Terconazole: Difference between revisions
m Infobox drug: rm/replace deprecated params. Fix unk parameters (rm some Chembox-params) (via AWB script) |
No edit summary |
||
| Line 2: | Line 2: | ||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| verifiedrevid = 470602308 |
| verifiedrevid = 470602308 |
||
| IUPAC_name = 1-[4-[ [('' |
| IUPAC_name = 1-[4-[ [(2''S'',4''S'')-2-(2,4-Dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-propan-2-yl-piperazine |
||
| image = Terconazole. |
| image = Terconazole structure.svg |
||
| width = 300 |
|||
<!--Clinical data--> |
<!--Clinical data--> |
||
| tradename = Terazol |
| tradename = Terazol |
||
| Line 11: | Line 13: | ||
| legal_status = |
| legal_status = |
||
| routes_of_administration = |
| routes_of_administration = |
||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| bioavailability = |
| bioavailability = |
||
| Line 16: | Line 19: | ||
| metabolism = |
| metabolism = |
||
| elimination_half-life = |
| elimination_half-life = |
||
<!--Identifiers--> |
<!--Identifiers--> |
||
| CAS_number_Ref = {{cascite|correct|??}} |
| CAS_number_Ref = {{cascite|correct|??}} |
||
| Line 35: | Line 39: | ||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
||
| ChEMBL = 1306 |
| ChEMBL = 1306 |
||
<!--Chemical data--> |
<!--Chemical data--> |
||
| C=26 | H=31 | Cl=2 | N=5 | O=3 |
| C=26 | H=31 | Cl=2 | N=5 | O=3 |
||
| Line 47: | Line 52: | ||
}} |
}} |
||
'''Terconazole''' is an [[ |
'''Terconazole''' is an [[antifungal]] drug used to treat [[vaginal yeast infection]]. It comes as a lotion or a suppository and disrupts the biosynthesis of fats in a yeast cell. It has a relatively broad spectrum compared to azole compounds but not triazole compounds. Testing shows that it is a suitable compound for prophylaxis for those that suffer from chronic vulvovaginal candidiasis. |
||
==Drug Class== |
==Drug Class== |
||
Terconazole is a triazole ketal with broad-spectrum antifungal/antimycotic tendencies. |
Terconazole is a triazole [[ketal]] with broad-spectrum antifungal/antimycotic tendencies. |
||
==History== |
==History== |
||
In 1940, the first commercial antifungal drug was available on the market. Before this, antifungal treatments were rare and expensive. |
In 1940, the first commercial antifungal drug was available on the market. Before this, antifungal treatments were rare and expensive. This treatment was called [[amphotericin B]]. It was effective in its function but was very toxic and only used for serious infections. The drug was infused into the bloodstream and could cause kidney damage and other side effects. The first azole compounds were administered to humans under strict care. These compounds were imidazoles, a molecule containing two non-adjacent nitrogen atoms in a 5 membered ring. These were synthesized in the late 60’s early 70’s. The first oral antimycotic imidazole called [[ketoconazole]] was available on the market in 1981. Triazole based drugs came shortly after and quickly gained popularity due to its broader spectrum of antifungal activity and less toxicity.<ref>{{cite journal|last1=Maertens|first1=J. A.|title=History of the Development of Azole Derivatives|journal=Clinical Microbiology and Infection|date=March 2004|volume=10|issue=1|pages=1–10|doi=10.1111/j.1470-9465.2004.00841.x}}</ref> Terconazole was the first triazole-based antifungal drug synthesised for human use. Janssen Pharmaceutica developed it in 1983.<ref>{{cite journal|last1=Heeres|first1=J|last2=Hendrickx|first2=R|last3=Van Cutsem|first3=J|title=Antimycotic azoles. 6. Synthesis and Antifungal Properties of Terconazole, a Novel Triazole Ketal|journal=Journal of Medicinal Chemistry|date=April 1983|volume=26|issue=4|pages=611–3|pmid=6834396}}</ref> Previously, all triazole based drugs targeted fungal infections related to plants from ''Candida'' species. Since creation, terconazole has been superseded by second-generation triazoles due to their even broader spectrum and higher activity levels against resistant pathogens like ''Aspergillus spp.''<ref name="Cauwenbergh">{{cite journal|last1=Cauwenbergh|first1=G|last2=Vanden Bossche|first2=H|title=Terconazole. Pharmacology of a New Antimycotic Agent|journal=The Journal of Reproductive Medicine|date=August 1989|volume=34|issue=8 Suppl.|pages=588–92|pmid=2677363}}</ref> It is still used as a treatment in cases of resistance to other drugs. |
||
==Indications== |
==Indications== |
||
Terconazole is approved to treat vulvovaginal candidiasis (vaginal thrush). It works as a broad spectrum antifungal and has shown to be an effective first-line treatment against other |
