity of air is about 1 J/g/K (even a bit less for CO2). On the other hand, the enthalpy of sublimation of CO2 is about 16.5kJ/mol = 375J/g (molar mass of 44g/mol). For CO2, you have as much "coldness" in a drop of temperature of 375°C as in the freezing from vapor to solid at atmospheric pressure. CO2 is a small fraction of the enclosed air, but freezing it can still absorb a good fraction of the "coldness" inside. TigraanClick here to contact me 09:33, 5 May 2017 (UTC)
- @Tigraan: Thanks. I found an alternative calculation that gives a more reasonable result (−51°C rather than −122°C) for the temperature drop in my initial example, in terms of initial temperature and pressure and final pressure: See equation 5 at https://www.physicsforums.com/insights/grandpa-chets-entropy-recipe/
- Given that −51°C isn't anywhere near the condensation temperature of CO2, I'm more concerned about the effects of water vapor. The application here is a water rocket, which has water and air in the bottle under pressure, so the air is likely saturated if we assume it's been under pressure for a while. After all the water is ejected, the water vapor in the remaining air likely has a modulating effect on the temperature, preventing it from getting too low.
- You have a good point about the mass of the bottle being orders of magnitude higher than the mass of the air. I haven't done the experiment myself of pumping up a bottle to high pressure and releasing it, but reports I've read don't include accounts of ice, but definitely visible fog forms in the bottle after all the air is released, and the skin of the bottle becomes cold. ~Anachronist (talk) 08:12, 6 May 2017 (UTC)
Blood compatibility in pregnancy
[edit]If I understand this correctly, thanks to placenta's two separate circulatory systems "no intermingling of maternal and fetal blood occurs in the placenta". But why some people whose blood type is somehow incompatible with their mother's one (myself included, e.g. in case of B-type son and his A-type mother) gestated well, while others got hemolytic disease of the newborn (ABO)? Thanks. 212.180.235.46 (talk) 16:24, 3 May 2017 (UTC)
- "During any pregnancy a small amount of the baby's blood can enter the mother's circulation.", according to our article on Rh_disease. My understanding is that "no intermingling... occurs" would be probably more correct as "almost no intermingling... occurs." It only takes trace exposure to develop antibodies, that's how/why many vaccines work. SemanticMantis (talk) 18:14, 3 May 2017 (UTC)
- Yes, there's a fairly narrow separation between the two circulatory systems, so small tears can allow for direct exchange. Due to the much greater volume of blood in the mother than the baby, this is far more likely to be a problem for the baby. StuRat (talk) 16:34, 3 May 2017 (UTC)
- Also, as the article on hemolytic disease of the newborn states, it can be caused by already-existing antibodies from the mother crossing to the fetus. Antibodies can cross the placenta; this is essential to providing the baby immune defenses in the first months of life. --47.138.161.183 (talk) 19:54, 3 May 2017 (UTC)
Would it make more sense for gas stations to sell by the pound or kilo?
[edit]The gallon of gas could be 40°F one month and 80°F in another. What technology(ies) dispense fuel by weight or mass? Sagittarian Milky Way (talk) 18:17, 3 May 2017 (UTC)
- Gasoline is normally stored in underground tanks, where the temp doesn't vary all that much. StuRat (talk) 20:16, 3 May 2017 (UTC)
- Not from hour to hour, no, but it does vary signfiicantly from season to season. In some countries (the sensible ones), gasoline pumps are required to be temperature-compensated, which means that in effect if is sold by mass even though the price is expressed in terms of volume. That is, if you pay for 20 liters you get whatever mass would have occupied 20 liters at the specified reference temperature. --76.71.6.254 (talk) 21:44, 3 May 2017 (UTC)
- I think it doesn't matter an awful lot. Gasoline retail is a highly competitive market, and all the competitors are affected the same way, so presumably the difference is folded into the price. However in principle there might be a small advantage to buying gas at the time of day when it would be expected to be coldest. --Trovatore (talk) 22:08, 3 May 2017 (UTC)
- Not from hour to hour, no, but it does vary signfiicantly from season to season. In some countries (the sensible ones), gasoline pumps are required to be temperature-compensated, which means that in effect if is sold by mass even though the price is expressed in terms of volume. That is, if you pay for 20 liters you get whatever mass would have occupied 20 liters at the specified reference temperature. --76.71.6.254 (talk) 21:44, 3 May 2017 (UTC)
- The article Fuel dispenser elaborates on StuRat's statement. ←Baseball Bugs What's up, Doc? carrots→ 23:05, 3 May 2017 (UTC)
- It elaborates on my statement, which is why I linked to it. --76.71.6.254 (talk) 02:50, 4 May 2017 (UTC)
- True. Your redirect threw me. ←Baseball Bugs What's up, Doc? carrots→ 03:34, 4 May 2017 (UTC)
- It elaborates on my statement, which is why I linked to it. --76.71.6.254 (talk) 02:50, 4 May 2017 (UTC)
- Even with the largest temperature variations that can reasonably be expected, the change in mass of gasoline per unit volume is unlikely to be much larger than 1%. Looie496 (talk) 14:42, 4 May 2017 (UTC)
- Snopes has some discussion on this, and related topics. AndrewWTaylor (talk) 09:14, 5 May 2017 (UTC)
- Nerds like me would like fuel to be sold by Joule (of the standard enthalpy of formation of the hydrocarbon molecules). But that is basically the same as selling it by mass for gasoline and diesel (which have a similar energy density).
- A real argument to sell or at least tax by weight rather than by volume exists in Europe. Unlike in the US, diesel fuel and the associated engine technology has a nontrivial market share for small vehicles. But diesel is significantly more dense than gasoline, which makes km-by-liter or tax-by-liter comparisons quite unfair. TigraanClick here to contact me 09:44, 5 May 2017 (UTC)
- In the United States, weights and measures are enforced by the individual states, who have all adopted the National Institute of Standards and Technology Handbook 44. On page 3-35 that document says that measuring devices for refined petroleum products may automatically compensate volumes to what the volume would be at 15°C. I don't know to what extent this is actually done at retail fuel stations. Jc3s5h (talk) 12:21, 5 May 2017 (UTC)
- Although while enforcement is state-level, one of the few powers that the federal government actually has is that of "fix[ing] the Standard of Weights and Measures"; you'd have to go to Congress, not any state legislature, to get a change in definition. Nyttend (talk) 10:54, 6 May 2017 (UTC)
Tubeless bike tires with holes instead
[edit]
Is there a name for these types of tires? I can't find it anywhere. Many thanks. Anna Frodesiak (talk) 19:10, 3 May 2017 (UTC)
- Interesting question - I have never seen these before. This article [5] calls them "flat-free", but other articles call totally solid tyres flat free, so enjoy the searching! DrChrissy (talk) 19:40, 3 May 2017 (UTC)
- Flat-free by Nexo company, eh? Okay. Thanks, DrChrissy. :) Anna Frodesiak (talk) 20:06, 3 May 2017 (UTC)
- Airless tire. I've never seen these on a bike before. Usually see them on heavy construction equipment, which our article mentions is due to the risk of puncture at construction sites. Someguy1221 (talk) 20:13, 3 May 2017 (UTC)
- Puncture-proof tires is the general term, at least in the games Mille Bornes. StuRat (talk) 20:15, 3 May 2017 (UTC)
- Aaaah "airless tire", okay. This calls for a few redirects. I couldn't find that article. Thanks, Someguy1221. Anna Frodesiak (talk) 20:19, 3 May 2017 (UTC)
- Considering run-flat tire, what would you suggest, or do for me while I make another coffee? :) Anna Frodesiak (talk) 20:22, 3 May 2017 (UTC)
- Airless, rather than run-flat. Run-flats are pneumatic, with a short-term backup if they're punctured. Andy Dingley (talk) 20:38, 3 May 2017 (UTC)
- Unfortunately, some of the articles I saw referred to solid rubber tyres as "airless". DrChrissy (talk) 20:53, 3 May 2017 (UTC)
- Why is that unfortunate, DrChrissy? Anna Frodesiak (talk) 20:58, 3 May 2017 (UTC)
- I thought it unfortunate because I think you are looking for a unique term. The articles seem to use terms interchangeably even though the tyres are clearly different. DrChrissy (talk) 21:09, 3 May 2017 (UTC)
- I understand. Okay, thanks, DrChrissy. Anna Frodesiak (talk) 21:22, 3 May 2017 (UTC)
- I thought it unfortunate because I think you are looking for a unique term. The articles seem to use terms interchangeably even though the tyres are clearly different. DrChrissy (talk) 21:09, 3 May 2017 (UTC)
- Why is that unfortunate, DrChrissy? Anna Frodesiak (talk) 20:58, 3 May 2017 (UTC)
- Unfortunately, some of the articles I saw referred to solid rubber tyres as "airless". DrChrissy (talk) 20:53, 3 May 2017 (UTC)
- Airless, rather than run-flat. Run-flats are pneumatic, with a short-term backup if they're punctured. Andy Dingley (talk) 20:38, 3 May 2017 (UTC)
- Considering run-flat tire, what would you suggest, or do for me while I make another coffee? :) Anna Frodesiak (talk) 20:22, 3 May 2017 (UTC)
I made Puncture-proof tires into a redirect. What other terms would people search when looking for airless tire? Anna Frodesiak (talk) 20:58, 3 May 2017 (UTC)
- Btw, if this is the standard tire for Mobike, it should be mentioned in article (hopefully sourced). 107.15.152.93 (talk) 21:22, 3 May 2017 (UTC)
- Mobike has that kind and also what appears to be solid tires with no air inside. See File:Mobike tire - 01.jpg. The other two big bike sharing models in Haikou have tubes and they are parked all over the city with flat tires. Regular tires and bike sharing programs don't mix. Anna Frodesiak (talk) 21:37, 3 May 2017 (UTC)
- I'm more wondering what other types of tires people might be thinking of when searching for "puncture-proof tire". Could be airless tire, solid tire (which has no article) or semi-pneumatic tire (also no article). Railway tires are also puncture-proof, but I doubt anyone would search for them with that term. Someguy1221 (talk) 21:23, 3 May 2017 (UTC)
- I agree, "solid tires" seems a better term. --Kharon (talk) 00:27, 4 May 2017 (UTC)
- There are also "puncture-proof" tires that are not solid or "semi-pneumatic" e.g. → [6] — 107.15.152.93 (talk) 02:19, 4 May 2017 (UTC)
Height of Parinacota?
[edit]So, different sources give different heights for Parinacota (volcano), including 6348 and 6380m. What is the most recent estimate? JoJo Eumerus mobile (talk) 21:37, 3 May 2017 (UTC)
- I'm not sure if this 2002 research paper gives a height [7], but it is freely accessible, discusses many features of event 8k years ago, and has lots of references to further scholarly works about the volcano. SemanticMantis (talk) 00:10, 4 May 2017 (UTC)
- I woke up early today and wrote this C program to parse Space Shuttle RADAR Topography Mission data:
:> cat print.c
#include <stdio.h>
#include <stdlib.h>
#include <unistd.h>
int main(int argc, char** argv)
{
FILE* infile = fopen("S18W069.hgt", "rb");
if (infile)
{
for (int i = 0; i < (1201*1201); i++) // I had only one cup of coffee at this point
{
int16_t h;
fread(&h, sizeof(int16_t),1, infile);
int16_t m = htons(h); // Space Shuttle SRTM data is big-endian - this code makes it work on Intel, PowerPC, SPARC, ...
fprintf(stdout, "%d \n", m);
}
fclose(infile);
}
return 0;
}
// Compile and run and sort: # cc -o a.x print.c ; ./a.x | sort | tail -n 1
- Here's the raw height file database from the United States Geological Survey, including some details about the binary data format; USGS SRTM database; and here's the quick start guide for the impatient; and more documentation for the literate reader who wishes to understand what's happening; and here's the data file for S18W069, including the vicinity of Parinacota volcano. Those heights are in meters referenced to the WGS84/EGM96 geoid.
- I got 5560 meters as the tallest measurement in that region (plus a bunch of junk "-1" data - "Data Voids", for those who read the manual!) So either the Space Shuttle disagrees with the location of the volcano, or the most accurate RADAR in the world did not publish data for the peak, or the reference geoid is really a weird shape. ... Hint: it is.
- Anybody who has access to a tool like MATLAB or python could take this raw data and produce a 3D rendering of the height-map, with very little extra effort, to see whether the data voids are near the center of the volcano's peak.
- For the record, I like flying near mountains, and so I am always concerned with how tall the rocks go. I have noticed that geological surveys, SRTM data, and aeronautical charts often disagree, sometimes by scary amounts of meters.
- The moral of this story is, when you want to measure mountains with meter accuracy, you need to start throwing around some really heavy-hitting science and technology: the final data product is way hard to interpret. "Meters above sea level" is a surprisingly abstract measure! This is why, when really smart scientists get together in a room together and decide how tall a mountain is, the most minute disagreement over the subtle details about the definition of the measurement will yield a variation of hundreds of meters. That doesn't necessarily mean the data is wrong or imprecise: it's just that you have to be very careful when you specify what you're measuring.
- If you fly an airplane and want to stay at least a few meters away from the ground, you need to make sure you're measuring your distance from the rocks the same way as the scientists who made your map!
- Nimur (talk) 13:42, 4 May 2017 (UTC)
- Well, this is embarrassing - everything in the southern hemisphere is backwards - so we actually want data file the data file for S19W069 or the data file for S19W070 (... latitude goes in descending order down south...). I'm pretty sure that I got the right data this time!
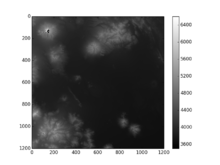

...the really real location, I think... - I got 6549 meters this time around (S19W069), and 6332 meters (S19W070), which both seem more reasonable; and I still got a bunch of data voids near the peak.
- So: I was incorrect in my first values - this is one among the many reasons I ought to know better than to post unreviewed data interpretations before breakfast. Nonetheless, some of my original points still stand: the SRTM data is really high-quality data, and it tells us a value that is potentially lots of meters different from other published data sources! The methodology we use to measure mountains can make a huge difference in the heights we report.
- Nimur (talk) 14:42, 4 May 2017 (UTC)
- Probably out of my depth here, but given that it's a volcano, and from a picture in its article appears to have retained a central, fairly circular crater, presumably there may be multiple locally highest points on the crater rim rather there being one at its centre – might this be causing difficulties with the measurements? You seem to hint at this above without saying so explicitly. {The poster formerly known as 87.81.230.195} 2.122.60.183 (talk) 15:01, 5 May 2017 (UTC)
- I think there probably is one single high point on the rim. Problem is that different people are giving different heights. Jo-Jo Eumerus (talk, contributions) 15:06, 5 May 2017 (UTC)
- How are they measuring the height? Barometric altimetry? Satellite RADAR? GPS? Complicated land-surveying and lots of trigonometry? Let's track down a specific source, and we can help you decipher the way they do their measurements. Are you looking at the data cited in our article? Nimur (talk) 15:46, 6 May 2017 (UTC)
- No idea. My impression is that mountaineers measure them with barometric methods and topographers from satellite images. I am in part asking because I was expanding the article and was not sure which height to cite. Jo-Jo Eumerus (talk, contributions) 19:48, 6 May 2017 (UTC)
- The infobox cites Wörner et al, The Nevados de Payachata volcanic region... (1990); but that author gives 6348 m - not 6380 m as listed in our info box! Further, he doesn't specify where that number comes from. But he does write a lot about the volcano's geology!
- On geological time-scales, a few meters here and there won't matter: mountains change, by erosion and orogeny and geologic uplift and glaciation and glacier loading and glacial eroding and cone collapse and ash accumulation and tephra build-up and lava flows, ... so, what's a few meters up and down?
- Nimur (talk) 12:33, 7 May 2017 (UTC)
- Um, it actually cites Davidson et al. 1990. Jo-Jo Eumerus (talk, contributions) 18:25, 7 May 2017 (UTC)
- Same author, different paper! Davidson is the third author on the one I've been reading, Wörner et al. So, I guess in the second paper, they amended the reported altitude? Nimur (talk) 02:12, 8 May 2017 (UTC)
- Um, it actually cites Davidson et al. 1990. Jo-Jo Eumerus (talk, contributions) 18:25, 7 May 2017 (UTC)
- No idea. My impression is that mountaineers measure them with barometric methods and topographers from satellite images. I am in part asking because I was expanding the article and was not sure which height to cite. Jo-Jo Eumerus (talk, contributions) 19:48, 6 May 2017 (UTC)
- How are they measuring the height? Barometric altimetry? Satellite RADAR? GPS? Complicated land-surveying and lots of trigonometry? Let's track down a specific source, and we can help you decipher the way they do their measurements. Are you looking at the data cited in our article? Nimur (talk) 15:46, 6 May 2017 (UTC)
- I think there probably is one single high point on the rim. Problem is that different people are giving different heights. Jo-Jo Eumerus (talk, contributions) 15:06, 5 May 2017 (UTC)
- Probably out of my depth here, but given that it's a volcano, and from a picture in its article appears to have retained a central, fairly circular crater, presumably there may be multiple locally highest points on the crater rim rather there being one at its centre – might this be causing difficulties with the measurements? You seem to hint at this above without saying so explicitly. {The poster formerly known as 87.81.230.195} 2.122.60.183 (talk) 15:01, 5 May 2017 (UTC)





