Iridium: Difference between revisions
m Dating maintenance tags: {{Huh?}} {{Which?}} |
→Catalysis: example with tertiary ref |
||
| Line 229: | Line 229: | ||
===Catalysis=== |
===Catalysis=== |
||
Iridium compounds are used as [[catalysis|catalysts]] in the [[Cativa process]] for [[carbonylation]] of [[methanol]] to produce [[acetic acid]].<ref name="ullmann-acetic">{{cite book|first=H.|last= Cheung| author2=Tanke, R. S.| author3=Torrence, G. P.|chapter=Acetic acid|title=Ullmann's Encyclopedia of Industrial Chemistry|publisher=Wiley|date=2000|doi=10.1002/14356007.a01_045|isbn= 978-3527306732}}</ref><ref name = "Jones">{{cite journal | last1 = Jones | first1 = Jane H. | title = The cativa™ process for the manufacture of acetic acid. | journal = Platinum Metals Review | volume = 44 | issue = 3 | year = 2000 | pages= 94–105 | url = https://technology.matthey.com/article/44/3/94-105/}}</ref> |
Iridium compounds are used as [[catalysis|catalysts]] in the [[Cativa process]] for [[carbonylation]] of [[methanol]] to produce [[acetic acid]].<ref name="ullmann-acetic">{{cite book|first=H.|last= Cheung| author2=Tanke, R. S.| author3=Torrence, G. P.|chapter=Acetic acid|title=Ullmann's Encyclopedia of Industrial Chemistry|publisher=Wiley|date=2000|doi=10.1002/14356007.a01_045|isbn= 978-3527306732}}</ref><ref name = "Jones">{{cite journal | last1 = Jones | first1 = Jane H. | title = The cativa™ process for the manufacture of acetic acid. | journal = Platinum Metals Review | volume = 44 | issue = 3 | year = 2000 | pages= 94–105 | url = https://technology.matthey.com/article/44/3/94-105/}}</ref> |
||
Iridium complexes are often active for [[hydrogenation]]. This property is the basis of the industrial route to the [[chiral]] [[herbicide]] [[(S)-metolachlor]]. As practiced by Syngenta on the scale of 10,000 tons/year, the complex [[ [Ir(COD)Cl]<sub>2</sub> in the presence of [[Josiphos]].<ref>{{cite book|editor=Matthias Beller, Hans-Ulrich Blaser|series=Topics in Organometallic Chemistry|volume=42|publisher=Springer|location=Berlin, Heidelberg|year=2012|isbn=978-3-642-32832-9}}</ref> |
|||
===Medical imaging=== |
===Medical imaging=== |
||
Revision as of 15:17, 15 October 2022
 | |||||||||||||||||||||||||||||||||
| Iridium | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ɪˈrɪdiəm/ | ||||||||||||||||||||||||||||||||
| Appearance | Silvery white | ||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Ir) | |||||||||||||||||||||||||||||||||
| Iridium in the periodic table | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Atomic number (Z) | 77 | ||||||||||||||||||||||||||||||||
| Group | group 9 | ||||||||||||||||||||||||||||||||
| Period | period 6 | ||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d7 6s2 | ||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 15, 2 | ||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||
| Melting point | 2719 K (2446 °C, 4435 °F) | ||||||||||||||||||||||||||||||||
| Boiling point | 4403 K (4130 °C, 7466 °F) | ||||||||||||||||||||||||||||||||
| Density (at 20° C) | 22.562 g/cm3 [3] | ||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 19 g/cm3 | ||||||||||||||||||||||||||||||||
| Heat of fusion | 41.12 kJ/mol | ||||||||||||||||||||||||||||||||
| Heat of vaporization | 564 kJ/mol | ||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.10 J/(mol·K) | ||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||
| Oxidation states | −3, –2, −1, 0, +1, +2, +3, +4, +5, +6, +7, +8, +9[4] | ||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.20 | ||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 136 pm | ||||||||||||||||||||||||||||||||
| Covalent radius | 141±6 pm | ||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) (cF4) | ||||||||||||||||||||||||||||||||
| Lattice constant | a = 383.92 pm (at 20 °C)[3] | ||||||||||||||||||||||||||||||||
| Thermal expansion | 6.47×10−6/K (at 20 °C)[3] | ||||||||||||||||||||||||||||||||
| Thermal conductivity | 147 W/(m⋅K) | ||||||||||||||||||||||||||||||||
| Electrical resistivity | 47.1 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[5] | ||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +25.6 × 10−6 cm3/mol (298 K)[6] | ||||||||||||||||||||||||||||||||
| Young's modulus | 528 GPa | ||||||||||||||||||||||||||||||||
| Shear modulus | 210 GPa | ||||||||||||||||||||||||||||||||
| Bulk modulus | 320 GPa | ||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 4825 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||
| Poisson ratio | 0.26 | ||||||||||||||||||||||||||||||||
| Mohs hardness | 6.5 | ||||||||||||||||||||||||||||||||
| Vickers hardness | 1760–2200 MPa | ||||||||||||||||||||||||||||||||
| Brinell hardness | 1670 MPa | ||||||||||||||||||||||||||||||||
| CAS Number | 7439-88-5 | ||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||
| Discovery and first isolation | Smithson Tennant (1803) | ||||||||||||||||||||||||||||||||
| Isotopes of iridium | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density of 22.56 g/cm3 (0.815 lb/cu in) as defined by experimental X-ray crystallography.[a] It is one of the most corrosion-resistant metals, even at temperatures as high as 2,000 °C (3,630 °F). However, corrosion-resistance is not quantifiable in absolute terms; although only certain molten salts and halogens are corrosive to solid iridium, finely divided iridium dust is much more reactive and can be flammable, whereas gold dust is not flammable but can be attacked by substances that iridium resists, such as aqua regia.
Iridium was discovered in 1803 among insoluble impurities in natural platinum. Smithson Tennant, the primary discoverer, named it after the Greek goddess Iris, personification of the rainbow, because of the striking and diverse colors of its salts. Iridium is one of the rarest elements in Earth's crust, with annual production and consumption of only 3 tonnes (6.6 thousand pounds). 191Ir and 193Ir are the only two naturally occurring isotopes of iridium, as well as the only stable isotopes; the latter is the more abundant.
The most important iridium compounds in use are the salts and acids it forms with chlorine, though iridium also forms a number of organometallic compounds used in industrial catalysis, and in research. Iridium metal is employed when high corrosion resistance at high temperatures is needed, as in high-performance spark plugs, crucibles for recrystallization of semiconductors at high temperatures, and electrodes for the production of chlorine in the chloralkali process. Iridium radioisotopes are used in some radioisotope thermoelectric generators.
Iridium is found in meteorites in much higher abundance than in the Earth's crust.[10] For this reason, the unusually high abundance of iridium in the clay layer at the Cretaceous–Paleogene boundary gave rise to the Alvarez hypothesis that the impact of a massive extraterrestrial object caused the extinction of dinosaurs and many other species 66 million years ago, now known to be produced by the impact that formed the Chicxulub crater. Similarly, an iridium anomaly in core samples from the Pacific Ocean suggested the Eltanin impact of about 2.5 million years ago.[11]
It is thought that the total amount of iridium in the planet Earth is much higher than that observed in crustal rocks, but as with other platinum-group metals, the high density and tendency of iridium to bond with iron caused most iridium to descend below the crust when the planet was young and still molten.
Characteristics
Physical properties

A member of the platinum group metals, iridium is white, resembling platinum, but with a slight yellowish cast. Because of its hardness, brittleness, and very high melting point, solid iridium is difficult to machine, form, or work; thus powder metallurgy is commonly employed instead.[12] It is the only metal to maintain good mechanical properties in air at temperatures above 1,600 °C (2,910 °F).[13] It has the 10th highest boiling point among all elements and becomes a superconductor at temperatures below 0.14 K (−273.010 °C; −459.418 °F).[14]
Iridium's modulus of elasticity is the second-highest among the metals, being surpassed only by osmium.[13] This, together with a high shear modulus and a very low figure for Poisson's ratio (the relationship of longitudinal to lateral strain), indicate the high degree of stiffness and resistance to deformation that have rendered its fabrication into useful components a matter of great difficulty. Despite these limitations and iridium's high cost, a number of applications have developed where mechanical strength is an essential factor in some of the extremely severe conditions encountered in modern technology.[13]
The measured density of iridium is only slightly lower (by about 0.12%) than that of osmium, the densest metal known.[15][16] Some ambiguity occurred regarding which of the two elements was denser, due to the small size of the difference in density and difficulties in measuring it accurately,[17] but, with increased accuracy in factors used for calculating density, X-ray crystallographic data yielded densities of 22.56 g/cm3 (0.815 lb/cu in) for iridium and 22.59 g/cm3 (0.816 lb/cu in) for osmium.[18]
Iridium is extremely brittle, to the point of being hard to weld because the heat-affected zone cracks, but it can be made more ductile by addition of small quantities of titanium and zirconium (0.2% of each apparently works well).[19]-
The Vickers hardness of pure platinum is 56 HV, whereas platinum with 50% of iridium can reach over 500 HV.[20][21]
Chemical properties
Iridium is the most corrosion-resistant metal known:[22] it is not attacked by acids, including aqua regia. In the presence of oxygen, it reacts with cyanide salts,[23]. Traditional oxidants also react, including the halogens and oxygen.[24] at higher temperatures.[25] Iridium also reacts directly with sulfur at atmospheric pressure to yield iridium disulfide.[26]
Compounds
| Oxidation states[b] | |
|---|---|
| −3 | [Ir(CO) 3]3− |
| −1 | [Ir(CO) 3(PPh 3)]− |
| 0 | Ir 4(CO) 12 |
| +1 | [Ir(CO)Cl(PPh 3) 2] |
| +2 | IrCp 2 |
| +3 | IrCl 3 |
| +4 | IrO 2 |
| +5 | Ir 4F 20 |
| +6 | IrF 6 |
| +7 | [(η2 -O 2)IrO 2]+ |
| +8 | IrO 4 |
| +9 | [IrO 4]+ [4] |

Iridium forms compounds in oxidation states between −3 and +9; the most common oxidation states are +3 and +4.[12] Well-characterized examples of the high +6 oxidation state are rare, but include IrF
6 and two mixed oxides Sr
2MgIrO
6 and Sr
2CaIrO
6.[12][27] In addition, it was reported in 2009 that iridium(VIII) oxide (IrO
4) was prepared under matrix isolation conditions (6 K in Ar) by UV irradiation of an iridium-peroxo complex. This species, however, is not expected to be stable as a bulk solid at higher temperatures.[28] The highest oxidation state (+9), which is also the highest recorded for any element, is only known in one cation, IrO+
4; it is only known as gas-phase species and is not known to form any salts.[4]
Iridium dioxide, IrO
2, a blue black solid, is the only well-characterized oxide of iridium.[12] A sesquioxide, Ir
2O
3, has been described as a blue-black powder which is oxidized to IrO
2 by HNO
3.[24] The corresponding disulfides, diselenides, sesquisulfides, and sesquiselenides are known, and IrS
3 has also been reported.[12] Iridium also forms iridates with oxidation states +4 and +5, such as K
2IrO
3 and KIrO
3, which can be prepared from the reaction of potassium oxide or potassium superoxide with iridium at high temperatures.[29]
Although no binary hydrides of iridium, Ir
xH
y are known, complexes are known that contain IrH4−
5 and IrH3−
6, where iridium has the +1 and +3 oxidation states, respectively.[30] The ternary hydride Mg
6Ir
2H
11 is believed to contain both the IrH4−
5 and the 18-electron IrH5−
4 anion.[31]
No monohalides or dihalides are known, whereas trihalides, IrX
3, are known for all of the halogens.[12] For oxidation states +4 and above, only the tetrafluoride, pentafluoride and hexafluoride are known.[12] Iridium hexafluoride, IrF
6, is a volatile and highly reactive yellow solid, composed of octahedral molecules. It decomposes in water and is reduced to IrF
4, a crystalline solid, by iridium black.[12] Iridium pentafluoride has similar properties but it is actually a tetramer, Ir
4F
20, formed by four corner-sharing octahedra.[12] Iridium metal dissolves in molten alkali-metal cyanides to produce the Ir(CN)3−
6 (hexacyanoiridate) ion.
Hexachloroiridic (IV) acid, H
2IrCl
6, and its ammonium salt are the most important iridium compounds from an industrial perspective.[32] They are involved in the purification of iridium and used as precursors for most other iridium compounds, as well as in the preparation of anode coatings. The IrCl2−
6 ion has an intense dark brown color, and can be readily reduced to the lighter-colored IrCl3−
6 and vice versa.[32] Iridium trichloride, IrCl
3, which can be obtained in anhydrous form from direct oxidation of iridium powder by chlorine at 650 °C,[32] or in hydrated form by dissolving Ir
2O
3 in hydrochloric acid, is often used as a starting material for the synthesis of other Ir(III) compounds.[12] Another compound used as a starting material is ammonium hexachloroiridate(III), (NH
4)
3IrCl
6. Iridium(III) complexes are diamagnetic (low-spin) and generally have an octahedral molecular geometry.[12]
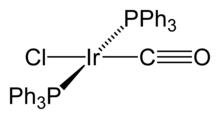
Organoiridium compounds contain iridium–carbon bonds where the metal is usually in lower oxidation states. For example, oxidation state zero is found in tetrairidium dodecacarbonyl, Ir
4(CO)
12, which is the most common and stable binary carbonyl of iridium.[12] In this compound, each of the iridium atoms is bonded to the other three, forming a tetrahedral cluster. Some organometallic Ir(I) compounds are notable enough to be named after their discoverers. One is Vaska's complex, IrCl(CO)[P(C
6H
5)
3]
2, which has the unusual property of binding to the dioxygen molecule, O
2.[33] Another one is Crabtree's catalyst, a homogeneous catalyst for hydrogenation reactions.[34] These compounds are both square planar, d8 complexes, with a total of 16 valence electrons, which accounts for their reactivity.[35]
An iridium-based organic LED material has been documented, and found to be much brighter than DPA or PPV, so could be the basis for flexible OLED lighting in the future.[36]
Isotopes
Iridium has two naturally occurring, stable isotopes, 191Ir and 193Ir, with natural abundances of 37.3% and 62.7%, respectively.[37] At least 37 radioisotopes have also been synthesized, ranging in mass number from 164 to 202. 192Ir, which falls between the two stable isotopes, is the most stable radioisotope, with a half-life of 73.827 days, and finds application in brachytherapy[38] and in industrial radiography, particularly for nondestructive testing of welds in steel in the oil and gas industries; iridium-192 sources have been involved in a number of radiological accidents. Three other isotopes have half-lives of at least a day—188Ir, 189Ir, and 190Ir.[37] Isotopes with masses below 191 decay by some combination of β+ decay, α decay, and (rare) proton emission, with the exception of 189Ir, which decays by electron capture. Synthetic isotopes heavier than 191 decay by β− decay, although 192Ir also has a minor electron capture decay path.[37] All known isotopes of iridium were discovered between 1934 and 2008, with the most recent discoveries being 200–202Ir.[39]
At least 32 metastable isomers have been characterized, ranging in mass number from 164 to 197. The most stable of these is 192m2Ir, which decays by isomeric transition with a half-life of 241 years,[37] making it more stable than any of iridium's synthetic isotopes in their ground states. The least stable isomer is 190m3Ir with a half-life of only 2 μs.[37] The isotope 191Ir was the first one of any element to be shown to present a Mössbauer effect. This renders it useful for Mössbauer spectroscopy for research in physics, chemistry, biochemistry, metallurgy, and mineralogy.[40]
History
Platinum group

The discovery of iridium is intertwined with that of platinum and the other metals of the platinum group. The first European reference to platinum appears in 1557 in the writings of the Italian humanist Julius Caesar Scaliger as a description of an unknown noble metal found between Darién and Mexico, "which no fire nor any Spanish artifice has yet been able to liquefy".[41] From their first encounters with platinum, the Spanish generally saw the metal as a kind of impurity in gold, and it was treated as such. It was often simply thrown away, and there was an official decree forbidding the adulteration of gold with platinum impurities.[42]


In 1735, Antonio de Ulloa and Jorge Juan y Santacilia saw Native Americans mining platinum while the Spaniards were travelling through Colombia and Peru for eight years. Ulloa and Juan found mines with the whitish metal nuggets and took them home to Spain. Antonio de Ulloa returned to Spain and established the first mineralogy lab in Spain and was the first to systematically study platinum, which was in 1748. His historical account of the expedition included a description of platinum as being neither separable nor calcinable. Ulloa also anticipated the discovery of platinum mines. After publishing the report in 1748, Ulloa did not continue to investigate the new metal. In 1758, he was sent to superintend mercury mining operations in Huancavelica.[41]
In 1741, Charles Wood,[43] a British metallurgist, found various samples of Colombian platinum in Jamaica, which he sent to William Brownrigg for further investigation.
In 1750, after studying the platinum sent to him by Wood, Brownrigg presented a detailed account of the metal to the Royal Society, stating that he had seen no mention of it in any previous accounts of known minerals.[44] Brownrigg also made note of platinum's extremely high melting point and refractoriness toward borax.[clarification needed] Other chemists across Europe soon began studying platinum, including Andreas Sigismund Marggraf,[45] Torbern Bergman, Jöns Jakob Berzelius, William Lewis, and Pierre Macquer. In 1752, Henrik Scheffer published a detailed scientific description of the metal, which he referred to as "white gold", including an account of how he succeeded in fusing platinum ore with the aid of arsenic. Scheffer described platinum as being less pliable than gold, but with similar resistance to corrosion.[41]
Discovery
Chemists who studied platinum dissolved it in aqua regia (a mixture of hydrochloric and nitric acids) to create soluble salts. They always observed a small amount of a dark, insoluble residue.[13] Joseph Louis Proust thought that the residue was graphite.[13] The French chemists Victor Collet-Descotils, Antoine François, comte de Fourcroy, and Louis Nicolas Vauquelin also observed the black residue in 1803, but did not obtain enough for further experiments.[13]
In 1803, British scientist Smithson Tennant (1761–1815) analyzed the insoluble residue and concluded that it must contain a new metal. Vauquelin treated the powder alternately with alkali and acids[22] and obtained a volatile new oxide, which he believed to be of this new metal—which he named ptene, from the Greek word πτηνός ptēnós, "winged".[46][47] Tennant, who had the advantage of a much greater amount of residue, continued his research and identified the two previously undiscovered elements in the black residue, iridium and osmium.[13][22] He obtained dark red crystals (probably of Na
2[IrCl
6]·nH
2O) by a sequence of reactions with sodium hydroxide and hydrochloric acid.[47] He named iridium after Iris (Ἶρις), the Greek winged goddess of the rainbow and the messenger of the Olympian gods, because many of the salts he obtained were strongly colored.[c][48] Discovery of the new elements was documented in a letter to the Royal Society on June 21, 1804.[13][49]
Metalworking and applications
British scientist John George Children was the first to melt a sample of iridium in 1813 with the aid of "the greatest galvanic battery that has ever been constructed" (at that time).[13] The first to obtain high-purity iridium was Robert Hare in 1842. He found it had a density of around 21.8 g/cm3 (0.79 lb/cu in) and noted the metal is nearly immalleable and very hard. The first melting in appreciable quantity was done by Henri Sainte-Claire Deville and Jules Henri Debray in 1860. They required burning more than 300 litres (79 US gal) of pure O
2 and H
2 gas for each 1 kilogram (2.2 lb) of iridium.[13]
These extreme difficulties in melting the metal limited the possibilities for handling iridium. John Isaac Hawkins was looking to obtain a fine and hard point for fountain pen nibs, and in 1834 managed to create an iridium-pointed gold pen. In 1880, John Holland and William Lofland Dudley were able to melt iridium by adding phosphorus and patented the process in the United States; British company Johnson Matthey later stated they had been using a similar process since 1837 and had already presented fused iridium at a number of World Fairs.[13] The first use of an alloy of iridium with ruthenium in thermocouples was made by Otto Feussner in 1933. These allowed for the measurement of high temperatures in air up to 2,000 °C (3,630 °F).[13]
In Munich, Germany in 1957 Rudolf Mössbauer, in what has been called one of the "landmark experiments in twentieth-century physics",[50] discovered the resonant and recoil-free emission and absorption of gamma rays by atoms in a solid metal sample containing only 191Ir.[51] This phenomenon, known as the Mössbauer effect resulted in the awarding of the Nobel Prize in Physics in 1961, at the age 32, just three years after he published his discovery.[52]
Occurrence
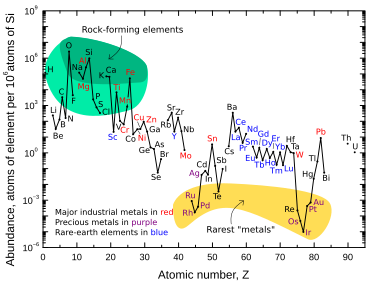

Iridium is one of the nine least abundant stable elements in Earth's crust, having an average mass fraction of 0.001 ppm in crustal rock; platinum is 10 times more abundant, gold is 40 times more abundant, and silver and mercury are 80 times more abundant.[12] Tellurium is about as abundant as iridium.[12] In contrast to its low abundance in crustal rock, iridium is relatively common in meteorites, with concentrations of 0.5 ppm or more.[54] The overall concentration of iridium on Earth is thought to be much higher than what is observed in crustal rocks, but because of the density and siderophilic ("iron-loving") character of iridium, it descended below the crust and into Earth's core when the planet was still molten.[32]
Iridium is found in nature as an uncombined element or in natural alloys; especially the iridium–osmium alloys, osmiridium (osmium-rich), and iridosmium (iridium-rich).[22] In the nickel and copper deposits, the platinum group metals occur as sulfides (i.e. (Pt,Pd)S), tellurides (i.e. PtBiTe), antimonides (PdSb), and arsenides (i.e. PtAs
2). In all of these compounds, platinum is exchanged by a small amount of iridium and osmium. As with all of the platinum group metals, iridium can be found naturally in alloys with raw nickel or raw copper.[55] A number of iridium-dominant minerals, with iridium as the species-forming element, are known. They are exceedingly rare and often represent the iridium analogues of the above-given ones. The examples are irarsite and cuproiridsite, to mention some.[56][57][58][59][60]
Within Earth's crust, iridium is found at highest concentrations in three types of geologic structure: igneous deposits (crustal intrusions from below), impact craters, and deposits reworked from one of the former structures. The largest known primary reserves are in the Bushveld igneous complex in South Africa,[61] (near the largest known impact structure, the Vredefort impact structure) though the large copper–nickel deposits near Norilsk in Russia, and the Sudbury Basin (also an impact crater) in Canada are also significant sources of iridium. Smaller reserves are found in the United States.[61] Iridium is also found in secondary deposits, combined with platinum and other platinum group metals in alluvial deposits. The alluvial deposits used by pre-Columbian people in the Chocó Department of Colombia are still a source for platinum-group metals. As of 2003, world reserves have not been estimated.[22]
Marine oceanography
Iridium is found within marine organisms, sediments, and the water column. The abundance of iridium in seawater[62] and organisms[63] is relatively low, as it does not readily form chloride complexes.[63] The abundance in organisms is about 20 parts per trillion, or about five orders of magnitude less than in sedimentary rocks at the Cretaceous–Paleogene (K–T) boundary.[63] The concentration of iridium in seawater and marine sediment is sensitive to marine oxygenation, seawater temperature, and various geological and biological processes.[64]
Iridium in sediments can come from cosmic dust, volcanoes, precipitation from seawater, microbial processes, or hydrothermal vents,[64] and its abundance can be strongly indicative of the source.[65][64] It tends to associate with other ferrous metals in manganese nodules.[62] Iridium is one of the characteristic elements of extraterrestrial rocks, and, along with osmium, can be used as a tracer element for meteoritic material in sediment.[66][67] Some of the mass extinctions, such as the Cretaceous extinction, can be identified by anomalously high concentrations of iridium in sediment, and these can be linked to major asteroid impacts.[68]
Cretaceous–Paleogene boundary presence

The Cretaceous–Paleogene boundary of 66 million years ago, marking the temporal border between the Cretaceous and Paleogene periods of geological time, was identified by a thin stratum of iridium-rich clay.[69] A team led by Luis Alvarez proposed in 1980 an extraterrestrial origin for this iridium, attributing it to an asteroid or comet impact.[69] Their theory, known as the Alvarez hypothesis, is now widely accepted to explain the extinction of the non-avian dinosaurs. A large buried impact crater structure with an estimated age of about 66 million years was later identified under what is now the Yucatán Peninsula (the Chicxulub crater).[70][71] Dewey M. McLean and others argue that the iridium may have been of volcanic origin instead, because Earth's core is rich in iridium, and active volcanoes such as Piton de la Fournaise, in the island of Réunion, are still releasing iridium.[72][73]
Production
| Year | Consumption (tonnes) |
Price (USD)[74] |
|---|---|---|
| 2001 | 2.6 | $415.25/ozt ($13.351/g) |
| 2002 | 2.5 | $294.62/ozt ($9.472/g) |
| 2003 | 3.3 | $93.02/ozt ($2.991/g) |
| 2004 | 3.60 | $185.33/ozt ($5.958/g) |
| 2005 | 3.86 | $169.51/ozt ($5.450/g) |
| 2006 | 4.08 | $349.45/ozt ($11.235/g) |
| 2007 | 3.70 | $444.43/ozt ($14.289/g) |
| 2008 | 3.10 | $448.34/ozt ($14.414/g) |
| 2009 | 2.52 | $420.4/ozt ($13.52/g) |
| 2010 | 10.40 | $642.15/ozt ($20.646/g) |
| 2011 | 9.36 | $1,035.87/ozt ($33.304/g) |
| 2012 | 5.54 | $1,066.23/ozt ($34.280/g) |
| 2013 | 6.16 | $826.45/ozt ($26.571/g) |
| 2014 | 6.1 | $556.19/ozt ($17.882/g) |
| 2015 | 7.81 | $544/ozt ($17.5/g) |
| 2016 | 7.71 | $586.90/ozt ($18.869/g) |
| 2017 | n.d. | $908.35/ozt ($29.204/g) |
| 2018 | n.d. | $1,293.27/ozt ($41.580/g) |
In 2018, worldwide production of iridium totaled 7,300 kilograms (16,100 lb).[75] In mid April 2021, iridium reached a price of US$6,400 per troy ounce on Metals Daily (a precious metals commodity listing). The price dropped to US$5158 for 2021. Iridium is also obtained commercially as a by-product from nickel and copper mining and processing. During electrorefining of copper and nickel, noble metals such as silver, gold and the platinum group metals as well as selenium and tellurium settle to the bottom of the cell as anode mud, which forms the starting point for their extraction.[74] To separate the metals, they must first be brought into solution. Several separation methods are available depending on the nature of the mixture; two representative methods are fusion with sodium peroxide followed by dissolution in aqua regia, and dissolution in a mixture of chlorine with hydrochloric acid.[32][61]
After the mixture is dissolved, iridium is separated from the other platinum group metals by precipitating ammonium hexachloroiridate ((NH
4)
2IrCl
6) or by extracting IrCl2−
6 with organic amines.[76] The first method is similar to the procedure Tennant and Wollaston used for their separation. The second method can be planned as continuous liquid–liquid extraction and is therefore more suitable for industrial scale production. In either case, the product is reduced using hydrogen, yielding the metal as a powder or sponge that can be treated using powder metallurgy techniques.[77][78]
Iridium prices have fluctuated over a considerable range. With a relatively small volume in the world market (compared to other industrial metals like aluminium or copper), the iridium price reacts strongly to instabilities in production, demand, speculation, hoarding, and politics in the producing countries. As a substance with rare properties, its price has been particularly influenced by changes in modern technology: The gradual decrease between 2001 and 2003 has been related to an oversupply of Ir crucibles used for industrial growth of large single crystals.[74][79] Likewise the prices above $1,000 per troy ounce ($32/g) between 2010 and 2014 have been explained with the installation of production facilities for single crystal sapphire used in LED backlights for TVs.[80]
| Country | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| World | 7,720 | 7,180 | 7,540 | 7,910 | 8,170 |
| 6,624 | 6,057 | 6,357 | 6,464 | 6,786 | |
| 598 | 619 | 586 | 845 | 836 | |
| 300 | 200 | 400 | 300 | 300 | |
| 200 | 300 | 200 | 300 | 250 |
The demand for iridium surged from 2.5 tonnes (5,500 lb) in 2009 to 10.4 tonnes (23,000 lb) in 2010, mostly because of electronics-related applications that saw a rise from 0.2 to 6 tonnes (440 to 13,230 lb) – iridium crucibles are commonly used for growing large high-quality single crystals, demand for which has increased sharply. This increase in iridium consumption is predicted to saturate due to accumulating stocks of crucibles, as happened earlier in the 2000s. Other major applications include spark plugs that consumed 0.78 tonnes (1,700 lb) of iridium in 2007, electrodes for the chloralkali process (1.1 t or 2,400 lb in 2007) and chemical catalysts (0.75 t or 1,700 lb in 2007).[74][82]
Applications
Ir metal and alloys
The high melting point, hardness and corrosion resistance of iridium and its alloys determine most of its applications. Resistance to heat and corrosion makes iridium an important alloying agent. Certain long-life aircraft engine parts are made of an iridium alloy, and an iridium–titanium alloy is used for deep-water pipes because of its corrosion resistance.[22] Iridium is also used as a hardening agent in platinum alloys.
Iridium are used, for example,[clarification needed] for multi-pored spinnerets, through which a plastic polymer melt is extruded to form fibers, such as rayon.[83] Osmium–iridium is used for compass bearings and for balances.[13]
Because of their resistance to arc erosion, iridium alloys are used for electrical contacts for some [which?] spark plugs,[84][85] and iridium-based spark plugs are particularly used in aviation.
Devices that must withstand extremely high temperatures are often made from iridium. For example, high-temperature crucibles made of iridium are used in the Czochralski process to produce oxide single-crystals (such as sapphires) for use in computer memory devices and in solid state lasers.[84][86] The crystals, such as gadolinium gallium garnet and yttrium gallium garnet, are grown by melting pre-sintered charges of mixed oxides under oxidizing conditions at temperatures up to 2,100 °C (3,810 °F).[13]
Catalysis
Iridium compounds are used as catalysts in the Cativa process for carbonylation of methanol to produce acetic acid.[87][88]
Iridium complexes are often active for hydrogenation. This property is the basis of the industrial route to the chiral herbicide (S)-metolachlor. As practiced by Syngenta on the scale of 10,000 tons/year, the complex [[ [Ir(COD)Cl]2 in the presence of Josiphos.[89]
Medical imaging
The radioisotope iridium-192 is one of the two most important sources of energy for use in industrial γ-radiography for non-destructive testing of metals.[90][91] Additionally, 192Ir is used as a source of gamma radiation for the treatment of cancer using brachytherapy, a form of radiotherapy where a sealed radioactive source is placed inside or next to the area requiring treatment. Specific treatments include high-dose-rate prostate brachytherapy, biliary duct brachytherapy, and intracavitary cervix brachytherapy.[22] iridium-192 is normally produced by neutron activation of isotop iridium-191 in natural-abundance iridium metal [92]
The use of iridium(III) complexes for imaging of mitochondria has been reviewed.[93]
Scientific

An alloy of 90% platinum and 10% iridium was used in 1889 to construct the International Prototype Metre and kilogram mass, kept by the International Bureau of Weights and Measures near Paris.[22] The meter bar was replaced as the definition of the fundamental unit of length in 1960 by a line in the atomic spectrum of krypton,[d][94] but the kilogram prototype remained the international standard of mass until 20 May 2019, when the kilogram was redefined in terms of the Planck constant.[95]
Iridium is often used as a coating for non-conductive materials in preparation for observation in scanning electron microscopes (SEM). The addition of a 2 to 20 nm (7.9×10−8 to 7.87×10−7 in) layer of iridium helps especially organic materials survive electron beam damage and reduces static charge build-up within the target area of the SEM beam's focal point.[96] A coating of iridium also increases the signal to noise ratio associated with secondary electron emission which is essential to using SEMs for X-Ray spectrographic composition analysis. While other metals can be used for coating objects for SEM use, iridium is the preferred coating when samples will be studied with a wide variety of imaging parameters.[97]
Iridium has been used in the radioisotope thermoelectric generators of unmanned spacecraft such as the Voyager, Viking, Pioneer, Cassini, Galileo, and New Horizons. Iridium was chosen to encapsulate the plutonium-238 fuel in the generator because it can withstand the operating temperatures of up to 2,000 °C (3,630 °F) and for its great strength.[13]
Another use concerns X-ray optics, especially X-ray telescopes.[98] The mirrors of the Chandra X-ray Observatory are coated with a layer of iridium 60 nm (2.4×10−6 in) thick. Iridium proved to be the best choice for reflecting X-rays after nickel, gold, and platinum were also tested. The iridium layer, which had to be smooth to within a few atoms, was applied by depositing iridium vapor under high vacuum on a base layer of chromium.[99]
Iridium is used in particle physics for the production of antiprotons, a form of antimatter. Antiprotons are made by shooting a high-intensity proton beam at a conversion target, which needs to be made from a very high density material. Although tungsten may be used instead, iridium has the advantage of better stability under the shock waves induced by the temperature rise due to the incident beam.[100]

Carbon–hydrogen bond activation (C–H activation) is an area of research on reactions that cleave carbon–hydrogen bonds, which were traditionally regarded as unreactive. The first reported successes at activating C–H bonds in saturated hydrocarbons, published in 1982, used organometallic iridium complexes that undergo an oxidative addition with the hydrocarbon.[101][102]
Iridium complexes are being investigated as catalysts for asymmetric hydrogenation. These catalysts have been used in the synthesis of natural products and able to hydrogenate certain difficult substrates, such as unfunctionalized alkenes, enantioselectively (generating only one of the two possible enantiomers).[103][104]
Iridium forms a variety of complexes of fundamental interest in triplet harvesting.[105][106][107]
As part of the cationic nucleic acid intercalators, Iridium-191 and Iridium-193 isotopes are used to detect nucleic acids in CyTOF experiments to analyse the presence or viability of nucleated cells in biological samples.[108]
Historical

Iridium–osmium alloys were used in fountain pen nib tips. The first major use of iridium was in 1834 in nibs mounted on gold.[13] Since 1944, the famous Parker 51 fountain pen was fitted with a nib tipped by a ruthenium and iridium alloy (with 3.8% iridium). The tip material in modern fountain pens is still conventionally called "iridium", although there is seldom any iridium in it; other metals such as ruthenium, osmium, and tungsten have taken its place.[109]
An iridium–platinum alloy was used for the touch holes or vent pieces of cannon. According to a report of the Paris Exhibition of 1867, one of the pieces being exhibited by Johnson and Matthey "has been used in a Whitworth gun for more than 3000 rounds, and scarcely shows signs of wear yet. Those who know the constant trouble and expense which are occasioned by the wearing of the vent-pieces of cannon when in active service, will appreciate this important adaptation".[110]
The pigment iridium black, which consists of very finely divided iridium, is used for painting porcelain an intense black; it was said that "all other porcelain black colors appear grey by the side of it".[111]
Precautions
Iridium in bulk metallic form is not biologically important or hazardous to health due to its lack of reactivity with tissues; there are only about 20 parts per trillion of iridium in human tissue.[22] Like most metals, finely divided iridium powder can be hazardous to handle, as it is an irritant and may ignite in air.[61] Very little is known about the toxicity of iridium compounds, primarily because it is used so rarely that few people come in contact with it and those who do only with very small amounts. However, soluble salts, such as the iridium halides, could be hazardous due to elements other than iridium or due to iridium itself.[38] At the same time, most iridium compounds are insoluble, which makes absorption into the body difficult.[22]
A radioisotope of iridium, 192
Ir, is dangerous, like other radioactive isotopes. The only reported injuries related to iridium concern accidental exposure to radiation from 192
Ir used in brachytherapy.[38] High-energy gamma radiation from 192
Ir can increase the risk of cancer. External exposure can cause burns, radiation poisoning, and death. Ingestion of 192Ir can burn the linings of the stomach and the intestines.[112] 192Ir, 192mIr, and 194mIr tend to deposit in the liver, and can pose health hazards from both gamma and beta radiation.[54]
Notes
- ^ At room temperature and standard atmospheric pressure, iridium has been calculated to have a density of 22.65 g/cm3 (0.818 lb/cu in), 0.04 g/cm3 (0.0014 lb/cu in) higher than osmium measured the same way.[8] Still, the experimental X-ray crystallography value is considered to be the most accurate, and as such iridium is considered to be the second densest element.[9]
- ^ Most common oxidation states of iridium are in bold. The right column lists one representative compound for each oxidation state.
- ^ Iridium literally means "of rainbows".
- ^ The definition of the meter was changed again in 1983. The meter is currently defined as the distance traveled by light in a vacuum during a time interval of 1⁄299,792,458 of a second.
References
- ^ "Standard Atomic Weights: Iridium". CIAAW. 2017.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ a b c Wang, Guanjun; Zhou, Mingfei; Goettel, James T.; Schrobilgen, Gary G.; Su, Jing; Li, Jun; Schlöder, Tobias; Riedel, Sebastian (2014). "Identification of an iridium-containing compound with a formal oxidation state of IX". Nature. 514 (7523): 475–477. Bibcode:2014Natur.514..475W. doi:10.1038/nature13795. PMID 25341786. S2CID 4463905.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Helmenstine, Anne Marie (May 6, 2022). "What is the Densest Element on the Periodic Table?". Thoughtco.com. Retrieved October 9, 2022.
- ^ Arblaster, J. W. (1989). "Densities of Osmium and Iridium Recalculations Based upon a Review of the Latest Crystallographic Data". Platinum Metals Rev. 33 (1): 14–16.
- ^ Becker, Luann (2002). "Repeated Blows" (PDF). Scientific American. 286 (3): 77–83. Bibcode:2002SciAm.286c..76B. doi:10.1038/scientificamerican0302-76. PMID 11857903. Retrieved January 19, 2016.
- ^ Kyte, Frank T.; Zhiming Zhou; John T. Wasson (1981). "High noble metal concentrations in a late Pliocene sediment". Nature. 292 (5822): 417–420. Bibcode:1981Natur.292..417K. doi:10.1038/292417a0. ISSN 0028-0836. S2CID 4362591.
- ^ a b c d e f g h i j k l m n Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth–Heinemann. pp. 1113–1143, 1294. ISBN 978-0-7506-3365-9. OCLC 213025882.
- ^ a b c d e f g h i j k l m n o p Hunt, L. B. (1987). "A History of Iridium". Platinum Metals Review. 31 (1): 32–41.
- ^ Kittel, C. (2004). Introduction to Solid State Physics (7th ed.). Wiley-India. ISBN 978-81-265-1045-0.
- ^ Arblaster, J. W. (1995). "Osmium, the Densest Metal Known". Platinum Metals Review. 39 (4): 164. Archived from the original on 2011-09-27. Retrieved 2008-10-02.
- ^ Cotton, Simon (1997). Chemistry of Precious Metals. Springer-Verlag New York, LLC. p. 78. ISBN 978-0-7514-0413-5.
- ^ Lide, D. R. (1990). CRC Handbook of Chemistry and Physics (70th ed.). Boca Raton (FL):CRC Press. ISBN 9780849304712.
- ^ Arblaster, J. W. (1989). "Densities of osmium and iridium: recalculations based upon a review of the latest crystallographic data" (PDF). Platinum Metals Review. 33 (1): 14–16. Archived from the original (PDF) on 2012-02-07. Retrieved 2008-09-17.
- ^ US 3293031A, Cresswell, Peter & Rhys, David, published 20/12/1966
- ^ Darling, A. S. (1960). "Iridium Platinum Alloys - A Critical Review Of Their Constitution And Properties". Platinum Metals Review. 4 (1): 18–26. Reviewed in "Iridium–Platinum Alloys". Nature. 186 (4720): 211. 1960. Bibcode:1960Natur.186Q.211.. doi:10.1038/186211a0. S2CID 4211238.
- ^ Biggs, T.; Taylor, S. S.; van der Lingen, E. (2005). "The Hardening of Platinum Alloys for Potential Jewellery Application". Platinum Metals Review. 49 (1): 2–15. doi:10.1595/147106705X24409.
- ^ a b c d e f g h i j Emsley, J. (2003). "Iridium". Nature's Building Blocks: An A–Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 201–204. ISBN 978-0-19-850340-8.
- ^ Emsley, John (2011). Nature's Building Blocks: An A–Z Guide to the Elements (New ed.). New York: Oxford University Press. ISBN 978-0-19-960563-7.
- ^ a b Perry, D. L. (1995). Handbook of Inorganic Compounds. CRC Press. pp. 203–204. ISBN 978-1439814611.
- ^ Lagowski, J. J., ed. (2004). Chemistry Foundations and Applications. Vol. 2. Thomson Gale. pp. 250–251. ISBN 978-0028657233.
- ^ Munson, Ronald A. (February 1968). "The Synthesis of Iridium Disulfide and Nickel diarsenide having the Pyrite Structure" (PDF). Inorganic Chemistry. 7 (2): 389–390. doi:10.1021/ic50060a047.
- ^ Jung, D.; Demazeau, Gérard (1995). "High Oxygen Pressure and the Preparation of New Iridium (VI) Oxides with Perovskite Structure: Sr
2MIrO
6 (M = Ca, Mg)". Journal of Solid State Chemistry. 115 (2): 447–455. Bibcode:1995JSSCh.115..447J. doi:10.1006/jssc.1995.1158. - ^ Gong, Y.; Zhou, M.; Kaupp, M.; Riedel, S. (2009). "Formation and Characterization of the Iridium Tetroxide Molecule with Iridium in the Oxidation State +VIII". Angewandte Chemie International Edition. 48 (42): 7879–7883. doi:10.1002/anie.200902733. PMID 19593837.
- ^ Gulliver, D. J.; Levason, W. (1982). "The chemistry of ruthenium, osmium, rhodium, iridium, palladium and platinum in the higher oxidation states". Coordination Chemistry Reviews. 46: 1–127. doi:10.1016/0010-8545(82)85001-7.
- ^ Holleman, A. F.; Wiberg, E.; Wiberg, N. (2001). Inorganic Chemistry (1st ed.). Academic Press. ISBN 978-0-12-352651-9. OCLC 47901436.
- ^ Černý, R.; Joubert, J.-M.; Kohlmann, H.; Yvon, K. (2002). "Mg
6Ir
2H
11, a new metal hydride containing saddle-like IrH5−
4 and square-pyramidal IrH4−
5 hydrido complexes". Journal of Alloys and Compounds. 340 (1–2): 180–188. doi:10.1016/S0925-8388(02)00050-6. - ^ a b c d e Renner, H.; Schlamp, G.; Kleinwächter, I.; Drost, E.; Lüschow, H. M.; Tews, P.; Panster, P.; Diehl, M.; et al. (2002). "Platinum group metals and compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a21_075. ISBN 978-3527306732.
- ^ Vaska, L.; DiLuzio, J.W. (1961). "Carbonyl and Hydrido-Carbonyl Complexes of Iridium by Reaction with Alcohols. Hydrido Complexes by Reaction with Acid". Journal of the American Chemical Society. 83 (12): 2784–2785. doi:10.1021/ja01473a054.
- ^ Crabtree, R. H. (1979). "Iridium compounds in catalysis". Accounts of Chemical Research. 12 (9): 331–337. doi:10.1021/ar50141a005.
- ^ Crabtree, R. H. (2005). The Organometallic Chemistry of the Transition Metals (PDF). Wiley. ISBN 978-0471662563. OCLC 224478241. Archived from the original (PDF) on 2012-11-19.
- ^ "Research and Development". Furuya Metal Co. 2013. Archived from the original on 2013-11-11.
- ^ a b c d e Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ a b c Mager Stellman, J. (1998). "Iridium". Encyclopaedia of Occupational Health and Safety. International Labour Organization. pp. 63.19. ISBN 978-92-2-109816-4. OCLC 35279504.
- ^ Robinson, R.; Thoennessen, M. (2012). "Discovery of tantalum, rhenium, osmium, and iridium isotopes". Atomic Data and Nuclear Data Tables. 98 (5): 911–932. arXiv:1109.0526. Bibcode:2012ADNDT..98..911R. doi:10.1016/j.adt.2011.09.003. S2CID 53992437.
- ^ Chereminisoff, N. P. (1990). Handbook of Ceramics and Composites. CRC Press. p. 424. ISBN 978-0-8247-8006-7.
- ^ a b c Weeks, M. E. (1968). Discovery of the Elements (7th ed.). Journal of Chemical Education. pp. 385–407. ISBN 978-0-8486-8579-9. OCLC 23991202.
- ^ Donald McDonald, Leslie B. Hunt (1982). A History of Platinum and its Allied Metals. Johnson Matthey Plc. pp. 7–8. ISBN 978-0-905118-83-3.
- ^ Dixon, Joshua; Brownrigg, William (1801). The literary life of William Brownrigg. To which are added an account of the coal mines near Whitehaven: And Observations on the means of preventing epidemic fevers. p. 52. Archived from the original on 24 March 2017.
- ^ Watson, Wm; Brownrigg, William (1749). "Several Papers concerning a New Semi-Metal, Called Platina; Communicated to the Royal Society by Mr. Wm. Watson F. R. S". Philosophical Transactions. 46 (491–496): 584–596. Bibcode:1749RSPT...46..584W. doi:10.1098/rstl.1749.0110. S2CID 186213277.
- ^ Marggraf, Andreas Sigismund (1760). Versuche mit dem neuen mineralischen Körper Platina del pinto genannt. Archived from the original on 24 March 2017.
- ^ Thomson, T. (1831). A System of Chemistry of Inorganic Bodies. Vol. 1. Baldwin & Cradock, London; and William Blackwood, Edinburgh. p. 693.
- ^ a b Griffith, W. P. (2004). "Bicentenary of Four Platinum Group Metals. Part II: Osmium and iridium – events surrounding their discoveries". Platinum Metals Review. 48 (4): 182–189. doi:10.1595/147106704x4844.
- ^ Weeks, M. E. (1968). Discovery of the Elements (7th ed.). Journal of Chemical Education. pp. 414–418. ISBN 978-0-8486-8579-9. OCLC 23991202.
- ^ Tennant, S. (1804). "On Two Metals, Found in the Black Powder Remaining after the Solution of Platina". Philosophical Transactions of the Royal Society of London. 94: 411–418. doi:10.1098/rstl.1804.0018. JSTOR 107152.
- ^ Trigg, G. L. (1995). "Recoilless Emission and Absorption of Radiation". Landmark Experiments in Twentieth Century Physics. Courier Dover Publications. pp. 179–190. ISBN 978-0-486-28526-9. OCLC 31409781.
- ^ Mössbauer, R. L. (1958). "Gammastrahlung in Ir191". Zeitschrift für Physik A (in German). 151 (2): 124–143. Bibcode:1958ZPhy..151..124M. doi:10.1007/BF01344210. S2CID 121129342.
- ^ Waller, I. (1964). "The Nobel Prize in Physics 1961: presentation speech". Nobel Lectures, Physics 1942–1962. Elsevier.
- ^ Scott, E. R. D.; Wasson, J. T.; Buchwald, V. F. (1973). "The chemical classification of iron meteorites—VII. A reinvestigation of irons with Ge concentrations between 25 and 80 ppm". Geochimica et Cosmochimica Acta. 37 (8): 1957–1983. Bibcode:1973GeCoA..37.1957S. doi:10.1016/0016-7037(73)90151-8.
- ^ a b "Iridium" (PDF). Human Health Fact Sheet. Argonne National Laboratory. 2005. Archived from the original (PDF) on March 4, 2012. Retrieved 2008-09-20.
- ^ Xiao, Z.; Laplante, A. R. (2004). "Characterizing and recovering the platinum group minerals—a review". Minerals Engineering. 17 (9–10): 961–979. doi:10.1016/j.mineng.2004.04.001.
- ^ "Irarsite: Mineral information, data and localities". Mindat.org. Retrieved 27 September 2022.
- ^ "Iridium: The mineralogy of Iridium". Mindat.org. Retrieved 27 September 2022.
- ^ "International Mineralogical Association - Commission on New Minerals, Nomenclature and Classification". nrmima.nrm.se. Archived from the original on 2019-08-10. Retrieved 2018-10-06.
- ^ "Cuproiridsite CuIr2S4" (PDF). Hanbook of mineralogy.org. Retrieved 3 March 2022.
- ^ Vitaly A. Stepanov; Valentina E. Kungurova; Vitaly I. Gvozdev (2010). "Irasite Discovery in Copper-Nickel Ores of Shanuch Deposit (KAMCHATKA)" (PDF). New Data on Minerals. 45: 23. Retrieved 3 March 2022.
- ^ a b c d Seymour, R. J.; O'Farrelly, J. I. (2001). "Platinum-group metals". Kirk Othmer Encyclopedia of Chemical Technology. Wiley. doi:10.1002/0471238961.1612012019052513.a01.pub2. ISBN 978-0471238966.
- ^ a b Goldberg, Hodge; Kay, V; Stallard, M; Koide, M (1986). "Some comparative marine chemistries of platinum and iridium". Applied Geochemistry. 1 (2): 227–232. Bibcode:1986ApGC....1..227G. doi:10.1016/0883-2927(86)90006-5.
- ^ a b c Wells, Boothe (1988). "Iridium in marine organisms". Geochimica et Cosmochimica Acta. 52 (6): 1737–1739. Bibcode:1988GeCoA..52.1737W. doi:10.1016/0016-7037(88)90242-6.
- ^ a b c Sawlowicz, Z (1993). "Iridium and other platinum-group elements as geochemical markers in sedimentary environments". Palaeogeography, Palaeoclimatology, Palaeoecology. 104 (4): 253–270. Bibcode:1993PPP...104..253S. doi:10.1016/0031-0182(93)90136-7.
- ^ Crocket, Macdougall; Harriss, R (1973). "Gold, palladium and iridium in marine sediments". Geochimica et Cosmochimica Acta. 37 (12): 2547–2556. Bibcode:1973GeCoA..37.2547C. doi:10.1016/0016-7037(73)90264-0.
- ^ Peucker-Ehrenbrink, B (2001). "Iridium and Osmium as Tracers of Extraterrestrial Matter in Marine Sediments". Accretion of Extraterrestrial Matter Throughout Earth's History. pp. 163–178. doi:10.1007/978-1-4419-8694-8_10. ISBN 978-1-4613-4668-5.
- ^ Barker, J; Edward, A (1968). "Accretion rate of cosmic matter from iridium and osmium contents of deep-sea sediments". Geochimica et Cosmochimica Acta. 32 (6): 627–645. Bibcode:1968GeCoA..32..627B. doi:10.1016/0016-7037(68)90053-7.
- ^ Colodner, D; Edmond, J (1992). "Post-depositional mobility of platinum, iridium and rhenium in marine sediments". Nature. 358 (6385): 402–404. Bibcode:1992Natur.358..402C. doi:10.1038/358402a0. S2CID 37386975.
- ^ a b Alvarez, L. W.; Alvarez, W.; Asaro, F.; Michel, H. V. (1980). "Extraterrestrial cause for the Cretaceous–Tertiary extinction" (PDF). Science. 208 (4448): 1095–1108. Bibcode:1980Sci...208.1095A. CiteSeerX 10.1.1.126.8496. doi:10.1126/science.208.4448.1095. PMID 17783054. S2CID 16017767.
- ^ Hildebrand, A. R.; Penfield, Glen T.; Kring, David A.; Pilkington, Mark; Zanoguera, Antonio Camargo; Jacobsen, Stein B.; Boynton, William V. (1991). "Chicxulub Crater; a possible Cretaceous/Tertiary boundary impact crater on the Yucatan Peninsula, Mexico". Geology. 19 (9): 867–871. Bibcode:1991Geo....19..867H. doi:10.1130/0091-7613(1991)019<0867:CCAPCT>2.3.CO;2.
- ^ Frankel, C. (1999). The End of the Dinosaurs: Chicxulub Crater and Mass Extinctions. Cambridge University Press. ISBN 978-0-521-47447-4. OCLC 40298401.
- ^ Ryder, G.; Fastovsky, D. E.; Gartner, S. (1996). The Cretaceous-Tertiary Event and Other Catastrophes in Earth History. Geological Society of America. p. 47. ISBN 978-0-8137-2307-5.
- ^ Toutain, J.-P.; Meyer, G. (1989). "Iridium-Bearing Sublimates at a Hot-Spot Volcano (Piton De La Fournaise, Indian Ocean)". Geophysical Research Letters. 16 (12): 1391–1394. Bibcode:1989GeoRL..16.1391T. doi:10.1029/GL016i012p01391.
- ^ a b c d Platinum-Group Metals. U.S. Geological Survey Mineral Commodity Summaries
- ^ Singerling, Sheryl A.; Schulte, Ruth F. (August 2021). "Platinum-Group Metals". 2018 Minerals Yearbook (PDF). USGS. p. 57.11.
- ^ Gilchrist, Raleigh (1943). "The Platinum Metals". Chemical Reviews. 32 (3): 277–372. doi:10.1021/cr60103a002. S2CID 96640406.
- ^ Ohriner, E. K. (2008). "Processing of Iridium and Iridium Alloys". Platinum Metals Review. 52 (3): 186–197. doi:10.1595/147106708X333827.
- ^ Hunt, L. B.; Lever, F. M. (1969). "Platinum Metals: A Survey of Productive Resources to industrial Uses" (PDF). Platinum Metals Review. 13 (4): 126–138. Archived from the original (PDF) on 2008-10-29. Retrieved 2008-10-01.
- ^ Hagelüken, C. (2006). "Markets for the catalysts metals platinum, palladium, and rhodium" (PDF). Metall. 60 (1–2): 31–42. Archived from the original (PDF) on March 4, 2009.
- ^ "Platinum 2013 Interim Review" (PDF). Platinum Today. Johnson Matthey. Retrieved 2014-01-10.
- ^ "Mineral Yearbook 2020 tables-only release". USGS.
- ^ Jollie, D. (2008). "Platinum 2008". Platinum Metals Rev. 52 (3). Johnson Matthey: 198–199. doi:10.1595/147106708X327428. ISSN 0268-7305. Retrieved 2022-09-26.
- ^ Egorova, R. V.; Korotkov, B. V.; Yaroshchuk, E. G.; Mirkus, K. A.; Dorofeev N. A.; Serkov, A. T. (1979). "Spinnerets for viscose rayon cord yarn". Fibre Chemistry. 10 (4): 377–378. doi:10.1007/BF00543390. S2CID 135705244.
- ^ a b Handley, J. R. (1986). "Increasing Applications for Iridium". Platinum Metals Review. 30 (1): 12–13.
- ^ Stallforth, H.; Revell, P. A. (2000). Euromat 99. Wiley-VCH. ISBN 978-3-527-30124-9.
- ^ Crookes, W. (1908). "On the Use of Iridium Crucibles in Chemical Operations". Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character. 80 (541): 535–536. Bibcode:1908RSPSA..80..535C. doi:10.1098/rspa.1908.0046. JSTOR 93031.
- ^ Cheung, H.; Tanke, R. S.; Torrence, G. P. (2000). "Acetic acid". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a01_045. ISBN 978-3527306732.
- ^ Jones, Jane H. (2000). "The cativa™ process for the manufacture of acetic acid". Platinum Metals Review. 44 (3): 94–105.
- ^ Matthias Beller, Hans-Ulrich Blaser, ed. (2012). Topics in Organometallic Chemistry. Vol. 42. Berlin, Heidelberg: Springer. ISBN 978-3-642-32832-9.
{{cite book}}: Missing or empty|title=(help) - ^ Halmshaw, R. (1954). "The use and scope of Iridium 192 for the radiography of steel". British Journal of Applied Physics. 5 (7): 238–243. Bibcode:1954BJAP....5..238H. doi:10.1088/0508-3443/5/7/302.
- ^ Hellier, Chuck (2001). Handbook of Nondestructive Evlaluation. The McGraw-Hill Companies. ISBN 978-0-07-028121-9.
- ^ Jean Pouliot; Luc Beaulieu (2010). "13 - Modern Principles of Brachytherapy Physics: From 2-D to 3-D to Dynamic Planning and Delivery". In Richard T. Hoppe; Theodore Locke Phillips; Mack Roach (eds.). Leibel and Phillips Textbook of Radiation Oncology (3rd ed.). W.B. Saunders. pp. 224–244. doi:10.1016/B978-1-4160-5897-7.00013-5. ISBN 9781416058977.
- ^ Tan, Cai-Ping; Wang, Jie; Ji, Liang-Nian; Mao, Zong-Wan (2021). "Chapter 14. Iridium(III) Complexes in Bio-Imaging Including Mitochrondria". Metal Ions in Bio-Imaging Techniques. Springer. pp. 403–423. doi:10.1515/9783110685701-020. S2CID 233701808.
- ^ Penzes, W. B. (2001). "Time Line for the Definition of the Meter". National Institute for Standards and Technology. Retrieved 2008-09-16.
- ^ General section citations: Recalibration of the U.S. National Prototype Kilogram, R. S. Davis, Journal of Research of the National Bureau of Standards, 90, No. 4, July–August 1985 (5.5 MB PDF Archived 2017-02-01 at the Wayback Machine); and The Kilogram and Measurements of Mass and Force, Z. J. Jabbour et al., J. Res. Natl. Inst. Stand. Technol. 106, 2001, 25–46 (3.5 MB PDF)
- ^ Höflinger, Gisela (2013-08-28). "Brief Introduction to Coating Technology for Electron Microscopy". Leica Microsystems. Leica Microsystems. Retrieved 22 April 2019.
- ^ Abdullah, S. Z.; Bérubé, Pierre R.; Horne, D.J. (2014). "SEM imaging of membranes: Importance of sample preparation and imaging parameters". Journal of Membrane Science. 463: 113–125. doi:10.1016/j.memsci.2014.03.048.
- ^ Ziegler, E.; Hignette, O.; Morawe, Ch.; Tucoulou, R. (2001). "High-efficiency tunable X-ray focusing optics using mirrors and laterally-graded multilayers". Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 467–468 (Part 2): 954–957. Bibcode:2001NIMPA.467..954Z. doi:10.1016/S0168-9002(01)00533-2.
- ^ "Face-to-Face with Jerry Johnston, CXC Program Manager & Bob Hahn, Chief Engineer at Optical Coating Laboratories, Inc., Santa Rosa, CA". Harvard-Smithsonian Center for Astrophysics; Chandra X-ray Center. 1995. Retrieved 2008-09-24.
- ^ Möhl, D. (1997). "Production of low-energy antiprotons". Zeitschrift Hyperfine Interactions. 109 (1–4): 33–41. Bibcode:1997HyInt.109...33M. doi:10.1023/A:1012680728257. S2CID 118043983.
- ^ a b Janowicz, A. H.; Bergman, R. G. (1982). "Carbon-hydrogen activation in completely saturated hydrocarbons: direct observation of M + R-H → M(R)(H)". Journal of the American Chemical Society. 104 (1): 352–354. doi:10.1021/ja00365a091.
- ^ a b Hoyano, J. K.; Graham, W. A. G. (1982). "Oxidative addition of the carbon-hydrogen bonds of neopentane and cyclohexane to a photochemically generated iridium(I) complex". Journal of the American Chemical Society. 104 (13): 3723–3725. doi:10.1021/ja00377a032.
- ^ Källström, K; Munslow, I; Andersson, P G (2006). "Ir-catalysed asymmetric hydrogenation: Ligands, substrates and mechanism". Chemistry: A European Journal. 12 (12): 3194–3200. doi:10.1002/chem.200500755. PMID 16304642.
- ^ Roseblade, S. J.; Pfaltz, A. (2007). "Iridium-catalyzed asymmetric hydrogenation of olefins". Accounts of Chemical Research. 40 (12): 1402–1411. doi:10.1021/ar700113g. PMID 17672517.
- ^ Wang, X.; Andersson, M. R.; Thompson, M. E.; Inganäsa, O. (2004). "Electrophosphorescence from substituted poly(thiophene) doped with iridium or platinum complex". Thin Solid Films. 468 (1–2): 226–233. Bibcode:2004TSF...468..226W. doi:10.1016/j.tsf.2004.05.095.
- ^ Tonzetich, Zachary J. (2002). "Organic Light Emitting Diodes—Developing Chemicals to Light the Future" (PDF). Journal of Undergraduate Research. 1 (1). Rochester University. Retrieved 2008-10-10.
- ^ Holder, E.; Langefeld, B. M. W.; Schubert, U. S. (2005-04-25). "New Trends in the Use of Transition Metal-Ligand Complexes for Applications in Electroluminescent Devices". Advanced Materials. 17 (9): 1109–1121. doi:10.1002/adma.200400284. S2CID 98683000.
- ^ "Fluidigm Cell-ID™ Intercalator-Ir".
- ^ Mottishaw, J. (1999). "Notes from the Nib Works—Where's the Iridium?". The PENnant. XIII (2).
- ^ Crookes, W., ed. (1867). "The Paris Exhibition". The Chemical News and Journal of Physical Science. XV: 182.
- ^ Pepper, J. H. (1861). The Playbook of Metals: Including Personal Narratives of Visits to Coal, Lead, Copper, and Tin Mines, with a Large Number of Interesting Experiments Relating to Alchemy and the Chemistry of the Fifty Metallic Elements. Routledge, Warne, and Routledge. p. 455.
- ^ "Radioisotope Brief: Iridium-192 (Ir-192)" (PDF). Radiation Emergencies. Centers for Disease Control and Prevention. 2004-08-18. Retrieved 2008-09-20.
External links
- Iridium at The Periodic Table of Videos (University of Nottingham)
- Iridium in Encyclopædia Britannica