Terconazole is approved to treat vulvovaginal candidiasis (vaginal thrush). It works as a broad spectrum antifungal and has shown to be an effective first-line treatment against other ''Candida'' species.<ref>{{cite journal|last1=Tolman|first1=EL|last2=Isaacson|first2=DM|last3=Rosenthale|first3=ME|last4=McGuire|first4=JL|last5=Van Cutsem|first5=J|last6=Borgers|first6=M|last7=Van den Bossche|first7=H|title=Anticandidal Activities of Terconazole, a broad-spectrum Antimycotic|journal=Antimicrobial Agents and Chemotherapy|date=June 1986|volume=29|issue=6|pages=986–91|pmid=3729366}}</ref> It also shows effectiveness against dermatomycoses in animal models.<ref>{{cite journal|last1=Sood|first1=G|last2=Nyirjesy|first2=P|last3=Weitz|first3=MV|last4=Chatwani|first4=A|title=Terconazole Cream for Non-Candida albicans Fungal Vaginitis: Results of a Retrospective Analysis|journal=Infectious Diseases in Obstetrics and Gynecology|date=2000|volume=8|issue=5–6|pages=240–3|pmid=11220485}}</ref> |
||
==Chemical Structure, Reactivity, Synthesis== |
==Chemical Structure, Reactivity, Synthesis== |
||
[[File:Terconazole synthesis.svg|thumb|center|700px|Terconazole synthesis: {{US patent|4144346}} {{US patent|4223036}} <ref>{{Cite doi|10.1021/jm00358a032}}</ref> {{Cite patent|DE|2804096}}]] |
[[File:Terconazole synthesis.svg|thumb|center|700px|Terconazole synthesis: {{US patent|4144346}} {{US patent|4223036}} <ref>{{Cite doi|10.1021/jm00358a032}}</ref> {{Cite patent|DE|2804096}}]] |
||
Terconazole has the chemical formula |
Terconazole has the chemical formula C<sub>26</sub>H<sub>31</sub>Cl<sub>2</sub>N<sub>5</sub>O<sub>3</sub>. The chemical name for terconazole is cis-1-{''p''-[|2-(2,4-dichlorophenyl)-2-(1''H''-1,2,4-triazol-1-ylmethyl)-1,3-dioxalan-4-yl|]methoxyphenyl}-4-isopropylpiperazine. Terconazole has a melting point of 126.3 °C. The molecular weight of terconazole is 532.462 g/mol. Terconazole is synthesized using two chemical compounds: ''cis''-[2(bromomethyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl] methyl benzoate and the sodium salt of triazole, created by mixing [[triazole]] with [[sodium hydride]]. These are put in a solution and catalyzed using [[dimethyl sulfate]] at 1300 °C to give many different types of triazole derivatives.<ref name="Cauwenbergh" /> These are purified using alcohol and [[chromatography]]. Terconazole is non-reactive except when exposed to strong oxidizing agents or strong bases due to the nitrogen attached to the triazole ring. It has been found to be photosensitive.<ref>{{cite journal|last1=Fromtling|first1=RA|title=Overview of Medically Important Antifungal Azole Derivatives|journal=Clinical Microbiology Reviews|date=April 1988|volume=1|issue=2|pages=187–217|pmid=3069196}}</ref> |
||
==Available Forms== |
==Available Forms== |
||
Terconazole is a white, odourless powder. It can be purchased commercially in the following forms: |
Terconazole is a white, odourless powder. It can be purchased commercially in the following forms: |
||
* Terconazole 0.4% cream 5 g applied intravaginally once a day for 7 days; |
|||
* Terconazole 0.8% cream 5 g applied intravaginally once a day for 3 days; |
|||
* Terconazole 80 mg vaginal suppository used once daily for 3 days.<ref>{{cite book|author1=Mosby|editor1-last=White|editor1-first=K|editor2-last=Watrous|editor2-first=J|title=Mosby's Drug Reference for Health Professions|date=2012|publisher=Elsevier|location=3251 Riverport Lane, St. Louis, Missouri, USA|isbn=978-0-323-09574-7|pages=1558–1559|edition=3rd|accessdate=10 July 2015|chapter=Terconazole}}</ref> |
|||
• Terconazole 80mg vaginal suppository used once daily for 3 days (Mosby, 2012). |
|||
==Mechanism of Action== |
==Mechanism of Action== |
||
Terconazole binds to the heme iron component on the cytochrome |
Terconazole binds to the heme iron component on the [[cytochrome P450]] enzyme [[lanosterol]] of fungi, also known as [[CYP3A4]]. The gene ERG11 controls lanosterol creation.<ref>{{cite journal|last1=Yoshida|first1=Y|title=Cytochrome P450 of Fungi: Primary Target for Azole Antifungal Agents|journal=Current Topics in Medical Mycology|date=1988|volume=2|pages=388–418|pmid=3288361}}</ref> Lanosterol is found within the yeast plasma membrane. It is a class of methylsterol. Within a normal yeast cell, lanosterol is demethylated using 14α-demethylation.<ref>{{cite journal|last1=Isaacson|first1=D. M.|last2=Tolman|first2=E. L.|last3=Tobia|first3=A. J.|last4=Rosenthale|first4=M. E.|last5=McGuire|first5=J. L.|last6=Bossche|first6=H. Vanden|last7=Janssen|first7=P. A. J.