COVID-19 testing: Difference between revisions
→Polymerase chain reaction: Add reference to qPCR as an abbreviation |
→Polymerase chain reaction: It's important to clarify that all these terms refer to the same thing, or people get confused when they see the different terms in the literature. |
||
| Line 15: | Line 15: | ||
===Current infection=== |
===Current infection=== |
||
====Polymerase chain reaction==== |
====Polymerase chain reaction==== |
||
[[Polymerase chain reaction]] (PCR) is a process that causes a very small well-defined segment of [[DNA]] to be [[DNA replication|amplified]], or multiplied many hundreds of thousands of times, so that there is enough of it to be detected and analyzed. Viruses such as SARS-CoV-2 do not contain [[DNA]] but only [[RNA]].<ref name=iaea_rt-pcr/> When a respiratory sample is collected from the person being tested it is treated with certain chemicals<ref>{{cite web |url=https://www.assaygenie.com/rna-extraction-for-covid-19-testing |title= RNA Extraction |publisher=AssayGenie |access-date=7 May 2020 }}</ref><ref name=iaea_rt-pcr/> which remove extraneous substances and extract only the RNA from the sample. [[Reverse transcription polymerase chain reaction]] (RT-PCR) is a technique that first uses [[Reverse transcriptase |reverse transcription]] to convert the extracted RNA into DNA and then uses PCR to amplify a piece of the resulting DNA, creating enough to be examined in order to determine if it matches the genetic code of SARS-CoV-2.<ref name=iaea_rt-pcr>{{cite web |url=https://www.iaea.org/newscenter/news/how-is-the-covid-19-virus-detected-using-real-time-rt-pcr |title= How is the COVID-19 Virus Detected using Real Time RT-PCR? |publisher= IAEA|access-date=5 May 2020|date = 27 March 2020}}</ref> [[Real-time polymerase chain reaction| Real-time PCR]] (qPCR)<ref>{{cite web |last1=Bustin |first1=Stephen A. |last2=Benes |first2=Vladimir |last3=Garson |first3=Jeremy A. |last4=Hellemans |first4=Jan |last5=Huggett |first5=Jim |last6=Kubista |first6=Mikael |last7=Mueller |first7=Reinhold |last8=Nolan |first8=Tania |last9=Pfaffl |first9=Michael W. |last10=Shipley |first10=Gregory L. |last11=Vandesompele |first11=Jo |last12=Wittwer |first12=Carl T. |title=The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments |url=https://academic.oup.com/clinchem/article/55/4/611/5631762 |website=Clinical Chemistry |pages=611–622 |language=en |doi=10.1373/clinchem.2008.112797 |date=1 April 2009}}</ref> provides advantages during the PCR portion of this process, including automating it and enabling high-throughput and more reliable instrumentation, and has become the preferred method.<ref>{{cite web |url= https://gene-quantification.de/bustin-mueller-qpcr-2005.pdf |title= Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis |publisher=Clinical Science |access-date=5 May 2020|date = 23 September 2005 }}</ref><ref>{{cite web |url=https://www.thermofisher.com/us/en/home/references/ambion-tech-support/rtpcr-analysis/general-articles/rt--pcr-the-basics.html |title= The Basics: RT-PCR |publisher=ThermoFisher Scientific |access-date=5 May 2020}}</ref> Altogether, the combined technique is sometimes abbreviated [[Reverse transcription polymerase chain reaction|qRT-PCR]]<ref name="pmid20204872">{{cite book |vauthors=Varkonyi-Gasic E, Hellens RP |title=Plant Epigenetics |chapter=qRT-PCR of Small RNAs |volume=631 |pages=109–22 |year=2010 |pmid=20204872 |doi=10.1007/978-1-60761-646-7_10 |series=Methods in Molecular Biology |isbn=978-1-60761-645-0 }}</ref> or rRT-PCR<ref>{{Cite web |url=https://www.fda.gov/media/136151/download|title=ACCELERATED EMERGENCY USE AUTHORIZATION (EUA) SUMMARY COVID-19 RT-PCR TEST (LABORATORY CORPORATION OF AMERICA) |last=|first= |date= |website=FDA |url-status=live |archive-url=|archive-date=|access-date=3 April 2020}}</ref> or RT-qPCR,<ref name="pmid20215014">{{cite journal |vauthors=Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M |title=A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines |journal=Methods |volume=50 |issue=4 |pages=S1–5 |date=April 2010 |pmid=20215014 |doi=10.1016/j.ymeth.2010.01.005 }}</ref> although sometimes just RT-PCR or PCR is used as an abbreviation. The test can be done on respiratory samples obtained by various methods, including a [[nasopharyngeal swab]] or [[sputum]] sample,<ref name="20200129cdc">{{cite web |url=https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html |title=Real-Time RT-PCR Panel for Detection 2019-nCoV |date=29 January 2020 |website=[[Centers for Disease Control and Prevention]] |access-date=1 February 2020 |archive-url=https://web.archive.org/web/20200130202031/https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html |archive-date=30 January 2020 |url-status=live }}</ref> as well as on [[saliva]].<ref name=sciencenews_saliva/> Results are generally available within a few hours to 2 days.<ref name="globenewswire1977226">{{cite web |url=https://www.globenewswire.com/news-release/2020/01/30/1977226/0/en/Curetis-Group-Company-Ares-Genetics-and-BGI-Group-Collaborate-to-Offer-Next-Generation-Sequencing-and-PCR-based-Coronavirus-2019-nCoV-Testing-in-Europe.html |title=Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe |date=30 January 2020 |website=GlobeNewswire News Room |access-date=1 February 2020 |archive-url=https://web.archive.org/web/20200131201626/https://www.globenewswire.com/news-release/2020/01/30/1977226/0/en/Curetis-Group-Company-Ares-Genetics-and-BGI-Group-Collaborate-to-Offer-Next-Generation-Sequencing-and-PCR-based-Coronavirus-2019-nCoV-Testing-in-Europe.html |archive-date=31 January 2020 |url-status=live }}</ref> The RT-PCR test performed with throat swabs is only reliable in the first week of the disease. Later on the virus can disappear in the throat while it continues to multiply in the lungs. For infected people tested in the second week, alternatively sample material can then be taken from the deep airways by suction catheter, or coughed up material (sputum) can be used.<ref name="chrdrosten20200326">{{cite web |last=Drosten|first=Christian|url=https://www.ndr.de/nachrichten/info/coronaskript146.pdf |title=Coronavirus-Update Folge 22 |date=26 March 2020 |website=NDR |access-date=2 April 2020 |archive-url=https://web.archive.org/web/20200331101408/https://www.ndr.de/nachrichten/info/coronaskript146.pdf |archive-date=31 March 2020 |url-status=live }}</ref> Saliva has been shown to be a common and transient medium for virus transmission<ref name=nature_saliva>{{cite web |url=https://www.nature.com/articles/s41368-020-0080-z |title= Saliva: potential diagnostic value and transmission of 2019-nCoV |publisher=Nature |access-date=6 May 2020|date = 17 April 2020}}</ref> and results provided to the FDA suggest that testing saliva may be as effective as nasal and throat swabs.<ref name=sciencenews_saliva>{{cite web |url=https://www.sciencenews.org/article/coronavirus-covid-19-where-things-stand-tests-testing-united-states |title= Here's where things stand on COVID-19 tests in the U.S. |publisher= ScienceNews|access-date=6 May 2020|date =17 April 2020 }}</ref> The [[FDA]] has granted [[Emergency Use Authorization]] for a test that collects [[saliva]] instead of using the traditional [[Nasopharyngeal swab|nasal swab]].<ref name=cnn_saliva>{{cite web |url=https://www.cnn.com/2020/04/14/health/coronavirus-test-saliva-fda-emergency-use-bn/index.html |title= FDA authorizes Covid-19 saliva test for emergency use |publisher=CNN |access-date=1 May 2020|date =14 April 2020 }}</ref> It is believed that this will reduce the risk for health care professionals,<ref name= Rutgers_saliva>{{cite web |url=https://www.rutgers.edu/news/new-rutgers-saliva-test-coronavirus-gets-fda-approval |title= New Rutgers Saliva Test for Coronavirus Gets FDA Approval |publisher=Rutgers.edu |access-date=1 May 2020|date =13 April 2020 }}</ref> will be much more comfortable for the patient,<ref name=cnn_saliva/> and will enable quarantined people to collect their own samples more efficiently.<ref name=Rutgers_saliva/> According to some experts it is too early to know the operating characteristics of the saliva test, and whether it will prove to be as sensitive as the [[nasopharyngeal swab]] test.<ref>{{cite web |url=https://newsnetwork.mayoclinic.org/discussion/covid-19-saliva-tests-what-is-the-benefit/ |title= COVID-19 saliva tests: What is the benefit? |publisher= Mayo Clinic|access-date=6 May 2020|date =16 April 2020 }}</ref><ref name=nature_saliva/> Some studies suggest that the diagnostic value of saliva depends on how saliva specimens are collected (from deep throat, from oral cavity, or from salivary glands).<ref name=nature_saliva/> A recent test conducted by the [[Yale University School of Public Health]] found that saliva yielded greater detection sensitivity and consistency throughout the course of infection when compared with samples taken with [[nasopharyngeal swab]]s.<ref>{{cite web |url=https://www.merckmanuals.com/home/resourcespages/select-covid-19-news |title= Yale University School of Public Health finds saliva samples promising alternative to nasopharyngeal swab |publisher= Merck Manual|access-date=6 April 2020|date = 29 April 2020}}</ref><ref>{{cite document |url=https://www.medrxiv.org/content/10.1101/2020.04.16.20067835v1 |title= Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs |publisher= Medrxiv|access-date=5 May 2020|date =22 April 2020 |doi= 10.1101/2020.04.16.20067835 |last1= Wyllie |first1= Anne Louise |last2= Fournier |first2= John |last3= Casanovas-Massana |first3= Arnau |last4= Campbell |first4= Melissa |last5= Tokuyama |first5= Maria |last6= Vijayakumar |first6= Pavithra |last7= Geng |first7= Bertie |last8= Muenker |first8= M. Catherine |last9= Moore |first9= Adam J. |last10= Vogels |first10= Chantal B. F. |last11= Petrone |first11= Mary E. |last12= Ott |first12= Isabel M. |last13= Lu |first13= Peiwen |last14= Lu-Culligan |first14= Alice |last15= Klein |first15= Jonathan |last16= Venkataraman |first16= Arvind |last17= Earnest |first17= Rebecca |last18= Simonov |first18= Michael |last19= Datta |first19= Rupak |last20= Handoko |first20= Ryan |last21= Naushad |first21= Nida |last22= Sewanan |first22= Lorenzo R. |last23= Valdez |first23= Jordan |last24= White |first24= Elizabeth B. |last25= Lapidus |first25= Sarah |last26= Kalinich |first26= Chaney C. |last27= Jiang |first27= Xiaodong |last28= Kim |first28= Daniel J. |last29= Kudo |first29= Eriko |last30= Linehan |first30= Melissa |displayauthors= 29 }}</ref> |
[[Polymerase chain reaction]] (PCR) is a process that causes a very small well-defined segment of [[DNA]] to be [[DNA replication|amplified]], or multiplied many hundreds of thousands of times, so that there is enough of it to be detected and analyzed. Viruses such as SARS-CoV-2 do not contain [[DNA]] but only [[RNA]].<ref name=iaea_rt-pcr/> When a respiratory sample is collected from the person being tested it is treated with certain chemicals<ref>{{cite web |url=https://www.assaygenie.com/rna-extraction-for-covid-19-testing |title= RNA Extraction |publisher=AssayGenie |access-date=7 May 2020 }}</ref><ref name=iaea_rt-pcr/> which remove extraneous substances and extract only the RNA from the sample. [[Reverse transcription polymerase chain reaction]] (RT-PCR) is a technique that first uses [[Reverse transcriptase |reverse transcription]] to convert the extracted RNA into DNA and then uses PCR to amplify a piece of the resulting DNA, creating enough to be examined in order to determine if it matches the genetic code of SARS-CoV-2.<ref name=iaea_rt-pcr>{{cite web |url=https://www.iaea.org/newscenter/news/how-is-the-covid-19-virus-detected-using-real-time-rt-pcr |title= How is the COVID-19 Virus Detected using Real Time RT-PCR? |publisher= IAEA|access-date=5 May 2020|date = 27 March 2020}}</ref> [[Real-time polymerase chain reaction| Real-time PCR]] (qPCR)<ref>{{cite web |last1=Bustin |first1=Stephen A. |last2=Benes |first2=Vladimir |last3=Garson |first3=Jeremy A. |last4=Hellemans |first4=Jan |last5=Huggett |first5=Jim |last6=Kubista |first6=Mikael |last7=Mueller |first7=Reinhold |last8=Nolan |first8=Tania |last9=Pfaffl |first9=Michael W. |last10=Shipley |first10=Gregory L. |last11=Vandesompele |first11=Jo |last12=Wittwer |first12=Carl T. |title=The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments |url=https://academic.oup.com/clinchem/article/55/4/611/5631762 |website=Clinical Chemistry |pages=611–622 |language=en |doi=10.1373/clinchem.2008.112797 |date=1 April 2009}}</ref> provides advantages during the PCR portion of this process, including automating it and enabling high-throughput and more reliable instrumentation, and has become the preferred method.<ref>{{cite web |url= https://gene-quantification.de/bustin-mueller-qpcr-2005.pdf |title= Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis |publisher=Clinical Science |access-date=5 May 2020|date = 23 September 2005 }}</ref><ref>{{cite web |url=https://www.thermofisher.com/us/en/home/references/ambion-tech-support/rtpcr-analysis/general-articles/rt--pcr-the-basics.html |title= The Basics: RT-PCR |publisher=ThermoFisher Scientific |access-date=5 May 2020}}</ref> Altogether, the combined technique has been described as real-time RT-PCR<ref name="pmid20515509">{{cite journal |vauthors=Kang XP, Jiang T, Li YQ, etal |title=A duplex real-time RT-PCR assay for detecting H5N1 avian influenza virus and pandemic H1N1 influenza virus |journal=Virol. J. |volume=7 |issue= |page=113 |year=2010 |pmid=20515509 |pmc=2892456 |doi=10.1186/1743-422X-7-113 }}</ref> or quantitative RT-PCR<ref name="pmid12325527">{{cite book | author = Joyce C | title = Quantitative RT-PCR. A review of current methodologies | series = Methods Mol. Biol. | volume = 193 | pages = 83–92 | year = 2002 | pmid = 12325527 | doi = 10.1385/1-59259-283-X:083 | isbn = 978-1-59259-283-8 }}</ref> and is sometimes abbreviated [[Reverse transcription polymerase chain reaction|qRT-PCR]]<ref name="pmid20204872">{{cite book |vauthors=Varkonyi-Gasic E, Hellens RP |title=Plant Epigenetics |chapter=qRT-PCR of Small RNAs |volume=631 |pages=109–22 |year=2010 |pmid=20204872 |doi=10.1007/978-1-60761-646-7_10 |series=Methods in Molecular Biology |isbn=978-1-60761-645-0 }}</ref> or rRT-PCR<ref>{{Cite web |url=https://www.fda.gov/media/136151/download|title=ACCELERATED EMERGENCY USE AUTHORIZATION (EUA) SUMMARY COVID-19 RT-PCR TEST (LABORATORY CORPORATION OF AMERICA) |last=|first= |date= |website=FDA |url-status=live |archive-url=|archive-date=|access-date=3 April 2020}}</ref> or RT-qPCR,<ref name="pmid20215014">{{cite journal |vauthors=Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M |title=A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines |journal=Methods |volume=50 |issue=4 |pages=S1–5 |date=April 2010 |pmid=20215014 |doi=10.1016/j.ymeth.2010.01.005 }}</ref> although sometimes just RT-PCR or PCR is used as an abbreviation. The test can be done on respiratory samples obtained by various methods, including a [[nasopharyngeal swab]] or [[sputum]] sample,<ref name="20200129cdc">{{cite web |url=https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html |title=Real-Time RT-PCR Panel for Detection 2019-nCoV |date=29 January 2020 |website=[[Centers for Disease Control and Prevention]] |access-date=1 February 2020 |archive-url=https://web.archive.org/web/20200130202031/https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html |archive-date=30 January 2020 |url-status=live }}</ref> as well as on [[saliva]].<ref name=sciencenews_saliva/> Results are generally available within a few hours to 2 days.<ref name="globenewswire1977226">{{cite web |url=https://www.globenewswire.com/news-release/2020/01/30/1977226/0/en/Curetis-Group-Company-Ares-Genetics-and-BGI-Group-Collaborate-to-Offer-Next-Generation-Sequencing-and-PCR-based-Coronavirus-2019-nCoV-Testing-in-Europe.html |title=Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe |date=30 January 2020 |website=GlobeNewswire News Room |access-date=1 February 2020 |archive-url=https://web.archive.org/web/20200131201626/https://www.globenewswire.com/news-release/2020/01/30/1977226/0/en/Curetis-Group-Company-Ares-Genetics-and-BGI-Group-Collaborate-to-Offer-Next-Generation-Sequencing-and-PCR-based-Coronavirus-2019-nCoV-Testing-in-Europe.html |archive-date=31 January 2020 |url-status=live }}</ref> The RT-PCR test performed with throat swabs is only reliable in the first week of the disease. Later on the virus can disappear in the throat while it continues to multiply in the lungs. For infected people tested in the second week, alternatively sample material can then be taken from the deep airways by suction catheter, or coughed up material (sputum) can be used.<ref name="chrdrosten20200326">{{cite web |last=Drosten|first=Christian|url=https://www.ndr.de/nachrichten/info/coronaskript146.pdf |title=Coronavirus-Update Folge 22 |date=26 March 2020 |website=NDR |access-date=2 April 2020 |archive-url=https://web.archive.org/web/20200331101408/https://www.ndr.de/nachrichten/info/coronaskript146.pdf |archive-date=31 March 2020 |url-status=live }}</ref> Saliva has been shown to be a common and transient medium for virus transmission<ref name=nature_saliva>{{cite web |url=https://www.nature.com/articles/s41368-020-0080-z |title= Saliva: potential diagnostic value and transmission of 2019-nCoV |publisher=Nature |access-date=6 May 2020|date = 17 April 2020}}</ref> and results provided to the FDA suggest that testing saliva may be as effective as nasal and throat swabs.<ref name=sciencenews_saliva>{{cite web |url=https://www.sciencenews.org/article/coronavirus-covid-19-where-things-stand-tests-testing-united-states |title= Here's where things stand on COVID-19 tests in the U.S. |publisher= ScienceNews|access-date=6 May 2020|date =17 April 2020 }}</ref> The [[FDA]] has granted [[Emergency Use Authorization]] for a test that collects [[saliva]] instead of using the traditional [[Nasopharyngeal swab|nasal swab]].<ref name=cnn_saliva>{{cite web |url=https://www.cnn.com/2020/04/14/health/coronavirus-test-saliva-fda-emergency-use-bn/index.html |title= FDA authorizes Covid-19 saliva test for emergency use |publisher=CNN |access-date=1 May 2020|date =14 April 2020 }}</ref> It is believed that this will reduce the risk for health care professionals,<ref name= Rutgers_saliva>{{cite web |url=https://www.rutgers.edu/news/new-rutgers-saliva-test-coronavirus-gets-fda-approval |title= New Rutgers Saliva Test for Coronavirus Gets FDA Approval |publisher=Rutgers.edu |access-date=1 May 2020|date =13 April 2020 }}</ref> will be much more comfortable for the patient,<ref name=cnn_saliva/> and will enable quarantined people to collect their own samples more efficiently.<ref name=Rutgers_saliva/> According to some experts it is too early to know the operating characteristics of the saliva test, and whether it will prove to be as sensitive as the [[nasopharyngeal swab]] test.<ref>{{cite web |url=https://newsnetwork.mayoclinic.org/discussion/covid-19-saliva-tests-what-is-the-benefit/ |title= COVID-19 saliva tests: What is the benefit? |publisher= Mayo Clinic|access-date=6 May 2020|date =16 April 2020 }}</ref><ref name=nature_saliva/> Some studies suggest that the diagnostic value of saliva depends on how saliva specimens are collected (from deep throat, from oral cavity, or from salivary glands).<ref name=nature_saliva/> A recent test conducted by the [[Yale University School of Public Health]] found that saliva yielded greater detection sensitivity and consistency throughout the course of infection when compared with samples taken with [[nasopharyngeal swab]]s.<ref>{{cite web |url=https://www.merckmanuals.com/home/resourcespages/select-covid-19-news |title= Yale University School of Public Health finds saliva samples promising alternative to nasopharyngeal swab |publisher= Merck Manual|access-date=6 April 2020|date = 29 April 2020}}</ref><ref>{{cite document |url=https://www.medrxiv.org/content/10.1101/2020.04.16.20067835v1 |title= Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs |publisher= Medrxiv|access-date=5 May 2020|date =22 April 2020 |doi= 10.1101/2020.04.16.20067835 |last1= Wyllie |first1= Anne Louise |last2= Fournier |first2= John |last3= Casanovas-Massana |first3= Arnau |last4= Campbell |first4= Melissa |last5= Tokuyama |first5= Maria |last6= Vijayakumar |first6= Pavithra |last7= Geng |first7= Bertie |last8= Muenker |first8= M. Catherine |last9= Moore |first9= Adam J. |last10= Vogels |first10= Chantal B. F. |last11= Petrone |first11= Mary E. |last12= Ott |first12= Isabel M. |last13= Lu |first13= Peiwen |last14= Lu-Culligan |first14= Alice |last15= Klein |first15= Jonathan |last16= Venkataraman |first16= Arvind |last17= Earnest |first17= Rebecca |last18= Simonov |first18= Michael |last19= Datta |first19= Rupak |last20= Handoko |first20= Ryan |last21= Naushad |first21= Nida |last22= Sewanan |first22= Lorenzo R. |last23= Valdez |first23= Jordan |last24= White |first24= Elizabeth B. |last25= Lapidus |first25= Sarah |last26= Kalinich |first26= Chaney C. |last27= Jiang |first27= Xiaodong |last28= Kim |first28= Daniel J. |last29= Kudo |first29= Eriko |last30= Linehan |first30= Melissa |displayauthors= 29 }}</ref> |
||
<gallery class="center" widths="200px" heights="200px" > |
<gallery class="center" widths="200px" heights="200px" > |
||
Revision as of 15:47, 8 May 2020
Template:Use Commonwealth English
| Part of a series on the |
| COVID-19 pandemic |
|---|
 |
|
|
|

COVID-19 testing can identify the SARS-CoV-2 virus and includes methods that detect the presence of virus itself (RT-PCR, isothermal nucleic acid amplification, antigen) and those that detect antibodies produced in response to infection. Detection of antibodies (serology) can be used both for diagnosis and population surveillance. Antibody tests show how many people have had the disease, including those whose symptoms were minor or who were asymptomatic. An accurate mortality rate of the disease and the level of herd immunity in the population can be determined from the results of this test. However, the duration and effectiveness of this immune response are still unclear,[1] and the rates of false positives and false negatives must be duly factored into the interpretation.
Due to limited testing, as of March 2020 no countries had reliable data on the prevalence of the virus in their population.[2] As of 24 May, the countries that published their testing data have on average performed a number of tests equal to only 2.6% of their population, and no country has tested samples equal to more than 17.3% of its population.[3] There are variations in how much testing has been done across countries.[4] This variability is also likely to be affecting reported case fatality rates, which have probably been overestimated in many countries, due to sampling bias.[5][6][7]
Test methods

There are two broad categories of test. Some look for the presence of the virus, e.g. the RT-PCR test. Others look for the antibodies which arise when a body is attacked by the virus. The purposes for which the test results are useful is different for the two kinds of test. Another option is to look for lung damage via either CT scan or low oxygen take up since testing via RT-PCR requires the virus to be established and there are a number of false negatives especially in the initial stages.
Current infection
Polymerase chain reaction
Polymerase chain reaction (PCR) is a process that causes a very small well-defined segment of DNA to be amplified, or multiplied many hundreds of thousands of times, so that there is enough of it to be detected and analyzed. Viruses such as SARS-CoV-2 do not contain DNA but only RNA.[9] When a respiratory sample is collected from the person being tested it is treated with certain chemicals[10][9] which remove extraneous substances and extract only the RNA from the sample. Reverse transcription polymerase chain reaction (RT-PCR) is a technique that first uses reverse transcription to convert the extracted RNA into DNA and then uses PCR to amplify a piece of the resulting DNA, creating enough to be examined in order to determine if it matches the genetic code of SARS-CoV-2.[9] Real-time PCR (qPCR)[11] provides advantages during the PCR portion of this process, including automating it and enabling high-throughput and more reliable instrumentation, and has become the preferred method.[12][13] Altogether, the combined technique has been described as real-time RT-PCR[14] or quantitative RT-PCR[15] and is sometimes abbreviated qRT-PCR[16] or rRT-PCR[17] or RT-qPCR,[18] although sometimes just RT-PCR or PCR is used as an abbreviation. The test can be done on respiratory samples obtained by various methods, including a nasopharyngeal swab or sputum sample,[19] as well as on saliva.[20] Results are generally available within a few hours to 2 days.[21] The RT-PCR test performed with throat swabs is only reliable in the first week of the disease. Later on the virus can disappear in the throat while it continues to multiply in the lungs. For infected people tested in the second week, alternatively sample material can then be taken from the deep airways by suction catheter, or coughed up material (sputum) can be used.[22] Saliva has been shown to be a common and transient medium for virus transmission[23] and results provided to the FDA suggest that testing saliva may be as effective as nasal and throat swabs.[20] The FDA has granted Emergency Use Authorization for a test that collects saliva instead of using the traditional nasal swab.[24] It is believed that this will reduce the risk for health care professionals,[25] will be much more comfortable for the patient,[24] and will enable quarantined people to collect their own samples more efficiently.[25] According to some experts it is too early to know the operating characteristics of the saliva test, and whether it will prove to be as sensitive as the nasopharyngeal swab test.[26][23] Some studies suggest that the diagnostic value of saliva depends on how saliva specimens are collected (from deep throat, from oral cavity, or from salivary glands).[23] A recent test conducted by the Yale University School of Public Health found that saliva yielded greater detection sensitivity and consistency throughout the course of infection when compared with samples taken with nasopharyngeal swabs.[27][28]
-
Demonstration of a nasopharyngeal swab for COVID-19 testing
-
Demonstration of a throat swab for COVID-19 testing
-
A PCR machine
Isothermal amplification assays
There are a number of isothermal nucleic acid amplification methods that in principle work like PCR, amplifying a piece of the virus's genome, but are faster than PCR because they don't involve repeated cycles of heating and cooling the sample.
Antigen
An antigen is the part of a pathogen that elicits an immune response. While RT-PCR tests look for RNA from the virus and antibody tests detect human antibodies that have been generated against the virus (detectable days or weeks after the infection sets in), antigen tests look for proteins from the surface of the virus. In the case of a coronavirus these are usually proteins from the surface spikes, and a nasal swab is used to collect samples from the nasal cavity.[29] One of the difficulties has been finding a protein target unique to SARS-CoV-2.[30] There are related coronaviruses that cause the common cold.[31]
Antigen tests are seen by many as the only way it will be possible to scale up testing to the numbers that will really be needed to detect acute infection on the scale required.[29] Isothermal nucleic acid amplification tests, such as the test from Abbot Labs, can only process one sample at a time per machine. RT-PCR tests are accurate but it takes too much time, energy and trained personnel to run the tests.[29] “There will never be the ability on a [PCR] test to do 300 million tests a day or to test everybody before they go to work or to school,” Deborah Birx, head of the White House Coronavirus Task Force, said on April 17. “But there might be with the antigen test.”[32]
An antigen test works by taking a nasal swab from a patient and exposing that to paper strips that contain artificial antibodies designed to bind to coronavirus antigens. Any antigens that are present will bind to the strips and give a visual readout. The process takes less than 30 minutes, can deliver results on the spot, and doesn't require expensive equipment or extensive training.[29]
The problem is that in the case of respiratory viruses often there is not enough of the antigen material present in the nasal swab to be detectable.[30] This would especially be true with people who are asymptomatic and who have very little if any nasal discharge. Unlike the RT-PCR test, which amplifies very small amounts of genetic material so that there is enough to detect, there is no amplification of viral proteins in an antigen test.[29][33] According to the World Health Organization (WHO) the sensitivity of similar antigen tests for respiratory diseases like the flu ranges between 34% and 80%. "Based on this information, half or more of COVID-19 infected patients might be missed by such tests, depending on the group of patients tested," the WHO said. Many scientists doubt whether an antigen test can be made reliable enough in time to be useful against Covid-19.[33]
Medical imaging
Chest CT scans are not recommended for routine screening. Radiologic findings in COVID19 are not specific.[34][35] Typical features on CT initially include bilateral multilobar ground-glass opacities with a peripheral or posterior distribution.[35] Subpleural dominance, crazy paving, and consolidation may develop as the disease evolves.[35][36]
-
Typical CT imaging findings
-
CT imaging of rapid progression stage
Past infection
Serology tests rely on drawn blood, not a nasal or throat swab, and can identify people who were infected and have already recovered from Covid-19, including those who didn't know that they had been infected.[37] Most serology tests are in the research stage of development.[38]
Part of the immune response to infection is the production of antibodies including IgM and IgG. According to the FDA, IgM antibodies to SARS-CoV-2 are generally detectable in blood several days after initial infection, although levels over the course of infection are not well characterized.[39] IgG antibodies to SARS-CoV-2 generally become detectable 10–14 days after infection although they may be detected earlier, and normally peak around 28 days after the onset of infection.[40][41] Since antibodies are slow to present they are not the best markers of acute infection, but as they may persist in the bloodstream for many years they are ideal for detecting historic infections.[29]
Antibody tests can be used to determine the percentage of the population that has contracted the disease and that is therefore presumed to be immune. However, it is still not clear how broad and how long and how effective this immune response is.[1][42] As of April 2020[update] "[s]ome countries are considering issuing so-called immunity passports or risk-free certificates to people who have antibodies against Covid-19, enabling them to travel or return to work assuming that they are protected against reinfection".[43] However, according to the World Health Organization as of 24 April 2020, “There is currently no evidence that people who have recovered from COVID-19 and have antibodies are protected from a second infection.”[44] One of the reasons for the uncertainty is that most, if not all, of the current Covid-19 antibody testing done at large scale is for detection of binding antibodies only and does not measure neutralizing antibodies.[45][46][47] A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic.[48] A binding antibody will bind to the pathogen but the pathogen remains infective; the purpose can be to flag the pathogen for destruction by the immune system.[49] It may even enhance infectivity by interacting with receptors on macrophages.[50] Since most Covid-19 antibody tests will return a positive result if they find only binding antibodies these tests cannot indicate that the person being tested has generated any NAbs which would give him or her protection against re-infection.[46][47]
It is normally expected that if binding antibodies are detected the person also has generated NAbs[47] and for many viral diseases total antibody responses correlate somewhat with NAb responses[51] but this is not yet known for Covid-19. A study of 175 people in China who had recovered from COVID‑19 and had mild symptoms reported that 10 individuals had produced no detectable NAbs at the time of discharge, nor did they develop NAbs thereafter. How these patients recovered without the help of NAbs and whether they were at risk of re-infection of SARS-CoV-2 was left for further exploration.[46][52] An additional source of uncertainty is that even if NAbs are present, several viruses, such as HIV, have evolved mechanisms to evade NAb responses. While this needs to be examined in the context of COVID-19 infection, past experiences with viral infection, in general, argue that in most recovered patients NAb level is a good indicator of protective immunity.[45]
It is presumed that once a person has been infected his or her chance of getting a second infection two to three months later is low, but how long that protective immunity might last is not known.[46] One study determined that reinfection at 29 days post-infection could not occur in SARS-CoV-2 infected rhesus macaques.[53] Studies have indicated that NAbs to the original SARS virus (the predecessor to the current SARS-CoV-2) can remain active in most people for two years[54] and are gone after six years.[55] Nevertheless, memory cells including Memory B cells and Memory T cells can last much longer and may have the ability to greatly lessen the severity of a reinfection.[55]
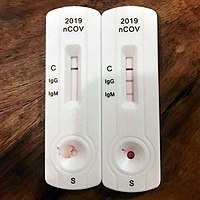
-
Blood from pipette is then placed onto a COVID-19 rapid diagnostic test device.
Infectivity
United States Centers for Disease Control and Prevention (CDC) updated the following information and recommendation in a "Decision Memo" on 3 May.[56] At this time, data are limited regarding how long after infection people continue to shed infectious SARV-CoV-2 RNA, and can therefore still infect others. Key findings are summarized here.
- Viral burden measured in upper respiratory specimens declines after onset of illness.[57]
- At this time, replication-competent virus has not been successfully cultured more than 9 days after onset of illness. The statistically estimated likelihood of recovering replication-competent virus approaches zero by 10 days.[58]
- As the likelihood of isolating replication-competent virus decreases, anti-SARS-CoV-2 IgM and IgG can be detected in an increasing number of persons recovering from infection.[59]
- Attempts to culture virus from upper respiratory specimens have been largely unsuccessful when viral burden is in low but detectable ranges (i.e., Ct values higher than 33-35)[56]
- Following recovery from clinical illness, many patients no longer have detectable viral RNA in upper respiratory specimens. Among those who continue to have detectable RNA, concentrations of detectable RNA 3 days following recovery are generally in the range at which replication-competent virus has not been reliably isolated by CDC.[60]
- No clear correlation has been described between length of illness and duration of post-recovery shedding of detectable viral RNA in upper respiratory specimens. [61]
- Infectious virus has not been cultured from urine or reliably cultured from feces;[62] these potential sources pose minimal if any risk of transmitting infection and any risk can be sufficiently mitigated by good hand hygiene.
For an emerging pathogen like SARS-CoV-2, the patterns and duration of illness and infectivity have not been fully described. However, available data indicate that shedding of SARS-CoV-2 RNA in upper respiratory specimens declines after onset of symptoms. At 10 days after illness onset, recovery of replication-competent virus in viral culture (as a proxy of the presence of infectious virus) is decreased and approaches zero. Although persons may produce PCR-positive specimens for up to 6 weeks,[63] it remains unknown whether these PCR-positive samples represent the presence of infectious virus. After clinical recovery, many patients do not continue to shed SARS-CoV-2 viral RNA. Among recovered patients with detectable RNA in upper respiratory specimens, concentrations of RNA after 3 days are generally in ranges where virus has not been reliably cultured by CDC. These data have been generated from adults across a variety of age groups and with varying severity of illness. Data from children and infants are not presently available.[56]
Approaches to testing

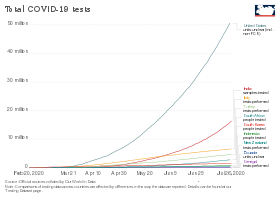
After issues with testing accuracy and capacity during January and February, the United States was testing 100,000 people per day by 27 March.[65] In comparison, several European countries have been conducting more daily tests per capita than the United States.[66][67] Three European countries are aiming to conduct 100,000 tests per day – Germany by mid-April, the United Kingdom by the end of April and France by the end of June. Germany has a large medical diagnostics industry, with over 100 testing labs that provided the technology and infrastructure to enable rapid increases in testing. The UK sought to diversify its life sciences companies into diagnostics to scale up testing capacity.[68]
In Germany, the National Association of Statutory Health Insurance Physicians said on 2 March, that it had a capacity for about 12,000 tests per day in the ambulatory setting and 10,700 had been tested in the prior week. Costs are borne by the health insurance when the test is ordered by a physician.[69] According to the president of the Robert Koch Institute, Germany has an overall capacity for 160,000 tests per week.[70] As of 19 March drive-in tests were offered in several large cities.[71] As of 26 March, the total number of tests performed in Germany was unknown, because only positive results are reported. Health minister Jens Spahn estimated 200,000 tests per week.[72] A first lab survey revealed that as of the end of March a total of at least 483,295 samples were tested and 33,491 samples (6.9%) tested positive for SARS-CoV-2.[73]
By the start of April, the United Kingdom was delivering around 10,000 swab tests per day. It set a target for 100,000 per day by the end of April, eventually rising to 250,000 tests per day.[68] The British NHS has announced that it is piloting a scheme to test suspected cases at home, which removes the risk of a patient infecting others if they come to a hospital or having to disinfect an ambulance if one is used.[74]
In drive-through testing for COVID‑19 for suspected cases, a healthcare professional takes sample using appropriate precautions.[75][76] Drive-through centers have helped South Korea do some of the fastest, most-extensive testing of any country.[77] Hong Kong has set up a scheme where suspected patients can stay home, "[the] emergency department will give a specimen tube to the patient", they spit into it, send it back and get a test result a while after.[78]
In Israel, researchers at Technion and Rambam Hospital developed and tested a method for testing samples from 64 patients simultaneously, by pooling the samples and only testing further if the combined sample is found to be positive.[79][80][81] Pool testing was then adopted in Israel, Germany, South Korea,[82] and Nebraska,[83] and the Indian states Uttar Pradesh,[84] West Bengal,[85] Punjab,[86] Chhattisgarh,[87] and Maharashtra.[88]
In Wuhan a makeshift 2000-sq-meter emergency detection laboratory named "Huo-Yan" (Chinese: 火眼, "Fire Eye") was opened on 5 February 2020 by BGI,[89][90] which can process over 10,000 samples a day.[91][90] With the construction overseen by BGI-founder Wang Jian and taking 5-days,[92] modelling has show cases in Hubei would have been 47% higher and the corresponding cost of tackling the quarantine would have doubled if this testing capacity had not come into operation.[citation needed] The Wuhan Laboratory has been promptly followed by Huo-Yan labs in Shenzhen, Tianjin, Beijing, and Shanghai, in a total of 12 cities across China. By 4 March 2020 the daily throughput totals were 50,000 tests per day.[93]
Open source, multiplexed designs released by Origami Assays have been released that can test as many as 1122 patient samples for COVID19 using only 93 assays.[94] These balanced designs can be run in small laboratories without the need for robotic liquid handlers.
By March, shortages and insufficient amounts of reagent has become a bottleneck for mass testing in the EU and UK[95] and the US.[96][97] This has led some authors to explore sample preparation protocols that involve heating samples at 98 °C (208 °F) for 5 minutes to release RNA genomes for further testing.[98][99]
On 31 March it was announced United Arab Emirates was now testing more of its population for Coronavirus per head than any other country, and was on track to scale up the level of testing to reach the bulk of the population.[100] This was through a combination of drive-through capability, and purchasing a population-scale mass-throughput laboratory from Group 42 and BGI (based on their "Huo-Yan" emergency detection laboratories in China). Constructed in 14 days, the lab is capable of conducting tens of thousands RT-PCR tests per day and is the first in the world of this scale to be operational outside of China.[101]
On 8 April 2020, In India, the Supreme Court of India ruled that private labs should be reimbursed at the appropriate time for COVID-19 tests [102] However private labs have stated that they are unable to scale up the testing because of the price cap put on the testing and labs being forced to make advance payment to suppliers while they receive deferred payment from hospitals.[103]
University of California San Francisco conducted PCR tests of 1,845 people in Bolinas, California on April 20–24, almost the entire town. No active infections were detected. Antibody testing was also performed but the results are not yet available.[104]
Production and volume
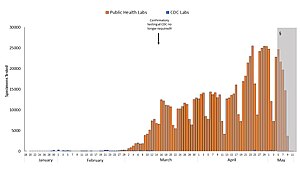
Blue: CDC lab
Orange: Public health lab
Gray: Data incomplete due to reporting lag
Not shown: Testing at private labs; total exceeded 100,000 per day by 27 March[105]
Different testing recipes targeting different parts of the coronavirus genetic profile were developed in China, France, Germany, Hong Kong, Japan, the United Kingdom, and the United States. The World Health Organization adopted the German recipe for manufacturing kits sent to low-income countries without the resources to develop their own. The German recipe was published on 17 January 2020; the protocol developed by the United States Centers for Disease Control and Prevention (CDC) was not available until 28 January, delaying available tests in the U.S.[106]
China[107] and the United States[108] had problems with the reliability of test kits early in the outbreak, and these countries and Australia[109] were unable to supply enough kits to satisfy demand and recommendations for testing by health experts. In contrast, experts say South Korea's broad availability of testing helped reduce the spread of the novel coronavirus. Testing capacity, largely in private sector labs, was built up over several years by the South Korean government.[110] On 16 March, the World Health Organization called for ramping up the testing programmes as the best way to slow the advance of COVID‑19 pandemic.[111][112]
High demand for testing due to wide spread of the virus caused backlogs of hundreds of thousands of tests at private U.S. labs, and supplies of swabs and chemical reagents became strained.[113]
Available tests
PCR based
When scientists from China first released information on the COVID‑19 viral genome on 11 January 2020, the Malaysian Institute for Medical Research (IMR) successfully produced the “primers and probes” specific to SARS-CoV-2 on the very same day. The IMR's laboratory in Kuala Lumpur had initiated early preparedness by setting up reagents to detect coronavirus using the rt-PCR method.[114] The WHO reagent sequence (primers and probes) released several days later was very similar to that produced in the IMR's laboratory, which was used to diagnose Malaysia's first COVID‑19 patient on 24 January 2020.[115]
Public Health England developed a test by 10 January,[116] using real-time RT-PCR (RdRp gene) assay based on oral swabs.[117] The test detected the presence of any type of coronavirus including specifically identifying SARS-CoV-2. It was rolled out to twelve laboratories across the United Kingdom on 10 February.[118] Another early PCR test was developed by Charité in Berlin, working with academic collaborators in Europe and Hong Kong, and published on 23 January. It used rtRT-PCR, and formed the basis of 250,000 kits for distribution by the World Health Organization (WHO).[119] The South Korean company Kogenebiotech developed a clinical grade, PCR-based SARS-CoV-2 detection kit (PowerChek Coronavirus) approved by Korea Centers for Disease Control and Prevention (KCDC) on 4 February 2020.[120] It looks for the "E" gene shared by all beta coronaviruses, and the RdRp gene specific to SARS-CoV-2.[121]
In China, BGI Group was one of the first companies to receive emergency use approval from China's National Medical Products Administration for a PCR-based SARS-CoV-2 detection kit.[122]
In the United States, the CDC distributed its SARS-CoV-2 Real Time PCR Diagnostic Panel to public health labs through the International Reagent Resource.[123] One of three genetic tests in older versions of the test kits caused inconclusive results due to faulty reagents, and a bottleneck of testing at the CDC in Atlanta; this resulted in an average of fewer than 100 samples a day being successfully processed throughout the whole of February 2020. Tests using two components were not determined to be reliable until 28 February 2020, and it was not until then that state and local laboratories were permitted to begin testing.[124] The test was approved by the FDA under an EUA.[citation needed]
US commercial labs began testing in early March 2020. As of 5 March 2020 LabCorp announced nationwide availability of COVID‑19 testing based on RT-PCR.[125] Quest Diagnostics similarly made nationwide COVID‑19 testing available as of 9 March 2020.[126]
In Russia, the first COVID‑19 test was developed by the State Research Center of Virology and Biotechnology VECTOR, production began on January 24.[127] On 11 February 2020 the test was approved by the Federal Service for Surveillance in Healthcare.[128]
On 12 March 2020, Mayo Clinic was reported to have developed a test to detect COVID‑19 infection.[129]
On 18 March 2020, the FDA issued EUA to Abbott Laboratories[130] for a test on Abbott's m2000 system; the FDA had previously issued similar authorization to Hologic,[131] LabCorp,[130] and Thermo Fisher Scientific.[132] On 21 March 2020, Cepheid similarly received an EUA from the FDA for a test that takes about 45 minutes on its GeneXpert system; the same system that runs the GeneXpert MTB/RIF.[133][134]
On 13 April, Health Canada approved a test from Spartan Bioscience. Institutions may "test patients" with a handheld DNA analyzer "and receive results without having to send samples away to a [central] lab."[135][136]
Isothermal nucleic amplification

On 27 March 2020, the FDA issued an Emergency Use Authorization for a test by Abbott Laboratories, called ID NOW COVID-19, that uses isothermal nucleic acid amplification technology instead of PCR.[137] The assay amplifies a unique region of the virus's RdRp gene; the resulting copies are then detected with "fluorescently-labeled molecular beacons".[138] The test kit uses the company's "toaster-size" ID NOW device which costs $12,000-$15,000.[139] The device can be used in laboratories or in patient care settings, and provides results in 13 minutes or less.[138] There are currently about 18,000 ID NOW devices in the U.S. and Abbott expects to ramp up manufacturing to deliver 50,000 ID NOW COVID-19 test kits per day.[140]
All visitors to U.S. President Donald Trump are required to undergo the test on site at the White House.[141]
In a study conducted by the Cleveland Clinic, the ID NOW COVID-19 test detected the virus only in 85.2% of the samples that contained it. According to the director of the study a test should be at least 95% reliable. Abbott said that the issue could have been caused by storing the samples in a special solution instead of inserting them directly into the testing machine.[142]
Serology tests
As of 24 April, six tests had been approved for diagnosis in the United States, all under FDA Emergency Use Authorization (EUA).[143] The tests are listed and described at the Johns Hopkins Center for Health Security. Other tests have been approved in other countries.[144]
In the United States, as of 28 April, Quest Diagnostics made a COVID-19 antibody test available for purchase to the general public through the QuestDirect service. Cost of the test is approximately US$130. The test requires the individual to visit a Quest Diagnostics location for a blood draw. Results are available days later.[145]
A number of countries are beginning large scale surveys of their populations using these tests.[146][147] A study in California conducted antibody testing in one county and estimated that the number of coronaviruses cases was between 2.5 and 4.2% of the population, or 50 to 85 times higher than the number of confirmed cases.[148][149]
In late March 2020, a number of companies received European approvals for their test kits. The testing capacity is several hundred samples within hours. The antibodies are usually detectable 14 days after the onset of the infection.[150]
WHO Emergency Use Listing
| Date listed | Product name | Manufacturer |
|---|---|---|
| 3 April 2020 | Cobas SARS-CoV-2 qualitative assay for use on the cobas 6800/8800 Systems | Roche Molecular Systems |
| 7 April 2020 | Coronavirus (COVID-19) genesig rtPCR assay | Primerdesign |
| 9 April 2020 | Abbott Realtime SARS-CoV-2[151] | Abbott Molecular |
| 24 April 2020 | PerkinElmer SARS-CoV-2 Real-time RT-PCR Assay[151] | SYM-BIO LiveScience |
As of 7 April 2020, the WHO had accepted two diagnostic tests for procurement under the Emergency Use Listing procedure (EUL) for use during the COVID‑19 pandemic, in order to increase access to quality-assured, accurate tests for the disease.[152] Both in vitro diagnostics, the tests are genesig Real-Time PCR Coronavirus (COVID‑19) manufactured by Primerdesign, and cobas SARS-CoV-2 Qualitative assay for use on the cobas® 6800/8800 Systems by Roche Molecular Systems. Approval means that these tests can also be supplied by the United Nations and other procurement agencies supporting the COVID‑19 response.[clarification needed]
Testing accuracy
In March 2020 China[107] reported problems with accuracy in their test kits.
In the United States, the test kits developed by the CDC had "flaws;" the government then removed the bureaucratic barriers that had prevented private testing.[108]
Spain purchased test kits from Chinese firm Shenzhen Bioeasy Biotechnology Co Ltd, but found that results were inaccurate. The firm explained that the incorrect results may be a result of a failure to collect samples or use the kits correctly. The Spanish ministry said it will withdraw the kits that returned incorrect results, and would replace them with a different testing kit provided by Shenzhen Bioeasy.[153]
80% of test kits the Czech Republic purchased from China gave wrong results.[154][155]
Slovakia purchased 1.2 million test kits from China which were found to be inaccurate. Prime Minister Matovič suggested these be dumped into the Danube.[156]
Ateş Kara of the Turkish Health Ministry said the test kits Turkey purchased from China had a "high error rate" and did not "put them into use."[157][158]
The UK purchased 3.5 million test kits from China but in early April 2020 announced these were not usable.[159][160][161]
On 21 April 2020, the Indian Council of Medical Research has advised Indian states to stop using the rapid antibody test kits purchased from China after receiving complaints from one state. Rajasthan health minister Raghu Sharma on 21 April said the kits gave only 5.4 percent accurate results against the expectation of 90 percent accuracy.[162]
Confirmatory testing
WHO recommends that countries that do not have testing capacity and national laboratories with limited experience on COVID‑19 send their first five positives and the first ten negative COVID‑19 samples to one of the 16 WHO reference laboratories for confirmatory testing.[163][164] Out of the 16 reference laboratories, 7 are in Asia, 5 in Europe, 2 in Africa, 1 in North America and 1 in Australia.[165]
Clinical effectiveness
Testing, followed with quarantine of those who tested positive and tracing of those with whom the SARS-CoV-2 positive people had had contact, resulted in positive outcomes.[clarification needed].[citation needed]
Italy
Researchers working in the Italian town of Vò, the site of the first COVID‑19 death in Italy, conducted two rounds of testing on the entire population of about 3,400 people, about ten days apart. About half the people testing positive had no symptoms, and all discovered cases were quarantined. With travel to the commune restricted, this eliminated new infections completely.[166]
Singapore
With aggressive contact tracing, inbound travel restrictions, testing, and quarantining, the COVID-19 pandemic in Singapore has proceeded much more slowly than in other developed countries [dubious – discuss], but without extreme restrictions like forced closure of restaurants and retail establishments. Many events have been cancelled, and Singapore did start advising residents to stay at home on 28 March, but schools reopened on time after holiday break on 23 March,[167] even though schools did close moving to "full home-based learning" on 8 April.[168]
U.S. aircraft carrier Theodore Roosevelt
After 94% of the 4,800 crew had been tested roughly 60 percent of the over 600 sailors who tested positive did not have symptoms.[169]
Russia
On 27 April, Russia passed the 3 million test mark and had 183,000 people under medical supervision because they were suspected of having the virus[170] with 87,147 people having tested positive for the virus and total confirmed deaths at 794.[171] On 28 April Anna Popova head of Federal Service for Surveillance in Healthcare (Roszdravnadzor) in a presidential update stated that there were now 506 laboratories testing and that 45% of those tested have no symptoms with the number having pneumonia reducing from 25% to 20% and only 5% of patients with a severe form. 40% of infections were from family members. The speed of people reporting illness has improved from 6 days to the day people find symptoms. Antibody testing was carried out on 3,200 Moscow doctors and 20% have immunity.[172]
Others
Several other countries, such as Iceland[173] and South Korea,[174] have also managed the pandemic with aggressive contact tracing, inbound travel restrictions, testing, and quarantining, but with less aggressive lock-downs. A statistical study has found that countries that have tested more, relative to the number of deaths, have much lower case fatality rates, probably because these countries are better able to detect those with only mild or no symptoms.[5] A subsequent study has also found that countries that have tested more widely also have a younger age distribution of cases, relative to the wider population.[175]
Research and development
A test which uses monoclonal antibodies which bind to the nucleocapsid protein (N protein) of the SARS-CoV-2 is being developed in Taiwan, with the hope that it can provide results in 15 to 20 minutes just like a rapid influenza test.[176] The World Health Organization raised concerns on 8 April that these tests need to be validated for the disease and are in a research stage only.[177] The United States Food and Drug Administration approved an antibody test on 2 April,[178] but some researchers warn that such tests should not drive public health decisions unless the percentage of COVID‑19 survivors who are producing neutralizing antibodies is also known.[42]
Virus testing statistics by country
The figures are influenced by the country's testing policy. If two countries are alike in every respect, including having the same spread of infection, and one of them has a shortage of testing capability, it may only test those showing symptoms while the other country having greater access to testing may test both those showing symptoms and others chosen at random. A country that only tests people showing symptoms will have a higher figure for “% (Percentage of positive tests)” than a country that also tests people chosen at random.[179] If two countries are alike in every respect, including which people they test, the one that tests more people will have a higher "Positive / million people."
| Country or region | Date[a] | Tested | Units[b] | Confirmed (cases) |
Confirmed / tested, % |
Tested / population, % |
Confirmed / population, % |
Ref. |
|---|---|---|---|---|---|---|---|---|
| 17 Dec 2020 | 154,767 | samples | 49,621 | 32.1 | 0.40 | 0.13 | [180] | |
| 18 Feb 2021 | 428,654 | samples | 96,838 | 22.6 | 15.0 | 3.4 | [181] | |
| 2 Nov 2020 | 230,553 | samples | 58,574 | 25.4 | 0.53 | 0.13 | [182][183] | |
| 23 Feb 2022 | 300,307 | samples | 37,958 | 12.6 | 387 | 49.0 | [184] | |
| 2 Feb 2021 | 399,228 | samples | 20,981 | 5.3 | 1.3 | 0.067 | [185] | |
| 6 Mar 2021 | 15,268 | samples | 832 | 5.4 | 15.9 | 0.86 | [186] | |
| 16 Apr 2022 | 35,716,069 | samples | 9,060,495 | 25.4 | 78.3 | 20.0 | [187] | |
| 29 May 2022 | 3,099,602 | samples | 422,963 | 13.6 | 105 | 14.3 | [188] | |
| 9 Sep 2022 | 78,548,492 | samples | 10,112,229 | 12.9 | 313 | 40.3 | [189] | |
| 1 Feb 2023 | 205,817,752 | samples | 5,789,991 | 2.8 | 2,312 | 65.0 | [190] | |
| 11 May 2022 | 6,838,458 | samples | 792,638 | 11.6 | 69.1 | 8.0 | [191] | |
| 28 Nov 2022 | 259,366 | samples | 37,483 | 14.5 | 67.3 | 9.7 | [192] | |
| 3 Dec 2022 | 10,578,766 | samples | 696,614 | 6.6 | 674 | 44.4 | [193] | |
| 24 Jul 2021 | 7,417,714 | samples | 1,151,644 | 15.5 | 4.5 | 0.70 | [194] | |
| 14 Oct 2022 | 770,100 | samples | 103,014 | 13.4 | 268 | 35.9 | [195] | |
| 9 May 2022 | 13,217,569 | samples | 982,809 | 7.4 | 139 | 10.4 | [196] | |
| 24 Jan 2023 | 36,548,544 | samples | 4,691,499 | 12.8 | 317 | 40.7 | [197] | |
| 8 Jun 2022 | 572,900 | samples | 60,694 | 10.6 | 140 | 14.9 | [198][199] | |
| 4 May 2021 | 595,112 | samples | 7,884 | 1.3 | 5.1 | 0.067 | [200] | |
| 28 Feb 2022 | 1,736,168 | samples | 12,702 | 0.73 | 234 | 1.71 | [201] | |
| 5 Jun 2022 | 4,358,669 | cases | 910,228 | 20.9 | 38.1 | 8.0 | [202] | |
| 27 Sep 2022 | 1,872,934 | samples | 399,887 | 21.4 | 54.7 | 11.7 | [203] | |
| 11 Jan 2022 | 2,026,898 | 232,432 | 11.5 | 89.9 | 10.3 | [204][205] | ||
| 19 Feb 2021 | 23,561,497 | samples | 10,081,676 | 42.8 | 11.2 | 4.8 | [206][207] | |
| 2 Aug 2021 | 153,804 | samples | 338 | 0.22 | 33.5 | 0.074 | [208] | |
| 2 Feb 2023 | 10,993,239 | samples | 1,295,524 | 11.8 | 158 | 18.6 | [209] | |
| 4 Mar 2021 | 158,777 | samples | 12,123 | 7.6 | 0.76 | 0.058 | [182][210] | |
| 5 Jan 2021 | 90,019 | 884 | 0.98 | 0.76 | 0.0074 | [211] | ||
| 1 Aug 2021 | 1,812,706 | 77,914 | 4.3 | 11.2 | 0.48 | [212] | ||
| 18 Feb 2021 | 942,685 | samples | 32,681 | 3.5 | 3.6 | 0.12 | [182] | |
| 26 Nov 2022 | 66,343,123 | samples | 4,423,053 | 6.7 | 175 | 11.7 | [213] | |
| 2 Mar 2021 | 99,027 | samples | 4,020 | 4.1 | 0.72 | 0.029 | [182][214] | |
| 1 Feb 2023 | 48,154,268 | samples | 5,123,007 | 10.6 | 252 | 26.9 | [215] | |
| 31 Jul 2020 | 160,000,000 | cases | 87,655 | 0.055 | 11.1 | 0.0061 | [216][217] | |
| 24 Nov 2022 | 36,875,818 | samples | 6,314,769 | 17.1 | 76.4 | 13.1 | [218][219] | |
| 2 Nov 2021 | 2,575,363 | samples | 561,054 | 21.8 | 51.5 | 11.2 | [220] | |
| 2 Feb 2023 | 5,481,285 | cases | 1,267,798 | 23.1 | 134 | 31.1 | [221] | |
| 2 Feb 2023 | 14,301,394 | samples | 1,112,470 | 7.8 | 126 | 9.8 | [222][223] | |
| 29 Jan 2023 | 27,820,163 | samples | 644,160 | 2.3 | 3,223 | 74.4 | [224] | |
| 1 Feb 2023 | 22,544,928 | samples | 4,590,529 | 20.4 | 211 | 42.9 | [225] | |
| 31 Jan 2023 | 67,682,707 | samples | 3,399,947 | 5.0 | 1,162 | 58.4 | [226][227] | |
| 28 Apr 2022 | 305,941 | 15,631 | 5.1 | 33.2 | 1.7 | [228] | ||
| 20 Jun 2022 | 209,803 | cases | 14,821 | 7.1 | 293 | 20.7 | [229] | |
| 22 Jul 2022 | 3,574,665 | samples | 626,030 | 17.5 | 32.9 | 5.8 | [230] | |
| 28 Feb 2021 | 124,838 | 25,961 | 20.8 | 0.14 | 0.029 | [182][231] | ||
| 23 Jul 2021 | 1,627,189 | samples | 480,720 | 29.5 | 9.5 | 2.8 | [232] | |
| 23 Jul 2021 | 3,137,519 | samples | 283,947 | 9.1 | 3.1 | 0.28 | [182][233] | |
| 18 Mar 2022 | 1,847,861 | samples | 161,052 | 8.7 | 28.5 | 2.5 | [234] | |
| 30 Jan 2023 | 403,773 | 17,113 | 4.2 | 30.8 | 1.3 | [235] | ||
| 31 Jan 2023 | 3,637,908 | samples | 613,954 | 16.9 | 274 | 46.2 | [236] | |
| 8 Dec 2021 | 415,110 | 49,253 | 11.9 | 36.5 | 4.3 | [237] | ||
| 24 Jun 2021 | 2,981,185 | samples | 278,446 | 9.3 | 2.6 | 0.24 | [238] | |
| 27 Feb 2022 | 774,000 | samples | 34,237 | 4.4 | 1,493 | 65.7 | [239] | |
| 2 Jan 2023 | 667,953 | samples | 68,848 | 10.3 | 74.5 | 7.7 | [240] | |
| 14 Jan 2022 | 9,042,453 | samples | 371,135 | 4.1 | 163 | 6.7 | [241] | |
| 15 May 2022 | 272,417,258 | samples | 29,183,646 | 10.7 | 417 | 44.7 | [242] | |
| 23 Jul 2021 | 958,807 | samples | 25,325 | 2.6 | 3.1 | 0.082 | [243] | |
| 15 Feb 2021 | 43,217 | samples | 4,469 | 10.3 | 2.0 | 0.21 | [244] | |
| 3 Nov 2021 | 4,888,787 | samples | 732,965 | 15.0 | 132 | 19.7 | [245] | |
| 7 Jul 2021 | 65,247,345 | samples | 3,733,519 | 5.7 | 77.8 | 4.5 | [246][247] | |
| 3 Jul 2021 | 1,305,749 | samples | 96,708 | 7.4 | 4.2 | 0.31 | [248] | |
| 18 Dec 2022 | 101,576,831 | samples | 5,548,487 | 5.5 | 943 | 51.5 | [249] | |
| 30 Jan 2022 | 164,573 | samples | 10,662 | 6.5 | 293 | 19.0 | [250] | |
| 11 May 2021 | 28,684 | 161 | 0.56 | 25.7 | 0.14 | [251] | ||
| 6 Jan 2023 | 6,800,560 | samples | 1,230,098 | 18.1 | 39.4 | 7.1 | [252] | |
| 21 Jul 2021 | 494,898 | samples | 24,878 | 5.0 | 3.8 | 0.19 | [182][253] | |
| 7 Jul 2022 | 145,231 | 8,400 | 5.8 | 7.7 | 0.45 | [254] | ||
| 15 Jun 2022 | 648,569 | cases | 66,129 | 10.2 | 82.5 | 8.4 | [255] | |
| 26 Nov 2022 | 223,475 | cases | 33,874 | 15.2 | 2.0 | 0.30 | [256] | |
| 26 Nov 2021 | 1,133,782 | samples | 377,859 | 33.3 | 11.8 | 3.9 | [257] | |
| 10 May 2022 | 11,394,556 | samples | 1,909,948 | 16.8 | 118 | 19.8 | [258] | |
| 9 Aug 2022 | 1,988,652 | samples | 203,162 | 10.2 | 546 | 55.8 | [259] | |
| 8 Jul 2022 | 866,177,937 | samples | 43,585,554 | 5.0 | 63 | 31.7 | [260][261] | |
| 3 Jul 2023 | 76,062,770 | cases | 6,812,127 | 9.0 | 28.2 | 2.5 | [262][263] | |
| 31 May 2022 | 52,269,202 | samples | 7,232,268 | 13.8 | 62.8 | 8.7 | [264] | |
| 3 Aug 2022 | 19,090,652 | samples | 2,448,484 | 12.8 | 47.5 | 6.1 | [265] | |
| 31 Jan 2023 | 12,990,476 | samples | 1,700,817 | 13.1 | 264 | 34.6 | [266] | |
| 17 Jan 2022 | 41,373,364 | samples | 1,792,137 | 4.3 | 451 | 19.5 | [267] | |
| 16 Mar 2023 | 269,127,054 | samples | 25,651,205 | 9.5 | 446 | 42.5 | [268] | |
| 3 Mar 2021 | 429,177 | samples | 33,285 | 7.8 | 1.6 | 0.13 | [269] | |
| 30 Sep 2022 | 1,184,973 | samples | 151,931 | 12.8 | 43.5 | 5.6 | [270] | |
| 1 Mar 2021 | 8,487,288 | 432,773 | 5.1 | 6.7 | 0.34 | [271] | ||
| 6 Jun 2021 | 7,407,053 | samples | 739,847 | 10.0 | 69.5 | 6.9 | [272] | |
| 28 May 2021 | 11,575,012 | samples | 385,144 | 3.3 | 62.1 | 2.1 | [273] | |
| 5 Mar 2021 | 1,322,806 | samples | 107,729 | 8.1 | 2.8 | 0.23 | [274] | |
| 31 May 2021 | 611,357 | cases | 107,410 | 17.6 | 33.8 | 5.9 | [275] | |
| 9 Mar 2022 | 7,754,247 | samples | 624,573 | 8.1 | 181 | 14.6 | [276] | |
| 10 Feb 2021 | 695,415 | samples | 85,253 | 12.3 | 10.7 | 1.3 | [277] | |
| 1 Mar 2021 | 114,030 | cases | 45 | 0.039 | 1.6 | 0.00063 | [278] | |
| 5 Sep 2021 | 3,630,095 | samples | 144,518 | 4.0 | 189 | 7.5 | [279] | |
| 14 Jun 2021 | 4,599,186 | samples | 542,649 | 11.8 | 67.4 | 8.0 | [280] | |
| 30 Mar 2022 | 431,221 | 32,910 | 7.6 | 21.5 | 1.6 | [281] | ||
| 17 Jul 2021 | 128,246 | 5,396 | 4.2 | 2.5 | 0.11 | [282] | ||
| 14 Apr 2022 | 2,578,215 | samples | 501,862 | 19.5 | 37.6 | 7.3 | [182][283] | |
| 31 Jan 2023 | 9,046,584 | samples | 1,170,108 | 12.9 | 324 | 41.9 | [284][285] | |
| 12 May 2022 | 4,248,188 | samples | 244,182 | 5.7 | 679 | 39.0 | [286] | |
| 19 Feb 2021 | 119,608 | cases | 19,831 | 16.6 | 0.46 | 0.076 | [287] | |
| 29 Nov 2022 | 624,784 | samples | 88,086 | 14.1 | 3.3 | 0.46 | [288] | |
| 7 Sep 2021 | 23,705,425 | cases | 1,880,734 | 7.9 | 72.3 | 5.7 | [289] | |
| 13 Mar 2022 | 2,216,560 | samples | 174,658 | 7.9 | 398 | 31.3 | [290][291] | |
| 7 Jul 2021 | 322,504 | samples | 14,449 | 4.5 | 1.6 | 0.071 | [182][292] | |
| 8 Sep 2021 | 1,211,456 | samples | 36,606 | 3.0 | 245 | 7.4 | [293] | |
| 16 Apr 2021 | 268,093 | 18,103 | 6.8 | 6.1 | 0.41 | [294] | ||
| 22 Nov 2020 | 289,552 | samples | 494 | 0.17 | 22.9 | 0.039 | [295] | |
| 15 Oct 2021 | 10,503,678 | cases | 3,749,860 | 35.7 | 8.2 | 2.9 | [296] | |
| 20 Apr 2022 | 3,213,594 | samples | 516,864 | 16.1 | 122 | 19.6 | [297] | |
| 10 Jul 2021 | 3,354,200 | cases | 136,053 | 4.1 | 100 | 4.1 | [298] | |
| 10 May 2021 | 394,388 | samples | 98,449 | 25.0 | 62.5 | 15.6 | [299][300] | |
| 6 Jan 2023 | 14,217,563 | cases | 1,272,299 | 8.9 | 38.5 | 3.4 | [301] | |
| 22 Jul 2021 | 688,570 | samples | 105,866 | 15.4 | 2.2 | 0.34 | [302] | |
| 16 Sep 2021 | 4,047,680 | samples | 440,741 | 10.9 | 7.4 | 0.81 | [303] | |
| 4 Jul 2022 | 1,062,663 | samples | 166,229 | 15.6 | 38.7 | 6.1 | [304] | |
| 26 Jul 2022 | 5,804,358 | samples | 984,475 | 17.0 | 20.7 | 3.5 | [305] | |
| 6 Jul 2021 | 14,526,293 | cases | 1,692,834 | 11.7 | 83.4 | 9.7 | [306] | |
| 3 Sep 2021 | 41,962 | samples | 136 | 0.32 | 15.7 | 0.050 | [307] | |
| 29 Jan 2023 | 7,757,935 | samples | 2,136,662 | 27.5 | 156 | 42.9 | [308][309] | |
| 22 Feb 2021 | 79,321 | cases | 4,740 | 6.0 | 0.35 | 0.021 | [310] | |
| 28 Feb 2021 | 1,544,008 | samples | 155,657 | 10.1 | 0.75 | 0.076 | [311] | |
| 25 Nov 2020 | 16,914 | cases | 0 | 0 | 0.066 | 0 | [312] | |
| 1 Jul 2021 | 881,870 | samples | 155,689 | 17.7 | 42.5 | 7.5 | [313][314] | |
| 12 Jul 2022 | 7,096,998 | samples | 103,034 | 1.5 | 2,177 | 31.6 | [315] | |
| 20 Jan 2022 | 9,811,888 | samples | 554,778 | 5.7 | 183 | 10.3 | [316] | |
| 28 Oct 2020 | 509,959 | samples | 114,434 | 22.4 | 11.0 | 2.5 | [317] | |
| 5 Mar 2021 | 9,173,593 | samples | 588,728 | 6.4 | 4.2 | 0.27 | [318] | |
| 5 Feb 2022 | 3,078,533 | samples | 574,105 | 18.6 | 60.9 | 11.4 | [319] | |
| 28 Jan 2023 | 7,475,016 | samples | 1,029,701 | 13.8 | 179 | 24.7 | [320] | |
| 17 Feb 2021 | 47,490 | cases | 961 | 2.0 | 0.53 | 0.011 | [321] | |
| 27 Mar 2022 | 2,609,819 | samples | 647,950 | 24.8 | 36.6 | 9.1 | [322] | |
| 17 Nov 2022 | 36,073,768 | samples | 4,177,786 | 11.6 | 109.9 | 12.7 | [323] | |
| 7 Jan 2023 | 34,402,980 | samples | 4,073,980 | 11.8 | 34.1 | 4.0 | [324][325] | |
| 27 Apr 2022 | 36,064,311 | samples | 5,993,861 | 16.6 | 94.0 | 15.6 | [326] | |
| 5 Jan 2022 | 27,515,490 | samples | 1,499,976 | 5.5 | 268 | 14.6 | [327] | |
| 11 Nov 2022 | 4,061,988 | cases | 473,440 | 11.7 | 141 | 16.4 | [328] | |
| 29 Jan 2021 | 5,405,393 | samples | 724,250 | 13.4 | 27.9 | 3.7 | [329] | |
| 6 Jun 2022 | 295,542,733 | samples | 18,358,459 | 6.2 | 201 | 12.5 | [330][331] | |
| 6 Oct 2021 | 2,885,812 | samples | 98,209 | 3.4 | 22.3 | 0.76 | [332] | |
| 26 Aug 2021 | 30,231 | cases | 995 | 3.3 | 57.6 | 1.9 | [333] | |
| 7 Oct 2022 | 212,132 | samples | 29,550 | 13.9 | 116.6 | 16.2 | [334] | |
| 28 Jan 2023 | 113,504 | cases | 9,585 | 8.4 | 103.0 | 8.7 | [335] | |
| 29 Jan 2023 | 192,613 | samples | 23,427 | 12.2 | 563 | 68.4 | [336] | |
| 26 Apr 2022 | 41,849,069 | samples | 753,632 | 1.8 | 120 | 2.2 | [337] | |
| 12 Jul 2021 | 624,502 | samples | 46,509 | 7.4 | 3.9 | 0.29 | [338] | |
| 2 Feb 2023 | 12,185,475 | cases | 2,473,599 | 20.3 | 175 | 35.5 | [339] | |
| 3 Aug 2021 | 16,206,203 | samples | 65,315 | 0.40 | 284 | 1.1 | [340][341] | |
| 2 Feb 2023 | 7,391,882 | samples | 1,861,034 | 25.2 | 135 | 34.1 | [342] | |
| 2 Feb 2023 | 2,826,117 | samples | 1,322,282 | 46.8 | 135 | 63.1 | [343] | |
| 24 May 2021 | 11,378,282 | cases | 1,637,848 | 14.4 | 19.2 | 2.8 | [344][345] | |
| 1 Mar 2021 | 6,592,010 | samples | 90,029 | 1.4 | 12.7 | 0.17 | [346] | |
| 26 May 2021 | 164,472 | 10,688 | 6.5 | 1.3 | 0.084 | [347] | ||
| 1 Jul 2021 | 54,128,524 | samples | 3,821,305 | 7.1 | 116 | 8.2 | [348][349] | |
| 30 Mar 2021 | 2,384,745 | samples | 93,128 | 3.9 | 10.9 | 0.43 | [350][351] | |
| 7 Jan 2021 | 158,804 | samples | 23,316 | 14.7 | 0.36 | 0.053 | [182] | |
| 24 May 2021 | 9,996,795 | samples | 1,074,751 | 10.8 | 96.8 | 10.4 | [352][353] | |
| 7 Nov 2022 | 23,283,909 | samples | 4,276,836 | 18.4 | 270 | 49.7 | [354] | |
| 3 Feb 2023 | 30,275,725 | samples | 8,622,129 | 28.48 | 128.3 | 36.528 | [355] | |
| 18 Nov 2020 | 3,880 | 509 | 13.1 | 0.0065 | 0.00085 | [182] | ||
| 4 Mar 2021 | 1,579,597 | cases | 26,162 | 1.7 | 2.3 | 0.038 | [356] | |
| 6 Jan 2023 | 807,269 | 39,358 | 4.9 | 9.4 | 0.46 | [357] | ||
| 3 Jan 2022 | 512,730 | cases | 92,997 | 18.1 | 37.6 | 6.8 | [358] | |
| 23 Aug 2021 | 2,893,625 | samples | 703,732 | 24.3 | 24.5 | 6.0 | [359] | |
| 2 Jul 2021 | 61,236,294 | samples | 5,435,831 | 8.9 | 73.6 | 6.5 | [360] | |
| 11 Feb 2021 | 852,444 | samples | 39,979 | 4.7 | 1.9 | 0.087 | [361] | |
| 24 Nov 2021 | 15,648,456 | samples | 3,367,461 | 21.5 | 37.2 | 8.0 | [362] | |
| 1 Feb 2023 | 198,685,717 | samples | 1,049,537 | 0.53 | 2,070 | 10.9 | [363] | |
| 19 May 2022 | 522,526,476 | samples | 22,232,377 | 4.3 | 774 | 32.9 | [364] | |
| 29 Jul 2022 | 929,349,291 | samples | 90,749,469 | 9.8 | 281 | 27.4 | [365][366] | |
| 16 Apr 2022 | 6,089,116 | samples | 895,592 | 14.7 | 175 | 25.8 | [367] | |
| 7 Sep 2020 | 2,630,000 | samples | 43,975 | 1.7 | 7.7 | 0.13 | [368] | |
| 30 Mar 2021 | 3,179,074 | samples | 159,149 | 5.0 | 11.0 | 0.55 | [369] | |
| 28 Aug 2022 | 45,772,571 | samples | 11,403,302 | 24.9 | 46.4 | 11.6 | [370] | |
| 10 Mar 2022 | 3,301,860 | samples | 314,850 | 9.5 | 19.0 | 1.8 | [371] | |
| 15 Oct 2022 | 2,529,087 | samples | 257,893 | 10.2 | 17.0 | 1.7 | [182][372] | |
| ||||||||
Virus testing statistics by country subdivision
Template:COVID-19 testing by country subdivision
References
![]() This article incorporates public domain material from Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19. Centers for Disease Control and Prevention. Retrieved 5 May 2020.
This article incorporates public domain material from Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19. Centers for Disease Control and Prevention. Retrieved 5 May 2020.
- ^ a b Abbasi, Jennifer (17 April 2020). "The Promise and Peril of Antibody Testing for COVID-19". JAMA. JAMA Network. doi:10.1001/jama.2020.6170. PMID 32301958. Retrieved 20 April 2020.
- ^ Ioannidis, John P.A. (17 March 2020). "A fiasco in the making? As the coronavirus pandemic takes hold, we are making decisions without reliable data". STAT. Retrieved 22 March 2020.
- ^ "Total tests for COVID-19 per 1,000 people". Our World in Data. Retrieved 16 April 2020.
- ^ "Iceland has tested more of its population for coronavirus than anywhere else. Here's what it learned". USA Today. 11 April 2020. Retrieved 16 April 2020.
- ^ a b Ward, D. (April 2020) "Sampling Bias: Explaining Wide Variations in COVID-19 Case Fatality Rates". WardEnvironment.
- ^ Henriques, Martha. "Coronavirus: Why death and mortality rates differ". bbc.com. Retrieved 8 April 2020.
- ^ Michaels, Jonathan A.; Stevenson, Matt D. (2020). "Explaining national differences in the mortality of Covid-19: Individual patient simulation model to investigate the effects of testing policy and other factors on apparent mortality" (Document). doi:10.1101/2020.04.02.20050633.
{{cite document}}: Cite document requires|publisher=(help); Unknown parameter|url=ignored (help) - ^ "Siouxsie Wiles & Toby Morris: What we don't know about Covid-19". The Spinoff. 6 May 2020. Retrieved 6 May 2020.
- ^ a b c "How is the COVID-19 Virus Detected using Real Time RT-PCR?". IAEA. 27 March 2020. Retrieved 5 May 2020.
- ^ "RNA Extraction". AssayGenie. Retrieved 7 May 2020.
- ^ Bustin, Stephen A.; Benes, Vladimir; Garson, Jeremy A.; Hellemans, Jan; Huggett, Jim; Kubista, Mikael; Mueller, Reinhold; Nolan, Tania; Pfaffl, Michael W.; Shipley, Gregory L.; Vandesompele, Jo; Wittwer, Carl T. (1 April 2009). "The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments". Clinical Chemistry. pp. 611–622. doi:10.1373/clinchem.2008.112797.
- ^ "Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis" (PDF). Clinical Science. 23 September 2005. Retrieved 5 May 2020.
- ^ "The Basics: RT-PCR". ThermoFisher Scientific. Retrieved 5 May 2020.
- ^ Kang XP, Jiang T, Li YQ, et al. (2010). "A duplex real-time RT-PCR assay for detecting H5N1 avian influenza virus and pandemic H1N1 influenza virus". Virol. J. 7: 113. doi:10.1186/1743-422X-7-113. PMC 2892456. PMID 20515509.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Joyce C (2002). Quantitative RT-PCR. A review of current methodologies. Methods Mol. Biol. Vol. 193. pp. 83–92. doi:10.1385/1-59259-283-X:083. ISBN 978-1-59259-283-8. PMID 12325527.
- ^ Varkonyi-Gasic E, Hellens RP (2010). "qRT-PCR of Small RNAs". Plant Epigenetics. Methods in Molecular Biology. Vol. 631. pp. 109–22. doi:10.1007/978-1-60761-646-7_10. ISBN 978-1-60761-645-0. PMID 20204872.
- ^ "ACCELERATED EMERGENCY USE AUTHORIZATION (EUA) SUMMARY COVID-19 RT-PCR TEST (LABORATORY CORPORATION OF AMERICA)". FDA. Retrieved 3 April 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M (April 2010). "A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines". Methods. 50 (4): S1–5. doi:10.1016/j.ymeth.2010.01.005. PMID 20215014.
- ^ "Real-Time RT-PCR Panel for Detection 2019-nCoV". Centers for Disease Control and Prevention. 29 January 2020. Archived from the original on 30 January 2020. Retrieved 1 February 2020.
- ^ a b "Here's where things stand on COVID-19 tests in the U.S." ScienceNews. 17 April 2020. Retrieved 6 May 2020.
- ^ "Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe". GlobeNewswire News Room. 30 January 2020. Archived from the original on 31 January 2020. Retrieved 1 February 2020.
- ^ Drosten, Christian (26 March 2020). "Coronavirus-Update Folge 22" (PDF). NDR. Archived (PDF) from the original on 31 March 2020. Retrieved 2 April 2020.
- ^ a b c "Saliva: potential diagnostic value and transmission of 2019-nCoV". Nature. 17 April 2020. Retrieved 6 May 2020.
- ^ a b "FDA authorizes Covid-19 saliva test for emergency use". CNN. 14 April 2020. Retrieved 1 May 2020.
- ^ a b "New Rutgers Saliva Test for Coronavirus Gets FDA Approval". Rutgers.edu. 13 April 2020. Retrieved 1 May 2020.
- ^ "COVID-19 saliva tests: What is the benefit?". Mayo Clinic. 16 April 2020. Retrieved 6 May 2020.
- ^ "Yale University School of Public Health finds saliva samples promising alternative to nasopharyngeal swab". Merck Manual. 29 April 2020. Retrieved 6 April 2020.
- ^ Wyllie, Anne Louise; Fournier, John; Casanovas-Massana, Arnau; Campbell, Melissa; Tokuyama, Maria; Vijayakumar, Pavithra; Geng, Bertie; Muenker, M. Catherine; Moore, Adam J.; Vogels, Chantal B. F.; Petrone, Mary E.; Ott, Isabel M.; Lu, Peiwen; Lu-Culligan, Alice; Klein, Jonathan; Venkataraman, Arvind; Earnest, Rebecca; Simonov, Michael; Datta, Rupak; Handoko, Ryan; Naushad, Nida; Sewanan, Lorenzo R.; Valdez, Jordan; White, Elizabeth B.; Lapidus, Sarah; Kalinich, Chaney C.; Jiang, Xiaodong; Kim, Daniel J.; Kudo, Eriko; Linehan, Melissa (22 April 2020). "Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs" (Document). Medrxiv. doi:10.1101/2020.04.16.20067835.
{{cite document}}: Unknown parameter|access-date=ignored (help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help); Unknown parameter|url=ignored (help) - ^ a b c d e f "Developing Antibodies and Antigens for COVID-19 Diagnostics". Technology Networks. 6 April 2020. Retrieved 30 April 2020.
- ^ a b "NIH launches competition to speed COVID-19 diagnostics". AAAS. 29 April 2020. Retrieved 1 May 2020.
- ^ "Coronaviruses and Acute Respiratory Syndromes (COVID-19, MERS, and SARS)". Merck. 1 April 2020. Retrieved 1 May 2020.
- ^ "Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing April 17, 2020". Whitehouse.gov. 17 April 2020. Retrieved 30 April 2020.
- ^ a b "What to know about the three main types of coronavirus tests". CNN. 29 April 2020. Retrieved 30 April 2020.
- ^ "ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection". American College of Radiology. 22 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ a b c Salehi, Sana; Abedi, Aidin; Balakrishnan, Sudheer; Gholamrezanezhad, Ali (14 March 2020). "Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients". American Journal of Roentgenology: 1–7. doi:10.2214/AJR.20.23034. ISSN 0361-803X. PMID 32174129.
Known features of COVID-19 on initial CT include bilateral multilobar ground-glass opacification (GGO) with a peripheral or posterior distribution, mainly in the lower lobes and less frequently within the right middle lobe.
- ^ Lee, Elaine Y. P.; Ng, Ming-Yen; Khong, Pek-Lan (24 February 2020). "COVID-19 pneumonia: what has CT taught us?". The Lancet Infectious Diseases. 0 (4): 384–385. doi:10.1016/S1473-3099(20)30134-1. ISSN 1473-3099. PMC 7128449. PMID 32105641. Retrieved 13 March 2020.
- ^ "The next frontier in coronavirus testing: Identifying the full scope of the pandemic, not just individual infections". STAT. 27 March 2020. Retrieved 30 April 2020.
- ^ "Tests that have been approved for research or surveillance purposes only". Johns Hopkins. 30 April 2020. Retrieved 1 May 2020.
- ^ "Cellex Emergency Use Authorization". FDA. 1 April 2020. Retrieved 10 April 2020.
- ^ "Will an Antibody Test Allow Us to Go Back to School or Work?". New York Times. 10 April 2020. Retrieved 15 April 2020.
- ^ "Mount Sinai Emergency Use Authorization". FDA. 15 April 2020. Retrieved 18 April 2020.
- ^ a b "What Immunity to COVID-19 Really Means". Scientific American. 10 April 2020. Archived from the original on 28 April 2020.
{{cite news}}: Cite has empty unknown parameter:|deadurl=(help) - ^ Lovelace Jr., Berkeley (27 April 2020). "WHO warns about coronavirus antibody tests as some nations consider issuing 'immunity passports' to recovered patients". CNBC.
- ^ ""Immunity passports" in the context of COVID-19". World Health Organization. 24 April 2020. Retrieved 28 April 2020.
- ^ a b "A SARS-CoV-2 surrogate virus neutralization test (sVNT) based on antibody-mediated blockage of ACE2-spike (RBD) protein-protein interaction". Research Square. 23 April 2020. Retrieved 28 April 2020.
- ^ a b c d "Will antibody tests for the coronavirus really change everything?". Nature. 18 April 2020. Retrieved 20 April 2020.
- ^ a b c "Q&A on COVID-19 Antibody Tests". factcheck.org. 27 April 2020. Retrieved 28 April 2020.
- ^ "Neutralising antibody". Biology-Online. 2008. Retrieved 4 July 2009.
- ^ Schmaljohn, AL (July 2013). "Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts". Current HIV Research. 11 (5): 345–53. doi:10.2174/1570162x113116660057. PMID 24191933.
- ^ "Virus neutralization by antibodies". Virology Blog. 24 July 2009. Retrieved 29 April 2020.
- ^ "expert reaction to announcement by Roche of its new serology test for COVID-19 antibodies". Science Media Centre. 17 April 2020. Retrieved 28 April 2020.
- ^ Wu, Fan; Wang, Aojie; Liu, Mei; Wang, Qimin; Chen, Jun; Xia, Shuai; Ling, Yun; Zhang, Yuling; Xun, Jingna; Lu, Lu; Jiang, Shibo; Lu, Hongzhou; Wen, Yumei; Huang, Jinghe (20 April 2020). "Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications" (Document). Medrxiv. doi:10.1101/2020.03.30.20047365.
{{cite document}}: Unknown parameter|access-date=ignored (help); Unknown parameter|url=ignored (help) - ^ "COVID-19 Reinfection Not a Concern, Monkey Study Suggests". Genetic Engineering & Biotechnology News. 23 March 2020. Retrieved 29 April 2020.
- ^ Cao, Wu-Chun; Liu, Wei; Zhang, Pan-He; Zhang, Fang; Richardus, Jan H. (13 September 2007). "Disappearance of Antibodies to SARS-Associated Coronavirus after Recovery". New England Journal of Medicine. 357 (11). NEJM: 1162–1163. doi:10.1056/NEJMc070348. PMID 17855683.
- ^ a b "Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study" (PDF). Journal of Immunology. 19 April 2011. Retrieved 1 May 2020.
- ^ a b c "Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19". CDC. 3 May 2020. Archived from the original on 4 May 2020.
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Midgley CM, Kujawski SA, Wong KK, Collins J, Epstein, Killerby ME, et al. (April 2020). "Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States". Nat. Med. doi:10.1038/s41591-020-0877-5. PMID 32327757.
- Young, Barnaby Edward; Ong, Sean Wei Xiang; Kalimuddin, Shirin; Low, Jenny G.; Tan, Seow Yen; Loh, Jiashen; Ng, Oon-Tek; Marimuthu, Kalisvar; Ang, Li Wei; Mak, Tze Minn; Lau, Sok Kiang; Anderson, Danielle E.; Chan, Kian Sing; Tan, Thean Yen; Ng, Tong Yong; Cui, Lin; Said, Zubaidah; Kurupatham, Lalitha; Chen, Mark I-Cheng; Chan, Monica; Vasoo, Shawn; Wang, Lin-Fa; Tan, Boon Huan; Lin, Raymond Tzer Pin; Lee, Vernon Jian Ming; Leo, Yee-Sin; Lye, David Chien (2020). "Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore". JAMA. 323 (15): 1488. doi:10.1001/jama.2020.3204. ISSN 0098-7484. PMID 32125362.
- Zou, Lirong; Ruan, Feng; Huang, Mingxing; Liang, Lijun; Huang, Huitao; Hong, Zhongsi; Yu, Jianxiang; Kang, Min; Song, Yingchao; Xia, Jinyu; Guo, Qianfang; Song, Tie; He, Jianfeng; Yen, Hui-Ling; Peiris, Malik; Wu, Jie (2020). "SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients". New England Journal of Medicine. 382 (12): 1177–1179. doi:10.1056/NEJMc2001737. ISSN 0028-4793. PMID 32074444.
- Wölfel, Roman; Corman, Victor M.; Guggemos, Wolfgang; Seilmaier, Michael; Zange, Sabine; Müller, Marcel A.; Niemeyer, Daniela; Jones, Terry C.; Vollmar, Patrick; Rothe, Camilla; Hoelscher, Michael; Bleicker, Tobias; Brünink, Sebastian; Schneider, Julia; Ehmann, Rosina; Zwirglmaier, Katrin; Drosten, Christian; Wendtner, Clemens (2020). "Virological assessment of hospitalized patients with COVID-2019". Nature. doi:10.1038/s41586-020-2196-x. ISSN 0028-0836. PMID 32235945.
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Wölfel et al. (2020)
- Arons, Melissa M.; Hatfield, Kelly M.; Reddy, Sujan C.; Kimball, Anne; James, Allison; Jacobs, Jesica R.; Taylor, Joanne; Spicer, Kevin; Bardossy, Ana C.; Oakley, Lisa P.; Tanwar, Sukarma; Dyal, Jonathan W.; Harney, Josh; Chisty, Zeshan; Bell, Jeneita M.; Methner, Mark; Paul, Prabasaj; Carlson, Christina M.; McLaughlin, Heather P.; Thornburg, Natalie; Tong, Suxiang; Tamin, Azaibi; Tao, Ying; Uehara, Anna; Harcourt, Jennifer; Clark, Shauna; Brostrom-Smith, Claire; Page, Libby C.; Kay, Meagan; Lewis, James; Montgomery, Patty; Stone, Nimalie D.; Clark, Thomas A.; Honein, Margaret A.; Duchin, Jeffrey S.; Jernigan, John A. (2020). "Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility". New England Journal of Medicine. doi:10.1056/NEJMoa2008457. ISSN 0028-4793. PMID 32329971.
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced Wölfel et al. (2020)
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Young et al. (2020)
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Midgley et al. (2020)
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Midgley et al. (2020)
- Wölfel et al. (2020)
- ^ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced Zhang, Sheng; Tong, Yi Xin; Xiao, Ai Tang (2020). "Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients". Clinical Infectious Diseases. doi:10.1093/cid/ciaa460. ISSN 1058-4838. PMC 7188124. PMID 32306036.
- ^ Roser, Max; Ritchie, Hannah; Ortiz-Ospina, Esteban (4 March 2020). "Coronavirus Disease (COVID-19) – Statistics and Research". Our World in Data – via ourworldindata.org.
- ^ "COVID-19: Tests per day". Our World in Data. Retrieved 15 April 2020.
- ^ "Daily COVID-19 tests per thousand people". Our World in Data. Retrieved 15 April 2020.
- ^ "Total tests for COVID-19 per 1,000 people". Our World in Data. Retrieved 15 April 2020.
- ^ a b "Coronavirus (COVID-19): Scaling up our testing programmes" (PDF). Department of Health and Social Care. 4 April 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ Nina Weber; Katherine Rydlink; Irene Berres (5 March 2020). "Coronavirus und Covid-19: So testet Deutschland". Der Spiegel (in German). Retrieved 23 March 2020.
- ^ Oltermann, Philip (22 March 2020). "Germany's low coronavirus mortality rate intrigues experts". The Guardian. ISSN 0261-3077. Retrieved 24 March 2020.
- ^ "Covid-19 – Tests auf das Coronavirus: Wann, wo und wie?". Deutschlandfunk (in German). 19 March 2020. Retrieved 24 March 2020.
- ^ Charisius, Hanno (26 March 2020). "Covid-19: Wie gut testet Deutschland?" (in German). Retrieved 26 March 2020.
- ^ "Coronavirus Disease 2019 Daily Situation Report of the Robert Koch Institute" (PDF). Robert Koch Institute. 26 March 2020. Retrieved 28 April 2020.
- ^ "NHS pilots home testing for coronavirus". MobiHealthNews. 24 February 2020. Archived from the original on 25 February 2020.
- ^ jkiger@postbulletin.com, Jeff Kiger. "Mayo Clinic starts drive-thru testing for COVID-19". PostBulletin.com. Retrieved 13 March 2020.
- ^ Hawkins, Andrew J. (11 March 2020). "Some states are offering drive-thru coronavirus testing". The Verge. Retrieved 13 March 2020.
- ^ "South Korea's Drive-Through Testing For Coronavirus Is Fast – And Free". npr. 11 March 2020. Retrieved 16 March 2020.
- ^ "In Age of COVID-19, Hong Kong Innovates To Test And Quarantine Thousands". NPR.org.
- ^ "Pooling method allows dozens of COVID-19 tests to run simultaneously". medicalxpress.com. Retrieved 24 March 2020.
- ^ "Israeli team has coronavirus test kit to test dozens of people at once". The Jerusalem Post | JPost.com. Retrieved 24 March 2020.
- ^ Staff, Israel21c (19 March 2020). "Israelis introduce method for accelerated COVID-19 testing". Israel21c. Retrieved 24 March 2020.
{{cite news}}: CS1 maint: numeric names: authors list (link) - ^ "[Coronavirus] Verified 'sample pooling' introduced to prevent herd infection in S. Korea". ajudaily.com. 9 April 2020. Retrieved 19 April 2020.
- ^ "Gov. Ricketts provides update on coronavirus testing". KMTV. 24 March 2020. Retrieved 19 April 2020.
- ^ "Latest coronavirus update: UP to begin 'pool testing' of Covid suspects". Free Press Journal. Retrieved 19 April 2020.
- ^ Sumati Yengkhom. "West Bengal to start pool testing of samples in low-risk zones". The Times of India. Retrieved 19 April 2020.
- ^ "Punjab launches pool testing". Retrieved 19 April 2020.
- ^ "'Chhattisgarh to adopt pool sample testing': Health minister TS Singh Deo on Covid-19". Hindustan Times. 15 April 2020. Retrieved 19 April 2020.
- ^ "Maharashtra to go for pool testing to defeat coronavirus". Deccan Herald. 12 April 2020. Retrieved 19 April 2020.
- ^ "Wuhan Test Lab Opens; CDC Ships Diagnostic Kits: Virus Update". Bloomberg. 5 February 2020. Retrieved 7 February 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ a b "China virus crisis deepens as whistleblower doctor dies". AFP.com. 27 February 2012. Retrieved 7 February 2020.
- ^ 日检测量达万份的"火眼"实验室连夜试运行.
- ^ "BGI's Coronavirus Response? Build a Lab in Wuhan". GEN – Genetic Engineering and Biotechnology News. 12 February 2020. Retrieved 27 March 2020.
- ^ "COVID-19 Local Laboratory Solution". BGI – Global. Retrieved 27 March 2020.
- ^ "Origami Assays". Origami Assays. 2 April 2020. Retrieved 7 April 2020.
- ^ "Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK –seventh update" (PDF). European Centre for Disease Prevention and Control. 25 March 2020. pp. 15–16. Retrieved 29 March 2020.
the current shortages of laboratory consumables and reagents affect diagnostic capacity and hamper the epidemic response at the national and local levels. The laboratories have experienced delayed or missing deliveries of swabbing material, plastic consumables, RNA extraction and RT-PCR reagents, and PPE. This is affecting laboratories in all EU/EEA countries.
{{cite web}}: CS1 maint: url-status (link) - ^ Baird, Robert P. (24 March 2020). "Why Widespread Coronavirus Testing Isn't Coming Anytime Soon". The New Yorker. Archived from the original on 28 March 2020. Retrieved 29 March 2020.
South Dakota, said that her state's public-health laboratory—the only lab doing COVID-19 testing in the state—had so much trouble securing reagents that it was forced to temporarily stop testing altogether. also noted critical shortages of extraction kits, reagents, and test kits
- ^ Ossola, Alexandra (25 March 2020). "Here are the coronavirus testing materials that are in short supply in the US". Quartz. Archived from the original on 26 March 2020. Retrieved 29 March 2020.
extract the virus's genetic material—in this case, RNA—using a set of chemicals that usually come in pre-assembled kits. "The big shortage is extraction kits" There are no easy replacements here: "These reagents that are used in extraction are fairly complex chemicals. They have to be very pure, and they have to be in pure solution"
- ^ Fomsgaard, Anders (27 March 2020). "Statens Serum Institut (SSI) solves essential COVID-19 testing deficiency problem". en.ssi.dk. Statens Serum Institut. Archived from the original on 29 March 2020.
several countries are in lack of the chemical reagents necessary to test their citizens for the disease.
- ^ "Danish researchers behind simple coronavirus test method". cphpost.dk. The Copenhagen Post. 28 March 2020. Archived from the original on 28 March 2020.
- ^ Sullivan (now, Helen; Rawlinson, earlier); Kevin; Gayle, Damien; Topping, Alexandra; Mohdin, and Aamna; Willsher, Kim; Wintour, Patrick; Wearden, Graeme; Greenfield, Patrick (31 March 2020). "Global confirmed virus death toll passes 40,000 – as it happened". The Guardian. ISSN 0261-3077. Retrieved 1 April 2020.
{{cite news}}: CS1 maint: multiple names: authors list (link) - ^ "VIDEO: UAE sets up COVID-19 detection lab in just 14 days". Gulf Today. 31 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ Private Labs Should Be Reimbursed At Appropriate Time For COVID-19 Tests, Says AIIMS RDA General Secretary
- ^ Covid-19: Private laboratories struggle with price caps, do only 17% tests
- ^ "Bolinas COVID-19 Testing Effort Detects No Active Infections". uscf.edu. 1 May 2020. Retrieved 3 May 2020.
- ^ Lee, Timothy B. (2 April 2020). "America's COVID-19 testing has stalled, and that's a big problem". Ars Technica.
- ^ "PolitiFact – Biden falsely says Trump administration rejected WHO coronavirus test kits (that were never offered)". @politifact.
- ^ a b Heartbreak in the Streets of Wuhan
- ^ a b "State figures on testing raise questions about efforts to contain outbreak". The BostonGlobe.com.
flaws with the testing kits first distributed by the federal government and bureaucratic hurdles that held up testing by private labs at hospitals, universities and testing chains
- ^ Davey, Melissa (14 March 2020). "Australian stocks of coronavirus testing kits 'rapidly deteriorating', says chief medical officer" – via www.theguardian.com.
- ^ "Experts Credit South Korea's Extensive Testing For Curbing Coronavirus Spread". NPR.org.
- ^ "'Test, Test, Test': WHO Chief's Coronavirus Message to World". The New York Times. 16 March 2020. Retrieved 16 March 2020.
- ^ Reuters, Source (16 March 2020). "'Test, test, test': WHO calls for more coronavirus testing – video". The Guardian. Retrieved 16 March 2020.
{{cite news}}:|last1=has generic name (help) - ^ "Coronavirus Testing Backlogs Continue As Laboratories Struggle To Keep Up With Demand". NPR.org.
- ^ "Laboratory Readiness for Detecting the 2019 novel coronavirus (2019-nCoV) infection in Malaysia". Director-General of Health, Malaysia. 9 February 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ "Malaysia must ramp up testing". The Star Malaysia. 26 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ "UK defends coronavirus response after Reuters investigation". Reuters. 9 April 2020. Retrieved 12 April 2020.
After developing a test for the new virus by Jan. 10
{{cite news}}: CS1 maint: url-status (link) - ^ "COVID-19 virus testing in NHS laboratories" (PDF). NHS England and NHS Improvement. 16 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ "PHE novel coronavirus diagnostic test rolled out across UK". GOV.UK. Retrieved 30 March 2020; "'Increased likelihood' of China virus reaching UK". BBC News. 23 January 2020. Retrieved 30 March 2020; "PHE tells patients with suspected coronavirus to call GP or NHS 111". The Pharmaceutical Journal. 27 January 2020. Retrieved 30 March 2020.
- ^ Sheridan, Cormac (19 February 2020). "Coronavirus and the race to distribute reliable diagnostics". Nature Biotechnology. 38 (4): 382–384. doi:10.1038/d41587-020-00002-2. PMID 32265548.
- ^ Jeong, Sei-im (28 February 2020). "Korea approves 2 more COVID-19 detection kits for urgent use". Korea Biomedical Review. Retrieved 12 March 2020.
- ^ "[NEW PRODUCT] COVID-19 KIT". kogene.co.kr. 27 February 2020. Archived from the original on 23 April 2020.
- ^ "BGI Sequencer, Coronavirus Molecular Assays Granted Emergency Use Approval in China". GenomeWeb. Retrieved 9 March 2020.
- ^ "About IRR". internationalreagentresource.org. Archived from the original on 20 April 2020.
- ^ Transcript for the CDC Telebriefing Update on COVID-19, 28 February 2020
- ^ "LabCorp Launches Test for Coronavirus Disease 2019 (COVID-19)". Laboratory Corporation of America Holdings.
- ^ "Covid19 : COVID-19". questdiagnostics.com.
- ^ Совещание по вопросам развития ситуации с коронавирусной инфекцией и мерам по её профилактике
- ^ "В России зарегистрирована отечественная тест-система для определения коронавируса". Interfax-Russia.ru. 14 February 2020.
- ^ Plumbo, Ginger. "Mayo Clinic develops test to detect COVID-19 infection". Mayo Clinic. Retrieved 13 March 2020.
- ^ a b "Emergency Use Authorization". FDA. 30 April 2020. Archived from the original on 29 April 2020.
- ^ "Hologic's Molecular Test for the Novel Coronavirus, SARS-CoV-2, Receives FDA Emergency Use Authorization". Hologic. 16 March 2020. Retrieved 13 April 2020.
- ^ "FDA Approves Abbott Laboratories Coronavirus Test, Company To Ship 150,000 Kits". IBTimes.com. 19 March 2020. Archived from the original on 20 March 2020.
- ^ "Sunnyvale company wins FDA approval for first rapid coronavirus test with 45-minute detection time". EastBayTimes.com. 21 March 2020. Archived from the original on 22 March 2020.
- ^ "Xpert® Xpress SARS-CoV-2 has received FDA Emergency Use Authorization". cepheid.com. Retrieved 13 April 2020.
- ^ "Health Canada approves new rapid COVID-testing kits". The Globe and Mail Inc. 13 April 2020.
- ^ "The power of DNA testing for everyone". Spartan Bioscience. Archived from the original on 22 April 2020. Retrieved 14 April 2020.
- ^ "Letter from FDA". FDA. 27 March 2020. Retrieved 2 April 2020.
- ^ a b ID NOW COVID-19, Instruction for Use, FDA
- ^ "The scramble for the rapid coronavirus tests everybody wants". Washington Post. 1 April 2020.
{{cite news}}: CS1 maint: url-status (link) - ^ "'A game changer': FDA authorizes Abbott Labs' portable, 5-minute coronavirus test the size of a toaster". USA Today. 28 March 2020. Retrieved 20 April 2020.
- ^ "To protect Trump, White House among first to use rapid coronavirus tests sought by communities". Washington Post. 7 April 2020.
{{cite news}}: CS1 maint: url-status (link) - ^ "Study Raises Questions About False Negatives From Quick COVID-19 Test". NPR. 21 April 2020. Retrieved 1 May 2020.
- ^ "Emergency Use Authorizations". FDA. 1 May 2020. Retrieved 1 May 2020.
- ^ "Tests that have been approved for diagnostic use in other countries". Johns Hopkins. 30 April 2020. Retrieved 1 May 2020.
- ^ "Quest Diagnostics Launches Consumer-Initiated COVID-19 Antibody Test Through QuestDirect™". Quest Diagnosics. 28 April 2020.
- ^ "NIH Begins Study to Quantify Undetected Cases of Coronavirus Infection | NIH: National Institute of Allergy and Infectious Diseases". niaid.nih.gov. Retrieved 11 April 2020.
- ^ Mandavilli, Apoorva; Thomas, Katie (10 April 2020). "Will an Antibody Test Allow Us to Go Back to School or Work?". The New York Times. Retrieved 11 April 2020.
- ^ Yadlowsky, Steve; Shah, Nigam; Steinhardt, Jacob (27 March 2020). "Estimation of SARS-CoV-2 Infection Prevalence in Santa Clara County". Medrxiv. medrxiv.org. doi:10.1101/2020.03.24.20043067. Retrieved 17 April 2020.
- ^ "Antibody tests suggest that coronavirus infections vastly exceed official counts". Nature. 19 April 2020. Retrieved 20 April 2020.
- ^ Fellmann F. (March 2020). (in German) "Jetzt beginnt die Suche nach den Genesenen". Tages Anzeiger. Retrieved 28 March 2020.
- ^ a b "WHO Emergency Use Listing for SARS‐CoV‐2 in vitro diagnostic products" (PDF). World Health Organization. Retrieved 4 May 2020.
- ^ "WHO lists two COVID-19 tests for emergency use". World Health Organization. Retrieved 10 April 2020.
- ^ "Chinese firm to replace exported coronavirus test kits deemed defective by Spain". 27 March 2020 – via www.reuters.com.
- ^ Morning, Prague (26 March 2020). "80% of Rapid COVID-19 Tests the Czech Republic Bought From China are Wrong".
- ^ VOJTĚCH BLAŽEK (23 March 2020). "Úřad dopředu psal, kdy mohou rychlotesty selhat. I tak je stát nasadil". Zeznam Zprávy (in Czech). Retrieved 7 April 2020.
Indeed, the rapid tests that arrived from China a few days ago do not really reliably detect the infection at an early stage
- ^ "Europe turned to China for coronavirus testing help. Why some are now regretting it". Fortune.
- ^ "Coronavirus test kits purchased from China are unreliable, says Science Committee member". duvarenglish.com.
- ^ "Coronavirus: Turkey rejects Chinese testing kits over inaccurate results". Middle East Eye.
- ^ Reporter, Chris Smyth, Whitehall Editor | Dominic Kennedy, Investigations Editor | Billy Kenber, Investigations. "Britain has millions of coronavirus antibody tests, but they don't work" – via www.thetimes.co.uk.
{{cite web}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ "Government's testing chief admits none of 3.5m coronavirus antibody kits work sufficiently". The Independent. 6 April 2020.
- ^ Chris Smyth; Dominic Kennedy; Billy Kenber (6 April 2020). "Britain has millions of coronavirus antibody tests, but they don't work". The Times. Retrieved 6 April 2020.
None of the antibody tests ordered by the government is good enough to use, the new testing chief has admitted. Professor John Newton said that tests ordered from China
- ^ "ICMR asks states not to use rapid test kits for two days". The Telegraph. 21 April 2020.
- ^ "National laboratories". who.int.
- ^ "PHE novel coronavirus diagnostic test rolled out across UK". GOV.UK. Retrieved 12 April 2020.
In addition to processing samples from suspected cases in this country, PHE is now working as a reference laboratory for WHO, testing samples from countries that do not have assured testing capabilities.
{{cite web}}: CS1 maint: url-status (link) - ^ "Specimen referral for COVID-19 – operational details of WHO reference laboratories providing confirmatory testing for COVID-19" (PDF). World Health Organization. Retrieved 29 March 2020.
- ^ "How an experiment helped one Italian town find 'submerged infections,' cut new COVID-19 cases to zero". National Post. 19 March 2020.
- ^ "COVID-19 outbreak: Petition to close schools in Singapore garners 7,700 signatures to date". msn.com.
- ^ "Coronavirus: Most workplaces to close, schools will move to full home-based learning from next week, says PM Lee". The Straits Times. 3 April 2020. Archived from the original on 23 April 2020.
{{cite news}}: Cite has empty unknown parameter:|deadurl=(help) - ^ "Coronavirus clue? Most cases aboard U.S. aircraft carrier are symptom-free". Reuters. 16 April 2020.
- ^ "Over 3 mln COVID-19 tests conducted in Russia". TASS. 27 April 2020. Retrieved 29 April 2020.
- ^ https://rospotrebnadzor.ru/about/info/news/news_details.php?ELEMENT_ID=14329
- ^ "Popova said explosive growth in incidence was not allowed due to measures taken". TASS. 28 April 2020. Retrieved 29 April 2020.
- ^ "COVID-19: First results of the voluntary screening in Iceland". Nordic Life Science – the leading Nordic life science news service. 27 March 2020. Retrieved 5 April 2020.
- ^ Normile, Dennis (17 March 2020). "Coronavirus cases have dropped sharply in South Korea. What's the secret to its success?". Science. Retrieved 5 April 2020.
- ^ Ward, Dan (April 2020) "Sampling Bias: Explaining Variations in Age Distributions of COVID-19 Cases". Technical Report. WardEnvironment. Bern, Switzerland. Retrieved 7 May 2020.
- ^ "Catching Virus Fast! Academia Sinica discovered useful antibodies for developing rapid immune based test kit of SARS-CoV-2 coronavirus". sinica.edu.tw. Academia Sinica. Archived from the original on 18 April 2020. Retrieved 12 March 2020.
{{cite news}}: Cite has empty unknown parameter:|deadurl=(help) - ^ "Advice on the use of point-of-care immunodiagnostic tests for COVID-19". who.int. Retrieved 11 April 2020.
- ^ Romano, Andrew. (7 April 2020). "Fauci once dismissed concerns about 'silent carriers' of coronavirus. Not anymore." Yahoo News Retrieved 17 April 2020.
- ^ "Want to know how many people have the coronavirus? Test randomly". The Conversation. 13 April 2020. Retrieved 7 May 2020.
- ^ "M&E – Health Information System General Directorate – National Diseases Surveillance and Response". MoPH Data Warehouse – Dashboard. 17 December 2020.
- ^ "COVID19/ Ministria e Shëndetësisë: 736 të vaksinuar, 3935 testime, 991 të shëruar, 1112 raste të reja dhe 17 humbje jete në 24 orët e fundit". Ministria e Shëndetësisë dhe Mbrojtjes Sociale [Ministry of Health and Social Protection] (in Albanian). 18 February 2021.
- ^ a b c d e f g h i j k l "Coronavirus Disease 2019 (COVID-19)". Africa CDC.
- ^ "Documentation: Rapport de Situation Sur L'Epidemie de Coronavirus COVID-19". Ministère de la Santé de la Population et de la Réforme Hospitalière [Ministry of Health, Population and Hospital Reform] (in French). 2 November 2020.
- ^ "COVID-19 Dashboard". Government of Andorra. 1 March 2022.
- ^ "COVID-19: Angola Com 58 Novas Infecções e 44 Recuperados". Agência Angola Press (in Portuguese). 4 March 2021.
- ^ "COVID-19 Antigua & Barbuda Dashboard". Official Facebook page of the Ministry of Health & The Environment, Antigua and Barbuda. 6 March 2021.
- ^ "Sala de Situaciόn Coronavirus online" (PDF). Argentina.gob.ar (in Spanish). 16 April 2022.
- ^ Կորոնավիրուսային հիվանդություն (COVID-19). Հիվանդությունների վերահսկման և կանխարգելման ազգային կենտրոն [National Center for Disease Control and Prevention] (in Armenian). 30 May 2022.
- ^ "Coronavirus (COVID-19) current situation and case numbers". Department of Health. 10 September 2022.
- ^ "Coronavirus". AGES Dashboard COVID19 (in German). 2 February 2023.
- ^ "Azərbaycanda Carı Vəzıyyət". Azərbaycan Respublikasının Nazirlər Kabineti [Cabinet of Ministers of the Republic of Azerbaijan] (in Azerbaijani). 11 May 2022.
- ^ "News and Press Releases: COVID-19 Report Update". Government of the Bahamas. 29 November 2022.
- ^ الموقع الرسمي للمستجدات الصحية، مملكة الب9رين. وزارة الصحة [Ministry of Health] (in Arabic). 3 December 2022.
- ^ "Bangladesh Covid-19 Update". Institute of Epidemiology, Disease Control and Research. 24 July 2021.
- ^ "COVID-19 Update". Barbados Government Information Service. 15 October 2022.
- ^ Официальный Минздрав. Официальный канал Министерства здравоохранения Республики Беларусь [Telegram channel of the Ministry of Health of the Republic of Belarus] (in Russian). 9 May 2022.
- ^ "Epistat COVID19 Belgian Dashboard". Sciensano. 25 January 2023.
- ^ "COVID-19 Update". Facebook account of the Ministry of Health and Wellness Belize. 1 November 2021.
- ^ "Coronavirus (COVID-19) By the Numbers". Statistical Institute of Belize. 9 June 2022.
- ^ "Informations coronavirus (covid-19)". Gouvernement de la République du Bénin [Government of the Republic of Benin] (in French). 5 May 2021.
- ^ "National Situational Update on COVID-19". Ministry of Health. 28 February 2022.
- ^ "Reporte COVID-19 en Bolivia". Ministerio de Salud [Ministry of Health] (in Spanish). 5 June 2022.
- ^ "Službene informacije o koronavirusu u BiH". Ministarstvo civilnih poslova Bosne i Hercegovine [Ministry of Civil Affairs of Bosnia and Herzegovina] (in Bosnian). 28 September 2022.
- ^ "COVID-19 Botswana Dashboard". Government of Botswana. 11 January 2022.
- ^ "BW government on Facebook". Government of Botswana. 3 December 2020.
- ^ "COVID-19 Testes". Ministério da Saúde [Ministry of Health] (in Portuguese). 19 February 2021.
- ^ "Coronavírus Brasil". Ministério da Saúde [Ministry of Health] (in Portuguese). 19 February 2021.
- ^ "Press Release on the Current Situation of the COVID-19 Infection in Brunei Darussalam". Ministry of Health Brunei Darussalam. 2 August 2021.
- ^ COVID-19: Единен информационен портал. COVID-19: Единен информационен портал [COVID-19: United information portal] (in Bulgarian). 3 February 2023.
- ^ "Communiqué Coronavirus (COVID-19) au Burkina Faso". Facebook account of the Service d'Information du Gouvernement (SIG) [Government Information Service] (in French). 5 March 2021.
- ^ "Update on COVID-19". Facebook account of the Ministère de la Santé Publique Burundi [Ministry of Public Health Burundi] (in French). 5 January 2021.
- ^ បច្ចុប្បន្នភាពនៃជំងឺកូរ៉ូណាថ្មី COVID-19 នៅប្រទេសកម្ពុជា. Communicable Disease Control Department, Ministry of Health (Cambodia) (in Khmer). 1 August 2021.
- ^ "Coronavirus disease (COVID-19): Outbreak update". Government of Canada. Retrieved 5 December 2022.
- ^ "Communiqué N*320 de la Coordination Nationale de Riposte Sanitaire". Official Facebook account of the Ministère de la Santé Publique du Tchad [Ministry of Public Health of Chad] (in French). 2 March 2021.
- ^ "Cifras Oficiales: COVID-19". Gobierno de Chile [Government of Chile] (in Spanish). 2 February 2023.
- ^ 我国核酸日检测能力达484万份. 中华人民共和国中央人民政府 [The Central People's Government of the People's Republic of China] (in Chinese). 6 August 2020.
- ^ "Aug 1: Daily briefing on novel coronavirus cases in China". National Health Commission of the People's Republic of China. 1 August 2020.
- ^ "#COVID19 en Colombia 28-01-2021". Instituto Nacional de Salud de Colombia [Colombia's National Institute of Health] (in Spanish). 17 January 2021.
- ^ "#ReporteCOVID19". Cuenta Oficial del Ministerio de Salud y Protección Social de Colombia [Official Account of Health and Social Protection Ministry of Columbia] (in Spanish). 24 November 2022.
- ^ "Situación Nacional COVID-19". Geovisión; Ministerio de Salud, Costa Rica [Ministry of Health, Costa Rica] (in Spanish). 2 November 2021.
- ^ "xxx novih slučajeva u protekla 24 sata". Koronavirus.hr (in Croatian). 3 February 2023.
- ^ "Covid19CubaData". Covid19CubaData (in Spanish). 21 July 2021.
- ^ "Coronavirus en Cuba". Ministerio de Salud Pública [Ministry of Public Health] (in Spanish). 3 February 2023.
- ^ Η εξάπλωση της COVID-19 στην Κύπρο. Πανεπιστήμιο Κύπρου [University of Cyprus] (in Greek). 3 February 2023.
- ^ "Přehled situace v ČR: COVID-19". Ministerstvo zdravotnictví České republiky [The Ministry of Health of the Czech Republic] (in Czech). 2 February 2023.
- ^ "Tal og overvågning over coronavirus/COVID-19 – Sundhedsstyrelsen". Sundhedsstyrelsen [The National Board of Health] (in Danish). 1 February 2023.
- ^ "Statens Serum Institut COVID-19 – Danmark". State20 Serum Institut [The National Board of Health] (in Danish). 15 November 2022.
- ^ "Poit de Presse Sur La Situation COVID19 Par Le Secrétaire De La Santé Dr Meeke Mohamed Moussa". Official Facebook account of the Ministere de la Santé de Djibouti [Djibouti Ministry of Health] (in French). 28 April 2022.
- ^ "Commonwealth of Dominica Coronavirus [COVID-19] Report". Facebook account of the Ministry of Health, Wellness and New Health Investment. 21 June 2022.
- ^ "Boletin Especial 484 COVID 19". Dirección General de Epidemiología [General Directorate of Epidemiology] (in Spanish). 23 July 2022.
- ^ "Situation Épidémiologique en RDC". Stop Coronavirus COVID-19 RDC (in French). 28 February 2021.
- ^ "Situación Nacional Por COVID-19 Infografía N°400" (PDF). Ministerio de Salud Pública [Ministry of Public Health] (in Spanish). 23 July 2021.
- ^ "facebook.com/EgyMohpSpokes". Facebook page for the Egyptian Ministry of Health and Population (MOHP) spokesperson (in Arabic). 23 July 2021.
- ^ "Situación nacional COVID-19". Gobierno de El Salvador [Government of El Salvador] (in Spanish). 19 March 2022.
- ^ "Estadísticas COVID-19" [Ministry of Health and Social Welfare]. Ministerio de Sanidad y Bienestar Social (in Spanish). Equatorial Guinea. 31 January 2023.
- ^ "Koroonakaart". Koroonakaart. 31 January 2023.
- ^ "COVID-19 Eswatini Dashboard". 8 December 2021.
- ^ የኢትዮጵያ የተቀናጀ የኮቪድ-19 መቆጣጠሪያ ስርዓት. covid19.et (in Amharic). 24 July 2021.
- ^ "Corona í Føroyum". Føroya Landsstýri [The Government of the Faroe Islands]. 27 February 2022.
- ^ "COVID-19 Update". Ministry o10 Health & Medical Services. Fiji. 2 January 2023.
- ^ "Confirmed coronavirus cases (COVID-19) in Finland". Terveyden ja hyvinvoinnin laitos (ArcGIS) [National Institute for Health and Welfare (ArcGIS)]. 14 January 2022.
- ^ "info coronavirus covid-19-carte et donnes covid 19 en france". Gouvernement.fr (in French). 15 May 2022.
- ^ "Situation Épidémiologique au Gabon". Info Covid19 Gabon (in French). 23 July 2021.
- ^ "The Gambia COVID-19 Outbreak Situational Report" (PDF). Ministry of Health. 15 February 2021.
- ^ COVID-19 სტატისტიკური მონაცემები. დაავადებათა კონტროლისა და საზოგადოებრივი ჯანმრთელობის ეროვნული ცენტრი [National Center for Disease Control and Public Health] (in Georgian). 3 November 2021.
- ^ "Robert Koch-Institut: COVID-19-Dashboard". Robert Koch-Institut [Robert Koch Institute]. 7 July 2021.
- ^ "Tabellen zu Testzahlen, Testkapazitäten und Probenrückstau pro Woche" (XLSX). Robert Koch-Institut [Robert Koch Institute]. 7 July 2021.
- ^ "Situation Update, COVID-19 Outbreak in Ghana". Ghana Health Service. 3 July 2021.
- ^ Ημερήσια έκθεση επιδημιολογικής επιτήρησης λοίμωξης από το νέο κορωνοϊό (COVID-19). Εθνικός Οργανισμός Δημόσιας Υγείας [National Public Health Organization] (in Greek). 20 December 2022.
- ^ "Coronavirus i Grønland". Naalakkersuisut [Government of Greenland] (in Danish). 30 January 2022.
- ^ "COVID-19 Update | Grenada Dashboard". Ministry of Health Grenada (Facebook). 11 May 2021.
- ^ "Situación de COVID-19 en Guatemala". Ministerio de Salud Pública y Asistencia Social [Ministry of Public Health and Social Assistance] (in Spanish). 7 January 2023.
- ^ "Republique de Guinee COVID-19 Décompte des cas". Official Twitter account of the Agence Nationale de Sécurité Sanitaire [National Agency for Health Security] (in French). 23 July 2021.
- ^ "Situação Epidemiológica Da Covid-19 Na Guiné-Bissau". Official Facebook page of the Alto Comissariado para o Covid-19 [High Commissioner for Covid-19] (in Portuguese). 8 July 2022.
- ^ "Guyana COVID-19 Dashboard". Ministry of Health. 16 June 2022.
- ^ "Surveillance de la COVID-19, Haiti, 2020-2021". Ministère de la Santé Publique et de la Population [Ministry of Public Health and Population] (in French). 7 December 2022.
- ^ "Estadística Nacional de Coronavirus COVID-19". Biblio3eca Virtual en Salud de Honduras [Virtual Health Library of Honduras] (in Spanish). 26 November 2021.
- ^ "Tájékoztató oldal a koronavírusról". Tájékoztató oldal a koronavírusról [Coronavirus Information Page] (in Hungarian). Cabinet Office of the Prime Minister. 11 May 2022.
- ^ "COVID-19 in Iceland – Statistics". Covid.is. 9 August 2022.
- ^ "SARS-CoV-2 (COVID-19) Testing: Status Update". Indian Council of Medical Research. Retrieved 19 September 2021.
- ^ "Ministry of Health and Family Welfare". Ministry of Health and Family Welfare. Retrieved 1 October 2021.
- ^ "Peta Sebaran". COVID-19 Handling and National Economic Recovery Committee. Retrieved 3 July 2023.
- ^ "Peta Sebaran". COVID-19 Handling and National Economic Recovery Committee. Retrieved 28 June 2023.
- ^ "Health Ministry's Updates on COVID-19". Government of the Islamic Republic of Iran. 1 June 2022.
- ^ "الموقف الوبائي اليومي لجائحة كورونا في العراق ليوم السبت الموافق ٥ كانون الاول ٢٠٢٠". وزارة الصحة العراقية (Facebook) [Iraqi Ministry of Health (Facebook)] (in Arabic). 3 August 2022.
- ^ "Ireland's COVID-19 Data Hub". gov.ie. 1 February 2023.
- ^ קורונה – לוח בקרה. נגיף הקורונה [Coronavirus] (in Hebrew). Ministry of Health. 17 January 2022.
- ^ "17 marzo 2023 – Aggiornamento casi Covid-19" (PDF). Dipartimento della Protezione Civile (GitHub) [Civil Protection Department (GitHub)] (in Italian). 16 March 2023.
- ^ "Point de la situation de la COVID-19 au 3/03/2021". Official Facebook channel of Le Ministère de la Santé et de l’Hygiène Publique [Ministry of Health and Public Hygiene, Ivory Coast] (in French). 3 March 2021.
- ^ "COVID-19 Clinical Management Summary". Ministry of Health & Wellness. 3 October 2022.
- ^ 新型コロナウイルス感染症の現在の状況と厚生労働省の対応について(令和3年3月1日版). 厚生労働省 [The Ministry of Health, Labour and Welfare] (in Japanese). 1 March 2021.
- ^ "corona.moh.gov.jo/en". Jordan Ministry of Health. 6 June 2021.
- ^ Данные по COVID-19 в Казахстане. Национальный центр общественного здравоохранения Министерства здравоохранения Республики Казахстан [National Center of Public Health of the Ministry of Healthcare of the Republic of Kazakhstan] (in Russian). 29 May 2021.
- ^ "twitter.com/MOH_Kenya". Official Twitter Account of the Ministry of Health Kenya. 5 March 2021.
- ^ "facebook.com/IKSHPK". Official Facebook account of the Instituti Kombëtar i Shëndetësisë Publike të Kosovës [National Institute of Public Health of Kosova] (in Albanian). 31 May 2021.
- ^ "twitter.com/KUWAIT_MOH". Kuwait Ministry of Health (Twitter). 9 March 2022.
- ^ За сутки проведено 3436 ПЦР-исследований на коронавирус. Insta official (in Kyrgyz). 10 February 2021.
- ^ "ຄະນະສະເພາະກິດ COVID-19". COVID-19 Task Force (in Lao). 1 March 2021.
- ^ "Covid-19 infekcijas izplatība Latvijā". Slimību profilakses un kontroles centrs (ArcGIS) [Center for Disease Prevention and Control (ArcGIS)] (in Latvian). 5 September 2021.
- ^ آخر اﻹحصاءات. فيروس كورونا: COVID-19 [Coronavirus: COVID-19] (in Arabic). Ministry of Information. 14 June 2021.
- ^ "COVID-19 Statistics". Official Twitter account of the National COVID-19 Secretariat (NACOSEC). 31 March 2022.
- ^ "#LiBCOVID19 Case Update". Official Facebook account of the National Public Health Institute of Liberia-NPHIL. 19 July 2021.
- ^ اليومي للوضع الوبائي المحلي لفيروس كورونا المستجد ليوم الأحد 28 فبراير 2021. Official Facebook account of the National Centre for Disease Control (NCDC) - Libya (in Arabic). 16 April 2022.
- ^ "Koronavirusas (COVID-19)". Lietuvos Respublikos sveikatos apsaugos ministerija [Ministry of Health of the Republic of Lithuania] (in Lithuanian). 1 February 2023.
- ^ "Korona Stop". Korona Stop. 16 May 2021.
- ^ "Coronavirus – Rapport Journalier" (PDF). La plate-forme de données luxembourgeoise [The luxembourgish data platform] (in French). Government of Luxembourg. 13 May 2022.
- ^ "COVID-19: Fivoaran'ny antontan'isa teto Madagasikara ny 13 Febroary ka hatramin'ny 19 Febroary 2021". Facebook account of the Ministère de la Santé Publique Madagascar [Ministry of Public Health Madagascar] (in French and Malagasy). 22 February 2021.
- ^ "COVID-19 Daily info update". Facebook page of the Ministry Of Health - Malawi. 29 November 2022.
- ^ "Situasi Terkini". Kementerian Kesihatan Malaysia [Ministry of Health Malaysia] (in Malay). 7 September 2021.
- ^ "COVID-19 Case Updates". Health Protection Agency (Twitter). 13 March 2022.
- ^ "COVID-19 Local Updates". Ministry of Health. 29 January 2021.
- ^ "Communique N°364 du Ministere de la Sante et du Développement Social Sur Le Suivi des Actions de Prevention et de Riposte Face a la Maladie a Coronavirus". Ministère de la Santé et du Développement Social du Mali [Ministry of Health and Social Development of Mali] (in French). 7 July 2021.
- ^ "COVID-19 Malta". Times of Malta (ArcGIS). 8 September 2021.
- ^ "المعطيات العامة للحالة الوبائية". Official Facebook page of the Ministère de la santé /وزارة الصحة [Ministry of Health] (in Arabic). Mauritania. 17 April 2021.
- ^ "Covid-19 : Communiqués". Republic of Mauritius. 23 October 2020.
- ^ "Covid-19 México". Gobierno de México [Government of Mexico] (in Spanish). 15 October 2021.
- ^ "Comunicate". Ministerul Sănătății Muncii și Protecției Sociale [Ministry of Health, Labour and Social Protection] (in Romanian). Moldova. 21 April 2022.
- ^ Нөхцөл байдлын мэдээ COVID-19. Эрүүл Мэндийн Яам [Ministry of Health] (in Mongolian). 10 July 2021.
- ^ "Uživo: COVID-19". Institut za javno zdravlje Crne Gore [Institute of Public Health of Montenegro] (in Montenegrin). 28 July 2020.
- ^ "Novosti". Institut za javno zdravlje Crne Gore [Institute of Public Health of Montenegro] (in Montenegrin). 11 May 2021.
- ^ مرض فيروس كورونا المستجد: الرصد الصحي بالمغرب. البوابة الرسمية لفيروس كورونا بالمغرب [The official portal of coronavirus in Morocco] (in Arabic). 7 January 2023.
- ^ "Boletim diário COVID-19 Nº379". Ministério da Saúde [Ministry of Health] (in Portuguese). 22 July 2021.
- ^ "Coronavirus Disease 2019 (COVID-19) Surveillance Dashboard (Myanmar)". Ministry of Health and Sports (in Burmese). 16 September 2021.
- ^ "COVID-19 update". Official Facebook account of the Ministry of Health and Social Services-Namibia. 5 July 2022.
- ^ "COVID-19 Dashboard". Ministry of Health and Population (Nepal). Retrieved 26 July 2022.
- ^ "Epidemiologische situatie van COVID-19 in Nederland" (PDF). Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment] (in Dutch). 6 July 2021.
- ^ "Info coronavirus Covid-19". Gouvernement de la Nouvelle-Calédonie [Government of New Caledonia] (in French). 4 September 2021.
- ^ "COVID-19: Testing data". Ministry of Health. 30 January 2023.
- ^ "COVID-19: Current cases". Ministry of Health. 30 January 2023.
- ^ "#Covid19Niger Bilan du 22/02/2021". Facebook account of the Ministère de la Santé Publique [Ministry of Public Health] (in French). 22 February 2021.
- ^ "Coronavirus COVID-19 Microsite". Nigeria Centre for Disease Control. 28 February 2021.
- ^ КНДР ввела максимальный уровень карантина. KBS World Radio (in Russian). 2 December 2020.
- ^ Регистрирани 237 Нови Случаи На Ковид 19 – Вкупно Дијагностицирани 84024, Оӡдравени 460 Пациенти – Починати 8 Лица. Министерство за здравство [Ministry of Health] (in Macedonian). 1 July 2021.
- ^ Во последните 24 часа. Министерство за здравство [Ministry of Health] (in Macedonian). 27 June 2021.
- ^ "COVID-19 Genel Durum". Kuzey Kıbrıs Türk Cumhuriyeti Sağlık Bakanlığı [Turkish Republic of Northern Cyprus Ministry of Health] (in Turkish). 13 July 2022.
- ^ "Dags- og ukerapporter om koronavirussykdom (covid-19)". Folkehelseinstituttet [Norwegian Institute of Public Health] (in Norwegian). 20 January 2022.
- ^ "Oman conducts over 500,000 COVID-19 tests since the start of pandemic". The Arabian Stories. 28 October 2020.
- ^ "Pakistan Cases Details". COVID-19 Health Advisory Platform. Ministry of National Health Services Regulations and Coordination. 5 March 2021.
- ^ فايروس كورونا (COVID-19) في فلسطين. فايروس كورونا (COVID-19) في فلسطين [Coronavirus (COVID-19) in Palestine] (in Arabic). 5 February 2022.
- ^ "Compartimos la actualización de datos sobre #COVID19 en nuestro país. Parte 1". Cuenta Oficial de Twitter del Ministerio de Salud de Panama [Official Twitter Account of the Ministry of Health Panama] (in Spanish). 31 January 2023.
- ^ "Official COVID-19 Info Website". Papua New Guinea Joint Agency Task Force, National Control Centre for COVID-19. 20 February 2021.
- ^ "Reportes – COVID19" (in Spanish). Ministe132 280rio de Salud Pública y Bienestar Social (Ministry of Public Health and Social Welfare). 28 March 2022.
- ^ "Sala Situacional". