Osteogenesis imperfecta: Difference between revisions
Psiĥedelisto (talk | contribs) onset is always at birth, not just "usually", this being a genetic disorder |
Psiĥedelisto (talk | contribs) →Genetics: add a cite, clarify that 90% of people with OI don't have _two_ mutations, because OI type I, the most common form, is most often a null allele in COL1A1…but rather it's just a bad translation from an article originally published in Portuguese which means "or" not "and" |
||
| Line 212: | Line 212: | ||
== Genetics == |
== Genetics == |
||
[[File:COL1A1 protein - PDB rendering based on 1y0f.jpg|thumb|An [[Collagen, type I, alpha 1|α1 type I collagen]] [[protein]]]] |
[[File:COL1A1 protein - PDB rendering based on 1y0f.jpg|thumb|An [[Collagen, type I, alpha 1|α1 type I collagen]] [[protein]]]] |
||
Osteogenesis imperfecta is a genetic |
Osteogenesis imperfecta is a group of genetic disorders, all of which cause bone fragility. OI has high [[genetic heterogeneity]], that is, many different genetic mutations can lead to the same or similar sets of observable symptoms ([[phenotypes]]).<ref>{{Cite journal|display-authors=6|vauthors=Duy BH, Zhytnik L, Maasalu K, Kändla I, Prans E, Reimann E, Märtson A, Köks S|date=2016-12-01|title=Mutation analysis of the ''COL1A1'' and ''COL1A2'' genes in Vietnamese patients with osteogenesis imperfecta|url=http://humgenomics.biomedcentral.com/articles/10.1186/s40246-016-0083-1|journal=Human Genomics|language=en|volume=10|issue=1|pages=27|doi=10.1186/s40246-016-0083-1|issn=1479-7364|pmc=4983065|pmid=27519266}}</ref> |
||
The main causes for developing the disorder are a result of mutations in the ''COL1A1'' and/or ''COL1A2'' genes which are jointly responsible for the production of [[collagen type I]].<ref>{{cite journal | vauthors = Palomo T, Vilaça T, Lazaretti-Castro M | title = Osteogenesis imperfecta: diagnosis and treatment | journal = Current Opinion in Endocrinology, Diabetes, and Obesity | volume = 24 | issue = 6 | pages = 381–388 | date = December 2017 | pmid = 28863000 | doi = 10.1097/MED.0000000000000367 | s2cid = 4555427 }}</ref> Approximately 90% of people with OI are [[heterozygous]] for mutations in either the ''COL1A1'' or ''COL1A2'' genes.<ref>{{cite journal | vauthors = Valadares ER, Carneiro TB, Santos PM, Oliveira AC, Zabel B | title = What is new in genetics and osteogenesis imperfecta classification? | journal = Jornal de Pediatria | volume = 90 | issue = 6 | pages = 536–41 | date = 18 July 2014 | pmid = 25046257 | doi = 10.1016/j.jped.2014.05.003 | doi-access = free }}</ref> There are several biological factors that are results of the dominant form of OI. These factors include: intracellular stress; abnormal tissue mineralization; abnormal cell to cell interactions; abnormal cell-[[Extracellular matrix|matrix]] interactions; a compromised cell matrix structure; and, abnormal interaction between non-collagenous [[proteins]] and collagen.<ref>{{cite journal | vauthors = Forlino A, Cabral WA, Barnes AM, Marini JC | title = New perspectives on osteogenesis imperfecta | journal = Nature Reviews. Endocrinology | volume = 7 | issue = 9 | pages = 540–57 | date = June 2011 | pmid = 21670757 | pmc = 3443407 | doi = 10.1038/nrendo.2011.81 }}</ref> |
|||
Previous research lead to the belief that OI was an [[autosomal dominant]] disorder with few other variations in genomes.<ref name=":1">{{cite journal | vauthors = Forlino A, Marini JC | title = Osteogenesis imperfecta | journal = Lancet | volume = 387 | issue = 10028 | pages = 1657–71 | date = April 2016 | pmid = 26542481 | pmc = 7384887 | doi = 10.1016/S0140-6736(15)00728-X }}</ref> However, with the lowering of the cost of [[DNA sequencing]] in the wake of 2003's [[Human Genome Project]],<ref>{{Cite journal|vauthors=Koromani F, Trajanoska K, Rivadeneira F, Oei L|date=2019-06-04|title=Recent Advances in the Genetics of Fractures in Osteoporosis|url=https://www.frontiersin.org/articles/10.3389/fendo.2019.00337/full|journal=Frontiers in Endocrinology|language=English|volume=|doi=10.3389/fendo.2019.00337|issn=1664-2392|pmc=6559287|pmid=31231309}}</ref> autosomal recessive forms of the disorder have been identified.<ref>{{cite journal | vauthors = Drögemüller C, Becker D, Brunner A, Haase B, Kircher P, Seeliger F, Fehr M, Baumann U, Lindblad-Toh K, Leeb T | display-authors = 6 | title = A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta | journal = PLOS Genetics | volume = 5 | issue = 7 | pages = e1000579 | date = July 2009 | pmid = 19629171 | pmc = 2708911 | doi = 10.1371/journal.pgen.1000579 | veditors = Barsh GS }}</ref> Recessive forms of OI relate heavily to defects in the collagen chaperones responsible for production of [[procollagen]] and the assembly of the related proteins.<ref>{{cite journal | vauthors = Rohrbach M, Giunta C | title = Recessive osteogenesis imperfecta: clinical, radiological, and molecular findings | journal = American Journal of Medical Genetics. Part C, Seminars in Medical Genetics | volume = 160C | issue = 3 | pages = 175–89 | date = August 2012 | pmid = 22791419 | doi = 10.1002/ajmg.c.31334 | s2cid = 28592112 }}</ref> Examples of [[Chaperone (protein)|collagen chaperones]] that are defective in patients with recessive forms of OI include [[Heat shock protein 47|chaperone HSP47]] ([[Cole-Carpenter syndrome]]) and [[FKBP65]].<ref name=":7">{{cite journal|vauthors=Marini JC, Blissett AR|date=August 2013|title=New genes in bone development: what's new in osteogenesis imperfecta|url=https://academic.oup.com/jcem/article/98/8/3095/2833222|journal=The Journal of Clinical Endocrinology and Metabolism|volume=98|issue=8|pages=3095–103|doi=10.1210/jc.2013-1505|pmc=3733862|pmid=23771926}}</ref> Mutations in these chaperones result in an improper [[Protein folding|folding]] pattern in the collagen 1 proteins which causes the recessive form of the disorder.<ref name=":7" /> There are three significant types of OI that are a result of mutations in the collagen [[Leprecan|prolyl 3-hydroxylation complex]] (components CRTAP, P3H1, and CyPB).<ref name=":7" /> These components are responsible for the modification of collagen α1(l)Pro986.<ref name=":7" /> Mutations in other genes such as ''SP7'', ''SERPINF1'', ''[[TMEM38B]]'' and ''BMP1'' can also lead to irregularly formed proteins and [[enzymes]] that result in other recessive types of osteogenesis imperfecta.<ref name=":7" /> |
Previous research lead to the belief that OI was an [[autosomal dominant]] disorder with few other variations in genomes.<ref name=":1">{{cite journal | vauthors = Forlino A, Marini JC | title = Osteogenesis imperfecta | journal = Lancet | volume = 387 | issue = 10028 | pages = 1657–71 | date = April 2016 | pmid = 26542481 | pmc = 7384887 | doi = 10.1016/S0140-6736(15)00728-X }}</ref> However, with the lowering of the cost of [[DNA sequencing]] in the wake of 2003's [[Human Genome Project]],<ref>{{Cite journal|vauthors=Koromani F, Trajanoska K, Rivadeneira F, Oei L|date=2019-06-04|title=Recent Advances in the Genetics of Fractures in Osteoporosis|url=https://www.frontiersin.org/articles/10.3389/fendo.2019.00337/full|journal=Frontiers in Endocrinology|language=English|volume=|doi=10.3389/fendo.2019.00337|issn=1664-2392|pmc=6559287|pmid=31231309}}</ref> autosomal recessive forms of the disorder have been identified.<ref>{{cite journal | vauthors = Drögemüller C, Becker D, Brunner A, Haase B, Kircher P, Seeliger F, Fehr M, Baumann U, Lindblad-Toh K, Leeb T | display-authors = 6 | title = A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta | journal = PLOS Genetics | volume = 5 | issue = 7 | pages = e1000579 | date = July 2009 | pmid = 19629171 | pmc = 2708911 | doi = 10.1371/journal.pgen.1000579 | veditors = Barsh GS }}</ref> Recessive forms of OI relate heavily to defects in the collagen chaperones responsible for production of [[procollagen]] and the assembly of the related proteins.<ref>{{cite journal | vauthors = Rohrbach M, Giunta C | title = Recessive osteogenesis imperfecta: clinical, radiological, and molecular findings | journal = American Journal of Medical Genetics. Part C, Seminars in Medical Genetics | volume = 160C | issue = 3 | pages = 175–89 | date = August 2012 | pmid = 22791419 | doi = 10.1002/ajmg.c.31334 | s2cid = 28592112 }}</ref> Examples of [[Chaperone (protein)|collagen chaperones]] that are defective in patients with recessive forms of OI include [[Heat shock protein 47|chaperone HSP47]] ([[Cole-Carpenter syndrome]]) and [[FKBP65]].<ref name=":7">{{cite journal|vauthors=Marini JC, Blissett AR|date=August 2013|title=New genes in bone development: what's new in osteogenesis imperfecta|url=https://academic.oup.com/jcem/article/98/8/3095/2833222|journal=The Journal of Clinical Endocrinology and Metabolism|volume=98|issue=8|pages=3095–103|doi=10.1210/jc.2013-1505|pmc=3733862|pmid=23771926}}</ref> Mutations in these chaperones result in an improper [[Protein folding|folding]] pattern in the collagen 1 proteins which causes the recessive form of the disorder.<ref name=":7" /> There are three significant types of OI that are a result of mutations in the collagen [[Leprecan|prolyl 3-hydroxylation complex]] (components CRTAP, P3H1, and CyPB).<ref name=":7" /> These components are responsible for the modification of collagen α1(l)Pro986.<ref name=":7" /> Mutations in other genes such as ''SP7'', ''SERPINF1'', ''[[TMEM38B]]'' and ''BMP1'' can also lead to irregularly formed proteins and [[enzymes]] that result in other recessive types of osteogenesis imperfecta.<ref name=":7" /> |
||
Revision as of 17:42, 18 August 2021
| Osteogenesis imperfecta | |
|---|---|
| Other names | Brittle bone disease,[1] Lobstein syndrome,[2] fragilitas ossium,[1] Vrolik disease,[1] osteopsathyrosis, Porak disease, Durante disease[3] |
 | |
| Blue sclerae, a classic symptom of most types of osteogenesis imperfecta | |
| Specialty | Pediatrics, medical genetics, osteology |
| Symptoms | Bones that break easily, blue tinge to the whites of the eye, short height, loose joints, hearing loss[1][4] |
| Onset | Birth[4] |
| Duration | Long term[4] |
| Causes | Genetic (autosomal dominant or de novo mutation)[1] |
| Diagnostic method | Based on symptoms, DNA testing[4] |
| Prevention | Pre-implantation genetic diagnosis |
| Treatment | Healthy lifestyle (exercise, no smoking), metal rods through the long bones[5] |
| Medication | Bisphosphonates[6] |
| Prognosis | Depends on the type[4] |
| Frequency | 1 in 10,000–20,000 people[1] |
Osteogenesis imperfecta (OI), also known as brittle bone disease, is a group of genetic disorders that mainly affect the bones.[1][7] It results in bones that break easily.[1] The range of symptoms may be mild to severe.[1] Symptoms found in various types of OI include a blue tinge to the whites of the eye (sclerae), short stature, loose joints, hearing loss, breathing problems[1][4] and problems with the teeth (dentinogenesis imperfecta).[8] Potentially life threatening complications include cervical artery dissection and aortic dissection.[9][10]
The underlying mechanism is usually a problem with connective tissue due to a lack of, or poorly formed, type I collagen.[1] In more than 90% of cases, OI occurs due to mutations in the COL1A1 or COL1A2 genes.[1] These genetic problems are often inherited from a person's parents in an autosomal dominant manner, but may also occur via a new mutation—de novo.[1] There are four main types, with type I being the least severe and type II the most severe.[1] As of 2020, eighteen different genes are known to cause the twenty documented types of OI.[11] Diagnosis is often based on symptoms and may be confirmed by a collagen biopsy and/or a DNA test.[4]
There is no cure.[4] Maintaining a healthy lifestyle by exercising and avoiding smoking can help prevent fractures.[5] Treatment may include acute care of broken bones, pain medication, physical therapy, mobility aids such as leg braces or wheelchairs, and rodding surgery,[5] a type of surgery that puts metal intramedullary rods along the long bones (such as the femur) in an attempt to strengthen them.[5] Evidence also supports the use of medications of the bisphosphonate class, such as pamidronate, to increase bone density.[12] Bisphosphonates are especially effective in children,[13] however it is unclear if they lead to increases in quality of life or decrease the incidence of fractures.[6]
OI affects about one in 10,000 to 20,000 people.[1] Outcomes depend on the genetic cause of the disorder (its type), but death during childhood from it is rare.[4] Moderate to severe OI primarily affects mobility; if rodding surgery is performed during childhood, those with more severe types of OI can gain the ability to walk.[14] The condition has been described since ancient history.[15] The Latinate term "osteogenesis imperfecta" came into use in 1895 and literally translates to "imperfect bone formation".[1][15]
Signs and symptoms
Orthopedic
The main symptom of OI is fragile, low mineral density bones; all types of OI have some bone involvement.[8] In moderate and especially severe OI, the long bones may be bowed, sometimes extremely so.[16] The weakness of the bones causes them to fracture easily; a study in Pakistan found an average of 5.8 fractures per year in untreated children.[17] Fractures typically occur much less after puberty, but may increase again in some after the fifth decade of life.[18]
Joint hypermobility is also a common sign of OI, thought to be because the affected genes are the same as those that cause some types of Ehlers–Danlos syndrome.[8]: 1513 (One OI mutation also causes combined Ehler–Danlos syndrome: "OIEDS1".)[19][20]
Hearing
By the age of 50, about 50% of adults with OI experience significant hearing loss, much earlier hearing loss as compared to the general population.[21] Hearing loss in OI may or may not be associated with visible deformities of the ossicles and inner ear.[22] Hearing loss frequently begins during the second, third, and fourth decades of life, and may be conductive, sensorineural, or mixed in nature.[23] If hearing loss does not occur by age 50, it is significantly less likely to occur in the years afterwards.[21]
Although rare, OI-related hearing loss can also begin in childhood; in a study of forty-five children aged four to sixteen, two were found to be affected, aged 11 and 15.[24]
Neurological
OI is associated with a number of neurological abnormalities, usually involving the central nervous system, due to deformation of the skeletal structures enveloping it. Neurological complications may adversely affect life expectancy, and neurosurgical intervention may be needed to correct severe complications.[25]
Gastrointestinal
OI may be associated with recurrent abdominal pain and chronic constipation, according to two studies on subjects affected by OI.[26][27][28]
Classification
There are two typing systems for OI in modern use. The first, created by David Sillence in 1979, classifies patients into four types, or syndromes, according to their clinical presentation, without taking into account the genetic cause of their disease.[29]: 114–115 The second system expands on the Sillence model, but assigns new numbered types genetically as they are found.[30] Therefore, people with OI can be described as having both a clinical type and a genetic type, which may or may not be equivalent.
Type I is the most common, and 90% of cases result from mutations to either COL1A1 or COL1A2.[1] Symptoms vary a lot between types, as well as vary from person to person, even in the same family.[31]
As of 2020, 20 genetic types of OI have been discovered:[11]
| Type | Description | Gene | OMIM | Mode of inheritance | Incidence |
|---|---|---|---|---|---|
| I | mild | Null COL1A1 allele | 166200 | autosomal dominant, 60% de novo[32] | 1 in 30,000[33] |
| II | severe and usually lethal in the perinatal period | COL1A1, COL1A2 | 166210 (IIA), 610854 (IIB) | autosomal dominant, ~100% de novo[32] | 1 in 40,000[34] to 1 in 100,000[33] |
| III | considered progressive and deforming | COL1A1, COL1A2 | 259420 | autosomal dominant, ~100% de novo[32] | 1 in 60,000[33] |
| IV | deforming, but with normal sclerae most of the time | COL1A1, COL1A2 | 166220 | autosomal dominant, 60% de novo[32] | |
| V | shares the same clinical features of IV, but has unique histologic findings ("mesh-like") | IFITM5 | 610967 | autosomal dominant[32][35] | |
| VI | shares the same clinical features of IV, but has unique histologic findings ("fish scale") | SERPINF1 | 610968 | autosomal recessive[32] | |
| VII | associated with cartilage associated protein | CRTAP | 610682 | autosomal recessive[32] | |
| VIII | severe to lethal, associated with the protein leprecan | LEPRE1 | 610915 | autosomal recessive | |
| IX | normal collagen but still severe, sometimes fatal, disease | PPIB | 259440 | autosomal recessive | |
| X | causes defects in the collagen chaperone protein HSP47 and severe disease | SERPINH1 | 613848 | autosomal recessive | |
| XI | clinically III, associated with the protein FKBP65 | FKBP10 | 610968 | autosomal recessive | |
| XII | causes hearing loss in most affected, related to a faulty osteoblast transcription factor | SP7 | 613849 | autosomal recessive | |
| XIII | moderate, related to faulty processing of collagen | BMP1 | 614856 | autosomal recessive | |
| XIV | of variable severity | TMEM38B | 615066 | autosomal recessive | |
| XV | clinically III, disrupts anabolic signaling of osteoblast cells | WNT1 | 615220 | autosomal recessive | |
| XVI | severe, sometimes lethal; causes missing transcription factor | CREB3L1 | 616229 | autosomal recessive | |
| XVII | severe, causes defective glycoprotein which binds to collagen | SPARC | 616507 | autosomal recessive | |
| XVIII | moderate, causes defects in FAM46A protein | TENT5A | 617952 | autosomal recessive | |
| XIX | moderate/severe, causes defective regulated intramembrane proteolysis | MBTPS2 | 301014 | X-linked recessive | |
| XX | clinically III, disrupts WNT signaling | MESD | 618644 | autosomal recessive |
- Type I
Collagen is of normal quality but is produced in insufficient quantities.[8]: 1516 Bones fracture more easily than in the general public, but not as easily as more severe types of OI; there might be scoliosis, albeit mild, with a low Cobb angle; the joints may be loose; blue sclerae may be apparent; hearing loss may occur; and, finally, there might be a slight decrease in height. Because cases exist missing one or more of these symptoms, OI type I in some cases goes undetected into adulthood.[8]: 1513–1514
IA and IB are defined to be distinguished by the absence or presence of dentinogenesis imperfecta respectively (characterized by opalescent teeth).[36]: 217 People with type I generally have a normal lifespan.[37]
- Type II
Collagen is not of a sufficient quality or quantity.[citation needed]
- Most cases result in death within the first year of life due to respiratory failure or intracerebral hemorrhage
- Severe respiratory problems due to underdeveloped lungs
- Severe bone deformity and small stature
Type II can be further subclassified into groups A, B, and C, which are distinguished by radiographic evaluation of the long bones and ribs. Type IIA demonstrates broad and short long bones with broad and beaded ribs. Type IIB demonstrates broad and short long bones with thin ribs that have little or no beading. Type IIC demonstrates thin and longer long bones with thin and beaded ribs.[citation needed]
- Type III
Collagen improperly formed, enough collagen is made but it is defective.[citation needed]
- Bones fracture easily, sometimes even before birth
- Bone deformity, often severe
- Respiratory problems possible
- Short stature, spinal curvature and sometimes barrel-shaped rib cage
- Triangular face[38]
- Loose joints (double-jointed)
- Poor muscle tone in arms and legs
- Discolouration of the sclera (the 'whites' of the eyes are blue)
- Early loss of hearing possible
Type III is distinguished among the other classifications as being the "progressive deforming" type, wherein a neonate presents with mild symptoms at birth and develops the aforementioned symptoms throughout life. Lifespans may be normal, albeit with severe physical handicapping.[citation needed]
- Type IV
Collagen quantity is sufficient, but is not of a high enough quality
- Bones fracture easily, especially before puberty
- Short stature, spinal curvature, and barrel-shaped rib cage
- Bone deformity is mild to moderate
- Early loss of hearing
Similar to type I, type IV can be further subclassified into types IVA and IVB characterized by absence (IVA) or presence (IVB) of dentinogenesis imperfecta.[36]: 217
- Type V

Having the same clinical features as type IV, it is distinguished histologically by "mesh-like" bone appearance. Further characterized by the "V triad" consisting of (a) radio-opaque band adjacent to growth plates, (b) hypertrophic calluses at fracture sites, and (c) calcification of the radio-ulnar interosseous membrane.[39]
OI type V can lead to calcification of the membrane between the two forearm bones, making it difficult to turn the wrist. Another symptom is abnormally large amounts of repair tissue (hyperplasic callus) at the site of fractures. Other features of this condition include radial head dislocation, long bone bowing, and mixed hearing loss.[40]
Cases of this type are caused by mutations in the IFITM5 gene.[40][35]
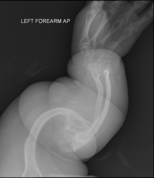
-
OI type V in an adult
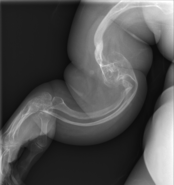
-
OI type V in a child
- Type VI
With the same clinical features as type IV, it is distinguished histologically by "fish-scale" bone appearance. Type VI has recently been found to be caused by a loss of function mutation in the SERPINF1 gene. SERPINF1, a member of the serpin family, is also known as pigment epithelium-derived factor (PEDF), the most powerful endogenous antiangiogenic factor in mammals.[citation needed]
- Type VII
In 2006, a recessive form called "type VII" was discovered (phenotype severe to lethal). Thus far it seems to be limited to a First Nations people in Quebec.[41] Mutations in the gene CRTAP causes this type.[42]
- Type VIII
OI caused by mutation in the gene LEPRE1 is classified as type VIII.[42]
- Type IX
Osteogenesis imperfecta type IX (OI9) is caused by homozygous or compound heterozygous mutation in the PPIB gene on chromosome 15q22.[43]
- Type X
Caused by homozygous mutation in the SERPINH gene on chromosome 11q13.[44]
- Type XI
OI caused by mutations in FKBP10 on chromosome 17q21.[45] The mutations cause a decrease in secretion of trimeric procollagen molecules. These mutations can also cause autosomal recessive Bruck syndrome which is similar to OI.
- Type XII
OI caused by a frameshift mutation in SP7. This mutation causes bone deformities, fractures, and delayed tooth eruption.[46]
- Type XIII
OI caused by a mutation in the bone morphogenic protein 1 (BMP1) gene.[47][48] This mutation causes recurrent fractures, high bone mass, and hyperextensive joints.[49]
- Type XIV
OI caused by mutations in the TMEM38B gene. This mutation causes recurrent fractures and osteopenia.
- Type XV
OI caused by homozygous or compound heterozygous mutations in the WNT1 gene on chromosome 12q13. It is autosomal recessive.[50][49]
- Type XVI
OI caused by mutations in the CREB3L1 gene. This mutation causes prenatal onset of recurrent fractures of the ribs and long bones, demineralization, decreased ossification of the skull, and blue sclerae. Family members who are heterozygous for OI XVI may have recurrent fractures, osteopenia and blue sclerae.[50]
- Type XVII
OI caused by homozygous mutation in the SPARC gene on chromosome 5q33.
- Type XVIII
OI caused by homozygous mutation in the FAM46A gene on chromosome 6q14. Characterized by congenital bowing of the long bones, wormian bones, blue sclerae, vertebral collapse, and multiple fractures in the first years of life.[51]
- Others
A family with recessive osteogenesis imperfecta has been reported to have a mutation in the TMEM38B gene on chromosome 9.[52] This gene encodes TRIC-B, a component of TRIC, a monovalent cation-specific channel involved in calcium release from intracellular stores.
It is extremely likely that there are other genes associated with this disease that have yet to be reported.
Genetics

Osteogenesis imperfecta is a group of genetic disorders, all of which cause bone fragility. OI has high genetic heterogeneity, that is, many different genetic mutations can lead to the same or similar sets of observable symptoms (phenotypes).[53]
The main causes for developing the disorder are a result of mutations in the COL1A1 and/or COL1A2 genes which are jointly responsible for the production of collagen type I.[54] Approximately 90% of people with OI are heterozygous for mutations in either the COL1A1 or COL1A2 genes.[55] There are several biological factors that are results of the dominant form of OI. These factors include: intracellular stress; abnormal tissue mineralization; abnormal cell to cell interactions; abnormal cell-matrix interactions; a compromised cell matrix structure; and, abnormal interaction between non-collagenous proteins and collagen.[56]
Previous research lead to the belief that OI was an autosomal dominant disorder with few other variations in genomes.[57] However, with the lowering of the cost of DNA sequencing in the wake of 2003's Human Genome Project,[58] autosomal recessive forms of the disorder have been identified.[59] Recessive forms of OI relate heavily to defects in the collagen chaperones responsible for production of procollagen and the assembly of the related proteins.[60] Examples of collagen chaperones that are defective in patients with recessive forms of OI include chaperone HSP47 (Cole-Carpenter syndrome) and FKBP65.[30] Mutations in these chaperones result in an improper folding pattern in the collagen 1 proteins which causes the recessive form of the disorder.[30] There are three significant types of OI that are a result of mutations in the collagen prolyl 3-hydroxylation complex (components CRTAP, P3H1, and CyPB).[30] These components are responsible for the modification of collagen α1(l)Pro986.[30] Mutations in other genes such as SP7, SERPINF1, TMEM38B and BMP1 can also lead to irregularly formed proteins and enzymes that result in other recessive types of osteogenesis imperfecta.[30]
Defects in the proteins pigment epithelium-derived factor (PEDF) and bone-restricted interferon-induced transmembrane protein (BRIL) are the causes of type V and VI osteogenesis imperfecta.[61] Defects in these proteins lead to defective bone mineralization which causes the characteristic brittle bones of osteogenesis imperfecta.[61] A single point mutation in the untranslated 5' (5'-UTR) region of the IFITM5 gene, which encodes BRIL, is linked directly to OI type V.[40][62]
In the rare case of type XIX, first discovered in 2016, OI is inherited as an X-linked genetic disorder, with its detrimental effects resulting ultimately from a mutation in the gene MBTPS2.[63] Genetic research is ongoing, and it is uncertain when all the genetic causes of OI will be identified, as the number of genes that need to be tested to rule out the disorder continue to increase.
Pathophysiology
People with OI are born with defective connective tissue, or without the ability to make it, usually because of a deficiency of type-I collagen.[64] This deficiency arises from an amino acid substitution of glycine to bulkier amino acids in the collagen triple helix structure. The larger amino acid side-chains create steric hindrance that creates a bulge in the collagen complex, which in turn influences both the molecular nanomechanics and the interaction between molecules, which are both compromised.[65] As a result, the body may respond by hydrolyzing the improper collagen structure. If the body does not destroy the improper collagen, the relationship between the collagen fibrils and hydroxyapatite crystals to form bone is altered, causing brittleness.[66] Another suggested disease mechanism is that the stress state within collagen fibrils is altered at the locations of mutations, where locally larger shear forces lead to rapid failure of fibrils even at moderate loads as the homogeneous stress state found in healthy collagen fibrils is lost.[65] These recent works suggest that OI must be understood as a multi-scale phenomenon, which involves mechanisms at the genetic, nano-, micro- and macro-level of tissues.
Most people with OI receive it from a parent, but in many cases it is a brand new (de novo or "sporadic") mutation in a family. Among a study of patients with survivable types of OI, OI type III is most often de novo (85%), followed by type IV (50%) and type I (34%).[67]: Table 1
Diagnosis

Diagnosis is typically based on medical imaging, including plain X-rays, and symptoms. Signs on medical imaging include abnormalities in all extremeties and the spine.[68]
An OI diagnosis can be confirmed through DNA or collagen testing, but in many cases, the occurrence of bone fractures with little trauma and the presence of other clinical features such as blue sclerae are sufficient for a diagnosis. A skin biopsy can be performed to determine the structure and quantity of type I collagen. DNA testing can confirm the diagnosis, however, it cannot exclude it because not all mutations causing OI are yet known and/or tested for. OI type II is often diagnosed by ultrasound during pregnancy, where already multiple fractures and other characteristic features may be visible. Relative to control, OI cortical bone shows increased porosity, canal diameter, and connectivity in micro-computed tomography.[69] Severe types of OI can usually be detected before birth by using an in vitro genetic testing technique.[70]
Genetic testing
In order to determine whether osteogenesis imperfecta is present, genetic sequencing of the most common problematic genes, COL1A1, COL1A2, and IFITM5, may be done;[71][72] if no mutation is found yet OI is still suspected, the other 10+ genes known to cause OI may be tested.[11] Duplication and deletion testing is also suggested to parents who suspect their child has OI.[71] The presence of frameshift mutations caused by duplications and deletions is generally the cause of increased severity within the disease.[71]
Differential diagnosis
An important differential diagnosis of OI is child abuse, as both may present with multiple fractures in various stages of healing.[8]: 1514 Differentiating them can be difficult, especially when no other characteristic features of OI are present. Other differential diagnoses include rickets, osteomalacia, and other rare skeletal syndromes.[8]: 1513
Treatment
There is no cure for osteogenesis imperfecta.[4] Maintaining a healthy lifestyle by exercising and avoiding smoking can help prevent fractures. Treatment may include care of broken bones, pain medication, physical therapy, braces or wheelchairs, and surgery. A type of surgery that puts metal rods through long bones may be done to strengthen them.[5]
Bone infections are treated as and when they occur with the appropriate antibiotics and antiseptics.
Bisphosphonates
In 1998, a clinical trial demonstrated the effectiveness of intravenous pamidronate, a bisphosphonate which had previously been used in adults to treat osteoporosis. In severe OI, pamidronate reduced bone pain, prevented new vertebral fractures, reshaped previously fractured vertebral bodies, and reduced the number of long-bone fractures.[73]
Although oral bisphosphonates are more convenient and cheaper, they are not absorbed as well, and intravenous bisphosphonates are generally more effective, although this is under study. Some studies have found oral and intravenous bisphosphonates, such as oral alendronate and intravenous pamidronate, equivalent.[74] In a trial of children with mild OI, oral risedronate increased bone mineral densities, and reduced nonvertebral fractures. However, it did not decrease new vertebral fractures.[75][76] A Cochrane review in 2016 concluded that though bisphosphonates seem to improve bone mineral density, it is uncertain whether this leads to a reduction in fractures or an improvement in the quality of life of individuals with osteogenesis imperfecta.[6]
Bisphosphonates are less effective for OI in adults.[13]
Surgery
Rodding
Metal rods can be surgically inserted in the long bones to improve strength, a procedure developed by Harold A. Sofield at Shriners Hospitals for Children in Chicago. During the late 1940s, Sofield, Chief of Staff at Shriners Hospitals in Chicago, worked there with large numbers of children with OI and experimented with various methods to strengthen the bones in these children.[77] In 1959, with Edward A. Miller, Sofield wrote a seminal article describing a solution that seemed radical at the time: breaking the bones ("fragmentation"), placing the broken bone fragments in a straight line ("realignment"), then placing stainless steel rods into the intramedullary canals of the long bones to stabilize and strengthen them ("rod fixation").[78] His treatment proved extremely useful for increasing the mobility of people with OI, and it has been adopted throughout the world and now forms the standard for surgical treatments for OI.
Rodding surgery is often done to increase mobility, and perhaps offer a path to walking, to patients with severe OI. A 2020 review in The Journal of Bone and Joint Surgery (JB&JS) found that around ⅔ of people with OI types III and IV (severe OI) have undergone some form of rodding surgery in their lives, at a mean age of 4⅒ and 7½ years respectively;[14]: Table I one possible explanation for this is that one half of children with type III could not walk at all without the surgery, as their limbs were more bowed, so required it earlier.[14]
In those with type III OI who had undergone rodding surgery, 79.5% had the femurs and tibias of both legs rodded.[14]: Table I The most common form of rods used are intramedullary (IM) rods, some of which, such as the Fassier–Duval IM rod, are telescoping.[79] Telescoping IM rods are widely used,[80] and the common Fassier–Duval IM rod is designed to be used to rod the femur, tibia, and humerus.[81]: 1 The surgery involves breaking the long bones in two, three, or more places, then putting the rod alongside the bone to keep it straight.[81]: 11
While telescoping IM rods are intended to grow along with both the femur and tibia in developing children; surgeons have a preference to use non-telescoping IM rods, such as Rush rods, in the tibia, which grows less comparatively—the JB&JS review found that while 69.7% of femurs were treated with telescoping IM rods, only 36.9% of tibiae were.[14]: Table IV
While the review in the JB&JS was able to correlate receiving rodding surgery with greater mobility across all types of OI, in patients with type IV, the surgery did not decrease the incidence of broken bones as compared to non-rodded patients—while type IV patients with rodded tibiae experienced 0.93 tibia fractures per year, patients with natural tibiae experienced only 0.81. However, in patients with type III, rodding surgery decreased the average number of tibia fractures per year from 0.84 to 0.57.[14]: Table V
Spinal
Spinal fusion can be performed to prevent or correct scoliosis, although the inherent bone fragility makes this operation more complex in OI patients than it does with patients who have adolescent idiopathic scoliosis.[82] Despite the risks, however, three Nemours–duPont orthopedic surgeons who specialize in the treatment of osteogenesis imperfecta recommend operating if the curve is greater than 50° after a child is past peak height velocity, as the spine's curve can continue to worsen even into adulthood.[83]: 104
Due to the risk involved, the same surgeons recommend that surgery for basilar impressions and basilar invaginations should only be carried out if the pressure being exerted on the spinal cord and brain stem is causing actual neurological symptoms.[83]: 106–107
Physiotherapy
Physiotherapy is used to strengthen muscles and improve motility in a gentle manner, while minimizing the risk of fracture. This often involves hydrotherapy, light resistance exercises, and the use of support cushions to improve posture. Individuals are encouraged to change positions regularly throughout the day to balance the muscles being used and the bones under pressure.
Exercise is generally recommended.[84]
Physical aids
With adaptive equipment such as crutches, motorized wheelchairs, splints, reach extenders, or modifications to the home, many individuals with OI can maintain a significant degree of autonomy.
Teeth
More than 1 in 2 people with OI also have dentinogenesis imperfecta (DI) - a congenital disorder of formation of dentine.[85] Dental treatment may pose as a challenge as a result of the various deformities, skeletal and dental, due to OI. Children with OI should go for a dental check-up as soon as their teeth erupt; this may minimize tooth structure loss as a result of abnormal dentine, and they should be monitored regularly to preserve their teeth and oral health.[85]
Many people with OI are treated with bisphosphonates, and there are several complications with dental procedures when a person is taking BP, namely bisphosphonate-related osteonecrosis of the jaw (BRONJ). However, no report of BRONJ in either a child or adult with OI was found in a 2016 review of the safety and efficacy of bisphosphonates for OI.[6]
Epidemiology
In the United States, the incidence of osteogenesis imperfecta is estimated to be one per 20,000 live births.[86] An estimated 20,000 to 50,000 people are affected by OI in the United States. The most common types are I, II, III and IV, while the rest are very rare.[87]
Frequency is approximately the same across groups, but for unknown reasons, the Shona and Ndebele of Zimbabwe seem to have a higher proportion of Type III to Type I than other groups.[88]
History
The condition, or types of it, has had various other names over the years and in different nations. Among some of the most common alternatives are Ekman-Lobstein syndrome, Vrolik syndrome, and the colloquial glass-bone disease. The name osteogenesis imperfecta dates to at least 1895[89] and has been the usual medical term in the 20th century to present. The current four type system began with Sillence in 1979.[29] An older system deemed less severe types "osteogenesis imperfecta tarda" while more severe forms were deemed "osteogenesis imperfecta congenita."[90] As this terminology did not differentiate well between the types, and all forms of osteogenesis are congenital, this naming convention has since fallen out of favor.
The condition has been found in an ancient Egyptian mummy from 1000 BC. The Norse king Ivar the Boneless may have had this condition, as well. The earliest studies of it began in 1788 with the Swede Olof Jakob Ekman. He described the condition in his doctoral thesis and mentioned cases of it going back to 1678, all in the same family, through three generations. Ekman's description of the condition mentioned dwarfism, bone fragility, and bowing of the bones.[91] In 1831, Edmund Axmann described it in himself and two brothers.[92] Jean Lobstein first described the mild form of the condition, today known as type I, in 1833, calling it "osteopsathyrosis idiopathica".[93]: 347 Willem Vrolik did work on the condition in the 1850s.[93]: 347 The idea that the adult and newborn forms were the same came in 1897 with Martin Benno Schmidt.[92]
Society and culture
United States
The Osteogenesis Imperfecta Foundation is a voluntary national health organization in the United States dedicated to helping people cope with the problems associated with osteogenesis imperfecta. The OI Foundation's mission is to improve the quality of life for people affected by OI through research, education, awareness, and mutual support.[94]
United Kingdom
The Brittle Bone Society is a UK charity that supports people with the condition.
Australia
The OI Society of Australia was foundation was founded in 1977. The aim is to offer information about the disease, support research, and to make the public aware of those with osteogenesis Imperfecta. The foundation holds a conference every two years to discuss educational events and support Wishbone Day.[95]
Canada
The Canadian Osteogenesis Imperfecta Society was established in 1983. It is an international non-profit organization that helps with assisted living for those affected by OI. They provide emotional support for patients and families, and support Canadian medical research in the causes of OI for all types involved. This organization also keeps an up-to-date library of medical research and findings of this disease for the public.[96]
Other animals
In dogs, OI is an autosomal recessive condition, meaning that dogs with two copies of the allele will be affected.[97] Beagles, Standard Wirehaired Dachshunds, Golden Retrievers, Poodles, Bedlington Terriers, Norwegian Elkhounds, and the Standard and Miniature Smooth haired Dachshund have all been known to be possible carriers of canine OI, as well as mice and some breeds of fish.[97] In Golden Retrievers, it is caused by a mutation in the COL1A1, and in Beagles, the COL1A2. A separate mutation in the SERPINH1 gene has been found to cause the condition in Dachshunds.[98] Many breed organizations and veterinarians offer OI tests to tell if a dog is a carrier of OI. Dogs who are heterozygous for OI should only be bred to non-carriers. Homozygous carriers should never be bred, unless it is to a non-carrier.[99]
Animal models of OI are critical to the diagnosis, treatment, and possible cure for their human counterparts. The experimental treatments and therapies used on animals play an important role in the successful treatment of OI in humans.[100] Although dogs, mice, fish, and humans are not genetically identical, some animal models have been officially recognized to represent the varying types of OI in humans. Research on animals may lead to human therapies for OI.[100]
References
- ^ a b c d e f g h i j k l m n o p q "Osteogenesis imperfecta". Genetics Home Reference. U.S. National Library of Medicine, National Institutes of Health. 2020-08-18. Retrieved 2021-08-15.
{{cite web}}: CS1 maint: url-status (link) - ^ William B (2006). Andrews' Diseases of the Skin: Clinical Dermatology (10th ed.). Saunders. p. 517. ISBN 978-0721629216.
- ^ "Brittle Bone Disorder". 1996. Retrieved 6 November 2018.
- ^ a b c d e f g h i j "Osteogenesis Imperfecta Overview". NIAMS. June 2015. Archived from the original on 18 October 2016. Retrieved 15 October 2016.
- ^ a b c d e "What Is Osteogenesis Imperfecta? Fast Facts: An Easy-to-Read Series of Publications for the Public". NIAMS. November 2014. Archived from the original on 18 October 2016. Retrieved 15 October 2016.
- ^ a b c d Dwan K, Phillipi CA, Steiner RD, Basel D (October 2016). "Bisphosphonate therapy for osteogenesis imperfecta". The Cochrane Database of Systematic Reviews. 10: CD005088. doi:10.1002/14651858.CD005088.pub4. PMC 6611487. PMID 27760454 – via National Center for Biotechnology Information.
- ^ "Osteogenesis imperfecta". rarediseases.info.nih.gov. Retrieved 2018-04-17.
- ^ a b c d e f g Rowe DW (2008). "Osteogenesis imperfecta". Principles of bone biology (3rd ed.). Amsterdam: Elsevier. p. 1513. ISBN 978-0-12-373884-4. OCLC 267135745.
Dentinogenesis imperfecta (DI) is most frequent in OI types III and IV, and overall, affects about 15% of OI patients among the different phenotypes.
- ^ Grond-Ginsbach C, Debette S (March 2009). "The association of connective tissue disorders with cervical artery dissections". Current Molecular Medicine. 9 (2): 210–4. doi:10.2174/156652409787581547. PMID 19275629. S2CID 6127483.
- ^ McNeeley MF, Dontchos BN, Laflamme MA, Hubka M, Sadro CT (December 2012). "Aortic dissection in osteogenesis imperfecta: case report and review of the literature". Emergency Radiology. 19 (6): 553–6. doi:10.1007/s10140-012-1044-1. PMID 22527359. S2CID 11109481.
- ^ a b c Marom R, Rabenhorst BM, Morello R (October 2020). "Osteogenesis imperfecta: an update on clinical features and therapies". European Journal of Endocrinology. 183 (4): R95–R106. doi:10.1530/EJE-20-0299. PMC 7694877. PMID 32621590.
- ^ Harrington J, Sochett E, Howard A (December 2014). "Update on the evaluation and treatment of osteogenesis imperfecta". Pediatric Clinics of North America. 61 (6): 1243–57. doi:10.1016/j.pcl.2014.08.010. PMID 25439022.
- ^ a b Chevrel G, Schott AM, Fontanges E, Charrin JE, Lina-Granade G, Duboeuf F, et al. (February 2006). "Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3-year randomized placebo-controlled trial". Journal of Bone and Mineral Research. 21 (2): 300–6. doi:10.1359/JBMR.051015. PMID 16418786. S2CID 34089615.
- ^ a b c d e f Rodriguez Celin M, Kruger KM, Caudill A, Nagamani SC, Harris GF, Smith PA (2020-09-11). "A Multicenter Study of Intramedullary Rodding in Osteogenesis Imperfecta". JB & JS Open Access. 5 (3): e20.00031. doi:10.2106/JBJS.OA.20.00031. PMC 7489747. PMID 32984750.
- ^ a b Kelly EB (2012). Encyclopedia of Human Genetics and Disease. ABC-CLIO. p. 613. ISBN 9780313387135. Archived from the original on 2017-11-05.
- ^ Johnson MT, Morrison S, Heeger S, Mooney S, Byers PH, Robin NH (February 2002). "A variant of osteogenesis imperfecta type IV with resolving kyphomelia is caused by a novel COL1A2 mutation". Journal of Medical Genetics. 39 (2): 128–32. doi:10.1136/jmg.39.2.128. PMC 1735034. PMID 11836364.
- ^ Atta I, Iqbal F, Lone SW, et al. "Journal of the College of Physicians and Surgeons Pakistan" (PDF). Journal of the College of Physicians and Surgeons Pakistan. 24 (9): 653–657.
- ^ Basel D, Steiner RD (June 2009). "Osteogenesis imperfecta: recent findings shed new light on this once well-understood condition". Genetics in Medicine. 11 (6): 375–85. doi:10.1097/GIM.0b013e3181a1ff7b. PMID 19533842.
- ^ Cabral WA, Makareeva E, Colige A, Letocha AD, Ty JM, Yeowell HN, et al. (2005-05-01). "Mutations Near Amino End of α1(I) Collagen Cause Combined Osteogenesis Imperfecta/Ehlers-Danlos Syndrome by Interference with N-propeptide Processing". Journal of Biological Chemistry. 280 (19): 19259–19269. doi:10.1074/jbc.m414698200. ISSN 0021-9258.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Combined osteogenesis imperfecta and Ehlers–Danlos syndrome 1; OIEDS1". Online Mendelian Inheritance in Man. Johns Hopkins University. 2020-12-02. 619115. Retrieved 2021-08-17.
{{cite web}}: Cite uses deprecated parameter|authors=(help)CS1 maint: url-status (link) - ^ a b Peterson CR, Monk EA, McAllion SJ (2001-04-01). "How common is hearing impairment in osteogenesis imperfecta?". The Journal of Laryngology & Otology. 115 (4): 280–282. doi:10.1258/0022215011907442. ISSN 0022-2151. PMID 11276328. S2CID 12303464.
- ^ Vernick D (2005-11-02). "OI Issues: Hearing Loss". Retrieved 4 November 2018.
- ^ Santos F, McCall AA, Chien W, Merchant S (December 2012). "Otopathology in Osteogenesis Imperfecta". Otology & Neurotology. 33 (9): 1562–6. doi:10.1097/MAO.0b013e31826bf19b. PMC 3498599. PMID 22996160.
- ^ Kuurila K, Grénman R, Johansson R, Kaitila I (July 2000). "Hearing loss in children with osteogenesis imperfecta". European Journal of Pediatrics. 159 (7): 515–9. doi:10.1007/s004310051322. PMID 10923226. S2CID 8729406.
- ^ Khandanpour N, Connolly DJ, Raghavan A, Griffiths PD, Hoggard N (2012-12-01). "Craniospinal abnormalities and neurologic complications of osteogenesis imperfecta: imaging overview". Radiographics. 32 (7): 2101–12. doi:10.1148/rg.327125716. PMID 23150860.
- ^ Violas P, Fassier F, Hamdy R, Duhaime M, Glorieux FH (September 2002). "Acetabular protrusion in osteogenesis imperfecta". Journal of Pediatric Orthopedics. 22 (5): 622–5. doi:10.1097/01241398-200209000-00010. PMID 12198464. S2CID 37895736.
- ^ Lee JH, Gamble JG, Moore RE, Rinsky LA (September 1995). "Gastrointestinal problems in patients who have type-III osteogenesis imperfecta". The Journal of Bone and Joint Surgery. American Volume. 77 (9): 1352–6. doi:10.2106/00004623-199509000-00010. PMID 7673285.
- ^ "Constipation and OI". OI Foundation. Retrieved 2019-06-04.
{{cite web}}: CS1 maint: url-status (link) - ^ a b Sillence DO, Senn A, Danks DM (April 1979). "Genetic heterogeneity in osteogenesis imperfecta". Journal of Medical Genetics. 16 (2): 101–16. doi:10.1136/jmg.16.2.101. PMC 1012733. PMID 458828.
- ^ a b c d e f Marini JC, Blissett AR (August 2013). "New genes in bone development: what's new in osteogenesis imperfecta". The Journal of Clinical Endocrinology and Metabolism. 98 (8): 3095–103. doi:10.1210/jc.2013-1505. PMC 3733862. PMID 23771926.
- ^ McKusick VA, Hamosh A (2018-10-23). "Osteogenesis imperfecta, type I; OI1". Online Mendelian Inheritance in Man. Johns Hopkins University. 166200. Retrieved 2021-08-17.
{{cite web}}: CS1 maint: url-status (link) - ^ a b c d e f g Steiner RD, Pepin MG, Byers PH, Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP (January 28, 2005). "COL1A1/2 Osteogenesis Imperfecta". In Adam MP, Ardinger HH, Pagon RA, et al. (eds.). GeneReviews [Internet]. University of Washington, Seattle. PMID 20301472. Archived from the original on 18 January 2017. Retrieved 26 March 2012.
- ^ a b c van Dijk FS, Cobben JM, Kariminejad A, Maugeri A, Nikkels PG, van Rijn RR, Pals G (December 2011). "Osteogenesis Imperfecta: A Review with Clinical Examples". Molecular Syndromology. 2 (1): 1–20. doi:10.1159/000332228. PMC 3343766. PMID 22570641.
- ^ Subramanian S, Viswanathan VK (2021). Osteogenesis Imperfecta. StatPearls. PMID 30725642.
{{cite book}}:|website=ignored (help) Last Update: February 3, 2019. - ^ a b Shapiro JR, Lietman C, Grover M, Lu JT, Nagamani SC, Dawson BC, et al. (July 2013). "Phenotypic variability of osteogenesis imperfecta type V caused by an IFITM5 mutation". Journal of Bone and Mineral Research. 28 (7): 1523–30. doi:10.1002/jbmr.1891. PMC 3688672. PMID 23408678.
- ^ a b Singer RB, Ogston SA, Paterson CR (2001). "Mortality in various types of osteogenesis imperfecta". Journal of Insurance Medicine. 33 (3): 216–20. PMID 11558400.
- ^ "Osteogenesis Imperfecta: Types, Symptoms & Management". Cleveland Clinic. 2021-05-05. Retrieved 2021-08-17.
{{cite web}}: CS1 maint: url-status (link) - ^ Chen H (2006). "Triangular face". Atlas of genetic diagnosis and counseling. Totowa, NJ: Humana Press. p. 771. ISBN 978-1-58829-681-8. Archived from the original on 8 June 2013.
- ^ Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, et al. (September 2000). "Type V osteogenesis imperfecta: a new form of brittle bone disease". Journal of Bone and Mineral Research. 15 (9): 1650–8. doi:10.1359/jbmr.2000.15.9.1650. PMID 10976985. S2CID 13748803.
- ^ a b c Cho TJ, Lee KE, Lee SK, Song SJ, Kim KJ, Jeon D, et al. (August 2012). "A single recurrent mutation in the 5'-UTR of IFITM5 causes osteogenesis imperfecta type V". American Journal of Human Genetics. 91 (2). Seoul National University Hospital: 343–8. doi:10.1016/j.ajhg.2012.06.005. PMC 3415533. PMID 22863190.
- ^ "Recessive Form of OI Discovered by Foundation-funded Researcher" (PDF). Archived from the original (PDF) on 2007-08-12. Retrieved 2008-04-09.
- ^ a b "Genetic Conditions: Osteogenesis imperfecta". Genetics Home Reference. U.S. National Library of Medicine. November 2007. Archived from the original on 2008-12-19 – via Medline Plus.
- ^ "OMIM Entry - # 259440 - OSTEOGENESIS IMPERFECTA, TYPE IX; OI9". www.omim.org. Retrieved 2020-08-10.
- ^ "OMIM Entry - # 613848 - OSTEOGENESIS IMPERFECTA, TYPE X; OI10". www.omim.org. Retrieved 2020-08-10.
- ^ "OMIM Entry - # 610968 - OSTEOGENESIS IMPERFECTA, TYPE XI; OI11". www.omim.org. Retrieved 2018-11-11.
- ^ Sam JE, Dharmalingam M (2017). "Osteogenesis Imperfecta". Indian Journal of Endocrinology and Metabolism. 21 (6): 903–908. doi:10.4103/ijem.IJEM_220_17. PMC 5729682. PMID 29285457.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "OMIM Entry # 616229 - OSTEOGENESIS IMPERFECTA, TYPE XVI; OI16". www.omim.org. Retrieved 2018-11-11.
- ^ Shapiro JR (2014). "Clinical and Genetic Classification of Osteogenesis Imperfecta and Epidemiology". Osteogenesis Imperfecta. Elsevier. pp. 15–22. doi:10.1016/b978-0-12-397165-4.00002-2. ISBN 9780123971654.
- ^ a b Sam JE, Dharmalingam M (2017-11-01). "Osteogenesis Imperfecta". Indian Journal of Endocrinology and Metabolism. 21 (6): 903–908. doi:10.4103/ijem.IJEM_220_17. PMC 5729682. PMID 29285457.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b "Springer Reference", OMIM (TM), Springer-Verlag, 2011, doi:10.1007/springerreference_36173[dead link]
- ^ "OMIM Entry - # 617952 - OSTEOGENESIS IMPERFECTA, TYPE XVIII; OI18". www.omim.org. Retrieved 2020-08-07.
- ^ Volodarsky M, Markus B, Cohen I, Staretz-Chacham O, Flusser H, Landau D, et al. (April 2013). "A deletion mutation in TMEM38B associated with autosomal recessive osteogenesis imperfecta". Human Mutation. 34 (4): 582–6. doi:10.1002/humu.22274. PMID 23316006. S2CID 6036441.
- ^ Duy BH, Zhytnik L, Maasalu K, Kändla I, Prans E, Reimann E, et al. (2016-12-01). "Mutation analysis of the COL1A1 and COL1A2 genes in Vietnamese patients with osteogenesis imperfecta". Human Genomics. 10 (1): 27. doi:10.1186/s40246-016-0083-1. ISSN 1479-7364. PMC 4983065. PMID 27519266.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Palomo T, Vilaça T, Lazaretti-Castro M (December 2017). "Osteogenesis imperfecta: diagnosis and treatment". Current Opinion in Endocrinology, Diabetes, and Obesity. 24 (6): 381–388. doi:10.1097/MED.0000000000000367. PMID 28863000. S2CID 4555427.
- ^ Valadares ER, Carneiro TB, Santos PM, Oliveira AC, Zabel B (18 July 2014). "What is new in genetics and osteogenesis imperfecta classification?". Jornal de Pediatria. 90 (6): 536–41. doi:10.1016/j.jped.2014.05.003. PMID 25046257.
- ^ Forlino A, Cabral WA, Barnes AM, Marini JC (June 2011). "New perspectives on osteogenesis imperfecta". Nature Reviews. Endocrinology. 7 (9): 540–57. doi:10.1038/nrendo.2011.81. PMC 3443407. PMID 21670757.
- ^ Forlino A, Marini JC (April 2016). "Osteogenesis imperfecta". Lancet. 387 (10028): 1657–71. doi:10.1016/S0140-6736(15)00728-X. PMC 7384887. PMID 26542481.
- ^ Koromani F, Trajanoska K, Rivadeneira F, Oei L (2019-06-04). "Recent Advances in the Genetics of Fractures in Osteoporosis". Frontiers in Endocrinology. doi:10.3389/fendo.2019.00337. ISSN 1664-2392. PMC 6559287. PMID 31231309.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Drögemüller C, Becker D, Brunner A, Haase B, Kircher P, Seeliger F, et al. (July 2009). Barsh GS (ed.). "A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta". PLOS Genetics. 5 (7): e1000579. doi:10.1371/journal.pgen.1000579. PMC 2708911. PMID 19629171.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rohrbach M, Giunta C (August 2012). "Recessive osteogenesis imperfecta: clinical, radiological, and molecular findings". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 160C (3): 175–89. doi:10.1002/ajmg.c.31334. PMID 22791419. S2CID 28592112.
- ^ a b Marini JC, Reich A, Smith SM (August 2014). "Osteogenesis imperfecta due to mutations in non-collagenous genes: lessons in the biology of bone formation". Current Opinion in Pediatrics. 26 (4): 500–7. doi:10.1097/MOP.0000000000000117. PMC 4183132. PMID 25007323.
- ^ Hanagata N (March 2016). "IFITM5 mutations and osteogenesis imperfecta". Journal of Bone and Mineral Metabolism. 34 (2): 123–31. doi:10.1007/s00774-015-0667-1. PMID 26031935. S2CID 3173191.
- ^ Lindert U, Cabral WA, Ausavarat S, Tongkobpetch S, Ludin K, Barnes AM, et al. (2016-07-06). "MBTPS2 mutations cause defective regulated intramembrane proteolysis in X-linked osteogenesis imperfecta". Nature Communications. 7 (1): 11920. doi:10.1038/ncomms11920. ISSN 2041-1723. PMC 4935805. PMID 27380894.
- ^ Rauch F, Glorieux FH (April 2004). "Osteogenesis imperfecta". Lancet. 363 (9418): 1377–85. doi:10.1016/S0140-6736(04)16051-0. PMID 15110498. S2CID 24081895.
- ^ a b Gautieri A, Uzel S, Vesentini S, Redaelli A, Buehler MJ (August 2009). "Molecular and mesoscale mechanisms of osteogenesis imperfecta disease in collagen fibrils". Biophysical Journal. 97 (3): 857–65. Bibcode:2009BpJ....97..857G. doi:10.1016/j.bpj.2009.04.059. PMC 2718154. PMID 19651044.
- ^ "Osteogenesis Imperfecta Foundation: Fast Facts". Archived from the original on 2007-06-28. Retrieved 2007-07-05.
- ^ Zhytnik L, Maasalu K, Duy BH, Pashenko A, Khmyzov S, Reimann E, et al. (March 2019). "De novo and inherited pathogenic variants in collagen-related osteogenesis imperfecta". Molecular Genetics & Genomic Medicine. 7 (3): e559. doi:10.1002/mgg3.559. PMC 6418448. PMID 30675999.
- ^ El-Sobky TA, Shawky RM, Sakr HM, Elsayed SM, Elsayed NS, Ragheb SG, Gamal R (15 November 2017). "A systematized approach to radiographic assessment of commonly seen genetic bone diseases in children: A pictorial review". J Musculoskelet Surg Res. 1 (2): 25. doi:10.4103/jmsr.jmsr_28_17. S2CID 79825711.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Jameson JR, Albert CI, Busse B, Smith PA, Harris GF (March 29, 2013). "3D micron-scale imaging of the cortical bone canal network in human osteogenesis imperfecta (OI)". In Weaver JB, Molthen RC (eds.). Medical Imaging 2013: Biomedical Applications in Molecular, Structural, and Functional Imaging. Vol. 8672. International Society for Optics and Photonics. pp. 86721L. doi:10.1117/12.2007209. S2CID 13876569.
- ^ Westgren M, Götherström C (September 2015). "Stem cell transplantation before birth - a realistic option for treatment of osteogenesis imperfecta?". Prenatal Diagnosis. 35 (9): 827–32. doi:10.1002/pd.4611. PMID 25962526. S2CID 10640427.
- ^ a b c Pepin MG, Byers PH (December 2015). "What every clinical geneticist should know about testing for osteogenesis imperfecta in suspected child abuse cases". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 169 (4): 307–13. doi:10.1002/ajmg.c.31459. PMID 26566591. S2CID 26045033.
- ^ "Is Osteogenesis Imperfecta Inherited?". 4 April 2014. Retrieved 7 November 2018.
- ^ Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R (October 1998). "Cyclic administration of pamidronate in children with severe osteogenesis imperfecta". The New England Journal of Medicine. 339 (14): 947–52. doi:10.1056/NEJM199810013391402. PMID 9753709. S2CID 19316414.Free full text
- ^ DiMeglio LA, Peacock M (January 2006). "Two-year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta". Journal of Bone and Mineral Research. 21 (1): 132–40. doi:10.1359/JBMR.051006. PMID 16355282. S2CID 12996685.
- ^ Bishop N, Adami S, Ahmed SF, Antón J, Arundel P, Burren CP, et al. (October 2013). "Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial". Lancet. 382 (9902): 1424–32. doi:10.1016/S0140-6736(13)61091-0. PMID 23927913. S2CID 25559791.
- ^ Ward LM, Rauch F (October 2013). "Oral bisphosphonates for paediatric osteogenesis imperfecta?". Lancet. 382 (9902): 1388–9. doi:10.1016/S0140-6736(13)61531-7. PMID 23927912. S2CID 5872511.
- ^ "A Leader in the Treatment of Osteogensis Imperfecta (OI)". Shriners International. Archived from the original on 2007-09-28. Retrieved 2007-07-05.
- ^ Sofield HA, Millar EA (1959-12-01). "Fragmentation, Realignment, and Intramedullary Rod Fixation of Deformities of the Long Bones in Children: A Ten-Year Appraisal". The Journal of Bone and Joint Surgery. 41 (8): 1371–1391. doi:10.2106/00004623-195941080-00001. ISSN 0021-9355.
- ^ "The Fassier-Duval Telescopic IM System". Pega Medical. Laval, Quebec. Retrieved 2021-08-15.
{{cite web}}: CS1 maint: url-status (link) - ^ Sterian A, Balanescu R, Barbilian A, Ulici A (2015). "Osteosynthesis in Osteogenesis Imperfecta, telescopic versus non-telescopic nailing". Journal of Medicine and Life. 8 (4): 563–5. PMC 4656972. PMID 26664490.
- ^ a b Frassier F, Duval P, Paley D (2021). "Fassier–Duval Telescopic IM System™ Surgical Technique" (PDF). Pega Medical. Laval, Quebec. FD-ST-EN Revision K. Retrieved 2021-08-15.
{{cite web}}: CS1 maint: url-status (link) - ^ Belyea CM, Knox JB (2020-01-01). "Spinal fusion in children with osteogenesis imperfecta: A nationwide retrospective comparative cohort study over a 12-year period". Current Orthopaedic Practice. 31 (1): 72–75. doi:10.1097/BCO.0000000000000805. ISSN 1940-7041. S2CID 209229412.
- ^ a b Wallace MJ, Kruse RW, Shah SA (February 2017). "The Spine in Patients With Osteogenesis Imperfecta". The Journal of the American Academy of Orthopaedic Surgeons. 25 (2): 100–109. doi:10.5435/JAAOS-D-15-00169. PMID 28009707.
- ^ "Osteogenesis Imperfecta Foundation | OIF.org". www.oif.org. Retrieved 2018-11-10.
- ^ a b Biria M, Abbas FM, Mozaffar S, Ahmadi R (July 2012). "Dentinogenesis imperfecta associated with osteogenesis imperfecta". Dental Research Journal. 9 (4): 489–94. PMC 3491340. PMID 23162594.
- ^ Plotkin H (29 February 2016). "Genetics of Osteogenesis Imperfecta". Archived from the original on 2010-12-30.
- ^ "Osteogenesis Imperfecta Panel". University of Nebraska Medical center. Retrieved 2019-07-30.
- ^ Viljoen D, Beighton P (August 1987). "Osteogenesis imperfecta type III: an ancient mutation in Africa?". American Journal of Medical Genetics. 27 (4): 907–12. doi:10.1002/ajmg.1320270417. PMID 3425600.
- ^ K. Buday, Beiträge zur Lehre von der Osteogenesis imperfecta (1895)
- ^ "Osteogenesis Imperfecta Foundation: Glossary". Archived from the original on 2007-08-07. Retrieved 2007-07-05.
- ^ Hektoen L (May 1903). "Anatomical Study of a Short-Limbed Dwarf, With Special Reference To Osteogenesis Imperfecta and Chondrodystrophia Foetalis. 1". The American Journal of the Medical Sciences. 125 (5). J.B. Lippincott, Company: 751–769. doi:10.1097/00000441-190305000-00001. ISBN 978-0-243-38392-4. S2CID 71836609.
- ^ a b synd/1743 at Who Named It?
- ^ a b Davel JG, Fichardt T, Van Der Spuy D (October 1956). "Osteogenesis imperfecta". Archives of Disease in Childhood. 31 (159): 346–53. doi:10.1136/adc.31.159.346. PMC 2011939. PMID 13363481.
- ^ "Mission – OI Foundation". Retrieved 2021-07-28.
- ^ "The OI Society of Australia". 2006. Retrieved 6 November 2018.
- ^ Sandor M (2008). "Genetic and Rare Diseases Information Center (GARD)". Retrieved 6 November 2018.
- ^ a b "Osteogenesis Imperfecta in Dogs - Symptoms, Causes, Diagnosis, Treatment, Recovery, Management, Cost". WagWalking. Retrieved 2018-11-07.
- ^ Eckardt J, Kluth S, Dierks C, Philipp U, Distl O (April 2013). "Population screening for the mutation associated with osteogenesis imperfecta in dachshunds". The Veterinary Record. 172 (14): 364. doi:10.1136/vr.101122. PMID 23315765. S2CID 34816198.
- ^ "Osteogenesis Imperfecta - CAG - Center for Animal Genetics". CAG - Center for Animal Genetics. Retrieved 2018-11-07.
- ^ a b Enderli TA, Burtch SR, Templet JN, Carriero A (September 2016). "Animal models of osteogenesis imperfecta: applications in clinical research". Orthopedic Research and Reviews. 8: 41–55. doi:10.2147/ORR.S85198. PMC 6209373. PMID 30774469.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- "Osteogenesis Imperfecta Overview". NIH Osteoporosis and Related Bone Diseases — National Resource Center. National Institutes of Health, U.S. Department of Health and Human Services.