|title=Selective Inhibition of 14α-Desmethyl Sterol Synthesis in Candida albicans by Terconazole, a New Triazole Antimycotic|journal=Journal of Antimicrobial Chemotherapy|date=1988|volume=21|issue=3|pages=333–343|doi=10.1093/jac/21.3.333}}</ref> This process creates [[zymosterol]]: a major constituent in the ergosterol biosynthesis pathway for the creation of cell membrane constituents in yeast. This structure provides the membrane with fluidity.<ref>{{cite web|title=C-4 Methylsterol Oxidase Activity|url=http://www.yeastgenome.org/go/GO:0000254/overview|website=Saccharomyces Genome Database|publisher=Stanford University, Stanford, CA 94305|accessdate=10 July 2015}}</ref> This occurs by transforming lanosterol into 4,4'-dimethyl cholesta-8,14,24-triene-3-β-ol. This stops respiration by prohibiting reduction of [[NADH]] to [[NAD]]. This stops biosynthesis of cell membrane products as well as transport and catabolism. Eventually, membrane fluidity and activity of membrane bound enzymes become depleted. It has also been shown to inhibit morphologic change of yeast as well as cell adherence and is directly toxic to yeast. Terconazole targets fungi specifically since humans do not use lanosterol in this pathway. This process does not affect all fungi such as ''[[Pneumocystis jirovecii]]'', which lacks lanosterol.<ref>{{cite journal|last1=Sobel|first1=Jack D.|title=Mini Review. Candida Vaginitis|journal=Infectious Diseases in Clinical Practice|date=1994|volume=3|issue=5|pages=334–9}}</ref> |
||
==Metabolism== |
==Metabolism== |
||
Absorption of terconazole is |
Absorption of terconazole is 5–8% in patients that have had a hysterectomy and 12–16% in other patients. In those that administered 0.8% terconazole, plasma concentrations of the drug remained quite low with the peak plasma concentration being 0.006 mcg at 6.6 hours. Those metabolism rates show similar results in pregnant vulvovaginal candidiasis, non-pregnant vulvovaginal candidiasis and healthy women. The half-life of terconazole in blood is recorded to be around 6.9 hours over a range of 4–11.3 hours). Radioactivity of plasma terconazole is low compared to terconazole at 0.6%. Excretion of radioactivity is via two routes, renal (32–53%) and fecal (47–52%). Metabolism is extensive and is highly protein bound (94.9%) with the degree of binding being independent of drug concentration.<ref>{{cite journal|last1=Sheehan|first1=DJ|last2=Hitchcock|first2=CA|last3=Sibley|first3=CM|title=Current and Emerging Azole Antifungal Agents|journal=Clinical Microbiology Reviews|date=January 1999|volume=12|issue=1|pages=40–79|pmid=9880474}}</ref> |
||
==Efficacy== |
==Efficacy== |
||
In a review of 19 studies, it was shown that short-term rates for intravaginally administered azole treatments show mycological cure (no presence of organism) in 80% of cases in a short term follow-up and 66% over long term follow-up |
In a review of 19 studies, it was shown that short-term rates for intravaginally administered azole treatments show mycological cure (no presence of organism) in 80% of cases in a short term follow-up and 66% over long term follow-up.<ref>{{cite journal|last1=Nurbhai|first1=M|last2=Grimshaw|first2=J|last3=Watson|first3=M|last4=Bond|first4=C|last5=Mollison|first5=J|last6=Ludbrook|first6=A|title=Oral Versus Intra-vaginal Imidazole and Triazole Anti-fungal Treatment of Uncomplicated Vulvovaginal Candidiasis (Thrush)|journal=The Cochrane Database of Systematic Reviews|date=17 October 2007|issue=4|pages=CD002845|pmid=17943774}}</ref> In a double-blind study by Slavin in 1992, terconazole showed a 75% mycological cure over a short-term period (7–14 days) and 100% mycological cure over a long-term period (28–34 days). This study focused on the drug as an 80 mg vaginal suppository, taken three times overnight by 10 women.<ref>{{cite journal|last1=Slavin|first1=MB|last2=Benrubi|first2=GI|last3=Parker|first3=R|last4=Griffin|first4=CR|last5=Magee|first5=MJ|title=Single Dose Oral Fluconazole vs Intravaginal Terconazole in Treatment of Candida Vaginitis. Comparison and Pilot Study|journal=The Journal of the Florida Medical Association|date=October 1992|volume=79|issue=10|pages=693–6|pmid=1460451}}</ref> In another placebo-controlled, double blind study by Schmidt et al., the efficacy of different concentrations of terconazole creams were tested. Cream was applied for three days to 24 women between the ages of 18–60. The results showed 0.8% terconazole mycologic cure rates were 83.3% within 1–3 days of starting treatment, 83.3% within 8–11 days of treatment and 58.3% within 30–35 days of treatment.<ref name="Schmidt">{{cite journal|last1=Schmidt|first1=C|last2=Sobel|first2=J|last3=Meriwether|first3=C|title=Comparison of 0.8% and 1.6% Terconazole Cream in Severe Vulvovaginal Candidiasis|journal=Obstetrics and Gynecology|date=September 1990|volume=76|issue=3 Pt 1|pages=414–6|pmid=2381618}}</ref> The efficacy of the suppository is more effective after a long-term follow-up than terconazole as a cream or other intravaginal treatments.<ref>{{cite book|last1=Maibach|first1=Howard I.|last2=Farage|first2=Miranda A. (ed.)|title=The Vulva: Anatomy, Physiology and Pathology|date=2006|publisher=Informa Healthcare USA, Inc.|location=270 Madison Avenue, New York, NY 10016; 2 Park Square, Milton Park, Abingdon, Oxon OX14 4RN, UK|isbn=978-0-8493-3608-9|pages=128–129|edition=1st|accessdate=10 July 2015|chapter=Vulvar Therapies: Evidence vs. Testimony. Fungal: Candidiasis.}}</ref> |
||
==Side Effects== |
==Side Effects== |
||
The most common side effects of terconazole include |
The most common side effects of terconazole include [[headache]]s, vulvar/vaginal irritation, [[rash]], [[itch]]ing, burning or discomfort.<ref>{{cite web|last1=Workowski|first1=KA|last2=Berman|first2=S|title=Sexually Transmitted Diseases Treatment Guidelines, 2010. Vol. 59. No. RR-12.|url=http://www.cdc.gov/mmwr/pdf/rr/rr5912.pdf|website=USA Centers for Disease Control and Prevention|publisher=Office of Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, GA 30333|accessdate=10 July 2015|date=December 17, 2010}}</ref> Other side effects may include abdominal pain or cramps, [[dysmenorrhea]], [[chills]], [[fever]] and allergic reactions. Flu-like symptoms have been recorded in those that take suppositories greater than 160 mg.<ref name="Schmidt" /> May cause birth defects if used in the first trimester.<ref>{{cite journal|last1=Faro|first1=S|last2=Apuzzio|first2=J|last3=Bohannon|first3=N|last4=Elliott|first4=K|last5=Martens|first5=MG|last6=Mou|first6=SM|last7=Phillips-Smith|first7=LE|last8=Soper|first8=DE|last9=Strayer|first9=A|last10=Young|first10=RL|title=Treatment Considerations in Vulvovaginal Candidiasis|journal=The Female Patient|date=March 1997|volume=22|issue=1|pages=1–17}}</ref> |
||
==Drug Interactions== |
==Drug Interactions== |
||
Terconazole may interact with the spermicide nonoxynol-9. A precipitate is formed upon combination of both drugs. Terconazole may weaken latex-based |
Terconazole may interact with the spermicide [[nonoxynol-9]]. A precipitate is formed upon combination of both drugs. Terconazole may weaken latex-based [[condom]]s.<ref>{{cite book|last1=Grayson|first1=ML (editor-in-chief)|last2=Crowe|first2=SM|last3=McCarthy|first3=JS|last4=Mills|first4=J|last5=Mouton|first5=JW|last6=Norrby|first6=SR|last7=Paterson|first7=DL|last8=Pfaller|first8=MA|title=Kucers' the Use of Antibiotics Sixth Edition: a Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs|date=2010|publisher=CRC Press. Taylor & Francis Group|location=6000 Broken Sound Parkway NW, Suite 300, Boca Raton, FL 33487-2742|isbn=978-0340927670|pages=1933–5|edition=6th}}</ref> |
||
==Safety== |
==Safety== |
||
Terconazole is not considered hazardous when handled under normal conditions. It is generally non-flammable and non-carcinogenic Generally is non-toxic, however, can emit toxic fumes when dust is set alight. Can cause respiratory distress as dust |
Terconazole is not considered hazardous when handled under normal conditions. It is generally non-flammable and non-carcinogenic. Generally is non-toxic, however, can emit toxic fumes when dust is set alight. Can cause respiratory distress as dust.<ref>{{cite book|last1=Mancano|first1=MA|last2=Gallagher|first2=JC|title=Frequently Prescribed Medications: Drugs You Need to Know|date=2013|publisher=Jones & Bartlett Learning|location=5 Wall Street, Burlington, MA 01803|isbn=978-1-4496-9884-3|page=312|edition=2nd}}</ref> Can be absorbed by embryo within the first trimester of pregnancy and cause birth defects. Cross inhibition shows that there may be some toxicity.<ref>{{cite web|author1=Melbourne Sexual Health Centre|title=Vaginal thrush|url=http://www.betterhealth.vic.gov.au/bhcv2/bhcarticles.nsf/pages/Thrush|website=Better Health Channer|publisher=State Government of Victoria|accessdate=10 July 2015}}</ref> |
||
==References== |
|||
Cauwenbergh, G., & Vanden Bossche, H. (1989). Terconazole. pharmacology of a new antimycotic agent.. Journal of Reproductive Medicine, 34(8), 588-592. |
|||
Faro, S., Apuzzio, J., Bohannon, N., Elliott, K., Martens, M. G., Mou, S. M., Young, R. L. (1997). Treatment considerations in vulvovaginal candidiasis. The Female Patient, 22(1), 1-17. |
|||
Fromtling, R. A. (1988). Overview of antifungal azoles. Clinical Microbiology Review, 1(1), 201. |
|||
Heeres, J., Hendrickx, R., & Van Cutsem, J. (1983). Antimycotic azoles: 6. synthesis & antifungal properties of terconazole, a novel triazole ketal. The Journal of Medical Chemistry, 26(1), 611-613. |
|||
Isaacson, D. M., Tolman, E. L., Tobia, A. J., Rosenthale, M. E., Mcguire, J. L., Van Den Bossche, H., & Janssen, P. A. (1987). Selective inhibition of 14α-desmethyl sterol synthesis in candida albicans by terconazole, a new triazole antimycotic. Journal of Antimicrobial Chemotherapy, 21(3), 333-343. |
|||
Maertens, J. A. (2004). History of the development of azole derivatives. Clinical Microbiology & Infection, 10(1), 1-10. doi:10.1111/j.1470-9465.2004.00841.x |
|||
Maibach, H. I., & Farage, M. A. (2006). Vulvar therapies: Evidence vs. testimony: Fungal: Candidiasis. In M. A. Farage (Ed.), The vulva: Anatomy, physiology, and pathology (1st ed., pp. 128-129). 270 Madison Avenue, New York, NY: Informa Healthcare USA Inc. |
|||
Mancano, M. A., & Gallagher, J. C. (2013). Antifungal, triazole. In T. Reilly (Ed.), Frequently prescribed medications (2nd ed., pp. 312). 5 Wall Street, Burlington, MA, USA: Jones & Bartlett Learning. |
|||
Melbourne Sexual Health Centre. (2011). Vaginal thrush. Retrieved 04/25, 2013, from http://www.betterhealth.vic.gov.au/bhcv2/bhcarticles.nsf/pages/Thrush |
|||
Mosby. (2012). Terconazole. In K. White, & J. Watrous (Eds.), Mosby's drug reference for health professions (3rd ed., pp. 1558-1559). 3251 Riverport Lane, St. Louis, Missouri, USA: Elsevier. |
|||
Nurbhai, M., Grimshaw, J., Watson, M., Bond, C. M., Mollison, J. A., & Ludbrook, A. (2009). Oral versus intra-vaginal imidazole and triazole anti-fungal treatment of uncomplicated vulvovaginal candidiasis (thrush) (review). The Chochrane Collaboration, 1(1), 1-54. |
|||
Pfaller, M. A. (2010). Terconazole. In M. L. Grayson (Ed.), Kucers' the use of antibiotics: A clinical review of antibacterial, antifungal, antiparasitic & antiviral drugs (6th ed., pp. 1933-1935). 1752 N Street, NW, Wahington, USA: ASM Press. |
|||
Saccharomyces Genome Database. (2013). C-4 methylsterol oxidase activity. Retrieved 02/03, 2013, from http://www.yeastgenome.org/cgi-bin/GO/goTerm.pl?goid=254#annosum |
|||
Schmidt, C., Sobel, J., & Meriwether, C. (1990). Comparison of 0.8% terconazole cream in severe vulvovaginal candidiasis. Obstetrics & Gynecology, 76(3), 414-416. |
|||
Sheehan, D. J., Hitchcock, C. A., & Sibley, C. M. (1999). Vulvovaginal candidiasis. Clinical microbiology reviews: Current & emerging azole antifungal treatments (12th ed., pp. 56-58). New York, NY, U.S.A.: American Society for microbiology. |
|||
Slavin, M. B., Benrubi, G. I., Parker, R., Griffin, C. R., & Magee, M. J. (1992). Single dose oral fluconazole vs intravaginal terconazole in treatment of candida vaginitis. comparison and pilot study.. The Journal of the Florida Medical Association, 79(10), 693-696. |
|||
Sobel, J. (1994). Mini review: Candida vaginitis. Infectious Diseases in Clinical Practice, 3(5), 334-339. |
|||
Sood, G., Nyirjesy, P., Weitz, M. V., & Chatwani, A. (2000). “Terconazole cream for non-candida albicans fungal vaginitis" results of a retrospective analysis . Diseases in Obstetrics & Gynecology, 8(1), 240-243. |
|||
Tolman, E. L., Isaacson, D. M., Rosenthale, M. E., McGuire, J. L., Van Cutsem, J., Borgers, M., & Van Den Bossche, H. (1986). Anticandidal activities of terconazole,a broad-spectrum antimycotic. Antimicrobial Agents & Chemotherapy, 29(6), 986-991. |
|||
Workowski, K. A., & Berman, S. (2010). Sexually transmitted diseases treatment guidelines, recommendations & reports. (Review No. 59). 1600 Clifton Rd. Atlanta, GA 30333, USA: Centers For Disease Control & Prevention. |
|||
Yoshida, Y. (1988). Cytochrome P450 of fungi: Primary target for azole anitfungal agents. Current Topics in Medical Mycology, 2(1), 388-389. doi:10.1007/978-1-4612-3730-3_1 |
|||
==References== |
==References== |
||
{{Reflist}} |
{{Reflist}} |
||
| ⚫ | |||
==See Also== |
|||
| ⚫ | |||
{{Gynecological anti-infectives and antiseptics}} |
{{Gynecological anti-infectives and antiseptics}} |
||
{{Antifungals}} |
{{Antifungals}} |
||
| ⚫ | |||
[[Category:Dioxolanes]] |
[[Category:Dioxolanes]] |
||
[[Category:Phenol ethers]] |
[[Category:Phenol ethers]] |
||
| ⚫ | |||
[[Category:Triazole antifungals]] |
[[Category:Triazole antifungals]] |
||
Revision as of 09:41, 10 July 2015
 | |
| Clinical data | |
|---|---|
| Trade names | Terazol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688022 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 94.9% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.061.573 |
| Chemical and physical data | |
| Formula | C26H31Cl2N5O3 |
| Molar mass | 532.462 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Terconazole is an antifungal drug used to treat vaginal yeast infection. It comes as a lotion or a suppository and disrupts the biosynthesis of fats in a yeast cell. It has a relatively broad spectrum compared to azole compounds but not triazole compounds. Testing shows that it is a suitable compound for prophylaxis for those that suffer from chronic vulvovaginal candidiasis.
Drug Class
Terconazole is a triazole ketal with broad-spectrum antifungal/antimycotic tendencies.
History
In 1940, the first commercial antifungal drug was available on the market. Before this, antifungal treatments were rare and expensive. This treatment was called amphotericin B. It was effective in its function but was very toxic and only used for serious infections. The drug was infused into the bloodstream and could cause kidney damage and other side effects. The first azole compounds were administered to humans under strict care. These compounds were imidazoles, a molecule containing two non-adjacent nitrogen atoms in a 5 membered ring. These were synthesized in the late 60’s early 70’s. The first oral antimycotic imidazole called ketoconazole was available on the market in 1981. Triazole based drugs came shortly after and quickly gained popularity due to its broader spectrum of antifungal activity and less toxicity.[1] Terconazole was the first triazole-based antifungal drug synthesised for human use. Janssen Pharmaceutica developed it in 1983.[2] Previously, all triazole based drugs targeted fungal infections related to plants from Candida species. Since creation, terconazole has been superseded by second-generation triazoles due to their even broader spectrum and higher activity levels against resistant pathogens like Aspergillus spp.[3] It is still used as a treatment in cases of resistance to other drugs.
Indications
Terconazole is approved to treat vulvovaginal candidiasis (vaginal thrush). It works as a broad spectrum antifungal and has shown to be an effective first-line treatment against other Candida species.[4] It also shows effectiveness against dermatomycoses in animal models.[5]
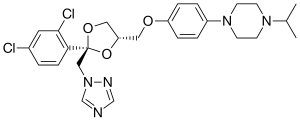
Chemical Structure, Reactivity, Synthesis

Terconazole has the chemical formula C26H31Cl2N5O3. The chemical name for terconazole is cis-1-{p-[|2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxalan-4-yl|]methoxyphenyl}-4-isopropylpiperazine. Terconazole has a melting point of 126.3 °C. The molecular weight of terconazole is 532.462 g/mol. Terconazole is synthesized using two chemical compounds: cis-[2(bromomethyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl] methyl benzoate and the sodium salt of triazole, created by mixing triazole with sodium hydride. These are put in a solution and catalyzed using dimethyl sulfate at 1300 °C to give many different types of triazole derivatives.[3] These are purified using alcohol and chromatography. Terconazole is non-reactive except when exposed to strong oxidizing agents or strong bases due to the nitrogen attached to the triazole ring. It has been found to be photosensitive.[7]
Available Forms
Terconazole is a white, odourless powder. It can be purchased commercially in the following forms:
- Terconazole 0.4% cream 5 g applied intravaginally once a day for 7 days;
- Terconazole 0.8% cream 5 g applied intravaginally once a day for 3 days;
- Terconazole 80 mg vaginal suppository used once daily for 3 days.[8]
Mechanism of Action
Terconazole binds to the heme iron component on the cytochrome P450 enzyme lanosterol of fungi, also known as CYP3A4. The gene ERG11 controls lanosterol creation.[9] Lanosterol is found within the yeast plasma membrane. It is a class of methylsterol. Within a normal yeast cell, lanosterol is demethylated using 14α-demethylation.[10] This process creates zymosterol: a major constituent in the ergosterol biosynthesis pathway for the creation of cell membrane constituents in yeast. This structure provides the membrane with fluidity.[11] This occurs by transforming lanosterol into 4,4'-dimethyl cholesta-8,14,24-triene-3-β-ol. This stops respiration by prohibiting reduction of NADH to NAD. This stops biosynthesis of cell membrane products as well as transport and catabolism. Eventually, membrane fluidity and activity of membrane bound enzymes become depleted. It has also been shown to inhibit morphologic change of yeast as well as cell adherence and is directly toxic to yeast. Terconazole targets fungi specifically since humans do not use lanosterol in this pathway. This process does not affect all fungi such as Pneumocystis jirovecii, which lacks lanosterol.[12]
Metabolism
Absorption of terconazole is 5–8% in patients that have had a hysterectomy and 12–16% in other patients. In those that administered 0.8% terconazole, plasma concentrations of the drug remained quite low with the peak plasma concentration being 0.006 mcg at 6.6 hours. Those metabolism rates show similar results in pregnant vulvovaginal candidiasis, non-pregnant vulvovaginal candidiasis and healthy women. The half-life of terconazole in blood is recorded to be around 6.9 hours over a range of 4–11.3 hours). Radioactivity of plasma terconazole is low compared to terconazole at 0.6%. Excretion of radioactivity is via two routes, renal (32–53%) and fecal (47–52%). Metabolism is extensive and is highly protein bound (94.9%) with the degree of binding being independent of drug concentration.[13]
Efficacy
In a review of 19 studies, it was shown that short-term rates for intravaginally administered azole treatments show mycological cure (no presence of organism) in 80% of cases in a short term follow-up and 66% over long term follow-up.[14] In a double-blind study by Slavin in 1992, terconazole showed a 75% mycological cure over a short-term period (7–14 days) and 100% mycological cure over a long-term period (28–34 days). This study focused on the drug as an 80 mg vaginal suppository, taken three times overnight by 10 women.[15] In another placebo-controlled, double blind study by Schmidt et al., the efficacy of different concentrations of terconazole creams were tested. Cream was applied for three days to 24 women between the ages of 18–60. The results showed 0.8% terconazole mycologic cure rates were 83.3% within 1–3 days of starting treatment, 83.3% within 8–11 days of treatment and 58.3% within 30–35 days of treatment.[16] The efficacy of the suppository is more effective after a long-term follow-up than terconazole as a cream or other intravaginal treatments.[17]
Side Effects
The most common side effects of terconazole include headaches, vulvar/vaginal irritation, rash, itching, burning or discomfort.[18] Other side effects may include abdominal pain or cramps, dysmenorrhea, chills, fever and allergic reactions. Flu-like symptoms have been recorded in those that take suppositories greater than 160 mg.[16] May cause birth defects if used in the first trimester.[19]
Drug Interactions
Terconazole may interact with the spermicide nonoxynol-9. A precipitate is formed upon combination of both drugs. Terconazole may weaken latex-based condoms.[20]
Safety
Terconazole is not considered hazardous when handled under normal conditions. It is generally non-flammable and non-carcinogenic. Generally is non-toxic, however, can emit toxic fumes when dust is set alight. Can cause respiratory distress as dust.[21] Can be absorbed by embryo within the first trimester of pregnancy and cause birth defects. Cross inhibition shows that there may be some toxicity.[22]
References
- ^ Maertens, J. A. (March 2004). "History of the Development of Azole Derivatives". Clinical Microbiology and Infection. 10 (1): 1–10. doi:10.1111/j.1470-9465.2004.00841.x.
- ^ Heeres, J; Hendrickx, R; Van Cutsem, J (April 1983). "Antimycotic azoles. 6. Synthesis and Antifungal Properties of Terconazole, a Novel Triazole Ketal". Journal of Medicinal Chemistry. 26 (4): 611–3. PMID 6834396.
- ^ a b Cauwenbergh, G; Vanden Bossche, H (August 1989). "Terconazole. Pharmacology of a New Antimycotic Agent". The Journal of Reproductive Medicine. 34 (8 Suppl.): 588–92. PMID 2677363.
- ^ Tolman, EL; Isaacson, DM; Rosenthale, ME; McGuire, JL; Van Cutsem, J; Borgers, M; Van den Bossche, H (June 1986). "Anticandidal Activities of Terconazole, a broad-spectrum Antimycotic". Antimicrobial Agents and Chemotherapy. 29 (6): 986–91. PMID 3729366.
- ^ Sood, G; Nyirjesy, P; Weitz, MV; Chatwani, A (2000). "Terconazole Cream for Non-Candida albicans Fungal Vaginitis: Results of a Retrospective Analysis". Infectious Diseases in Obstetrics and Gynecology. 8 (5–6): 240–3. PMID 11220485.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/jm00358a032, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/jm00358a032instead. - ^ Fromtling, RA (April 1988). "Overview of Medically Important Antifungal Azole Derivatives". Clinical Microbiology Reviews. 1 (2): 187–217. PMID 3069196.
- ^ Mosby (2012). "Terconazole". In White, K; Watrous, J (eds.). Mosby's Drug Reference for Health Professions (3rd ed.). 3251 Riverport Lane, St. Louis, Missouri, USA: Elsevier. pp. 1558–1559. ISBN 978-0-323-09574-7.
{{cite book}}:|access-date=requires|url=(help)CS1 maint: location (link) - ^ Yoshida, Y (1988). "Cytochrome P450 of Fungi: Primary Target for Azole Antifungal Agents". Current Topics in Medical Mycology. 2: 388–418. PMID 3288361.
- ^ Isaacson, D. M.; Tolman, E. L.; Tobia, A. J.; Rosenthale, M. E.; McGuire, J. L.; Bossche, H. Vanden; Janssen, P. A. J. (1988). "Selective Inhibition of 14α-Desmethyl Sterol Synthesis in Candida albicans by Terconazole, a New Triazole Antimycotic". Journal of Antimicrobial Chemotherapy. 21 (3): 333–343. doi:10.1093/jac/21.3.333.
- ^ "C-4 Methylsterol Oxidase Activity". Saccharomyces Genome Database. Stanford University, Stanford, CA 94305. Retrieved 10 July 2015.
- ^ Sobel, Jack D. (1994). "Mini Review. Candida Vaginitis". Infectious Diseases in Clinical Practice. 3 (5): 334–9.
- ^ Sheehan, DJ; Hitchcock, CA; Sibley, CM (January 1999). "Current and Emerging Azole Antifungal Agents". Clinical Microbiology Reviews. 12 (1): 40–79. PMID 9880474.
- ^ Nurbhai, M; Grimshaw, J; Watson, M; Bond, C; Mollison, J; Ludbrook, A (17 October 2007). "Oral Versus Intra-vaginal Imidazole and Triazole Anti-fungal Treatment of Uncomplicated Vulvovaginal Candidiasis (Thrush)". The Cochrane Database of Systematic Reviews (4): CD002845. PMID 17943774.
- ^ Slavin, MB; Benrubi, GI; Parker, R; Griffin, CR; Magee, MJ (October 1992). "Single Dose Oral Fluconazole vs Intravaginal Terconazole in Treatment of Candida Vaginitis. Comparison and Pilot Study". The Journal of the Florida Medical Association. 79 (10): 693–6. PMID 1460451.
- ^ a b Schmidt, C; Sobel, J; Meriwether, C (September 1990). "Comparison of 0.8% and 1.6% Terconazole Cream in Severe Vulvovaginal Candidiasis". Obstetrics and Gynecology. 76 (3 Pt 1): 414–6. PMID 2381618.
- ^ Maibach, Howard I.; Farage, Miranda A. (ed.) (2006). "Vulvar Therapies: Evidence vs. Testimony. Fungal: Candidiasis.". The Vulva: Anatomy, Physiology and Pathology (1st ed.). 270 Madison Avenue, New York, NY 10016; 2 Park Square, Milton Park, Abingdon, Oxon OX14 4RN, UK: Informa Healthcare USA, Inc. pp. 128–129. ISBN 978-0-8493-3608-9.
{{cite book}}:|access-date=requires|url=(help);|first2=has generic name (help)CS1 maint: location (link) - ^ Workowski, KA; Berman, S (December 17, 2010). "Sexually Transmitted Diseases Treatment Guidelines, 2010. Vol. 59. No. RR-12" (PDF). USA Centers for Disease Control and Prevention. Office of Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, GA 30333. Retrieved 10 July 2015.
- ^ Faro, S; Apuzzio, J; Bohannon, N; Elliott, K; Martens, MG; Mou, SM; Phillips-Smith, LE; Soper, DE; Strayer, A; Young, RL (March 1997). "Treatment Considerations in Vulvovaginal Candidiasis". The Female Patient. 22 (1): 1–17.
- ^ Grayson, ML (editor-in-chief); Crowe, SM; McCarthy, JS; Mills, J; Mouton, JW; Norrby, SR; Paterson, DL; Pfaller, MA (2010). Kucers' the Use of Antibiotics Sixth Edition: a Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs (6th ed.). 6000 Broken Sound Parkway NW, Suite 300, Boca Raton, FL 33487-2742: CRC Press. Taylor & Francis Group. pp. 1933–5. ISBN 978-0340927670.
{{cite book}}:|first1=has generic name (help)CS1 maint: location (link) - ^ Mancano, MA; Gallagher, JC (2013). Frequently Prescribed Medications: Drugs You Need to Know (2nd ed.). 5 Wall Street, Burlington, MA 01803: Jones & Bartlett Learning. p. 312. ISBN 978-1-4496-9884-3.
{{cite book}}: CS1 maint: location (link) - ^ Melbourne Sexual Health Centre. "Vaginal thrush". Better Health Channer. State Government of Victoria. Retrieved 10 July 2015.
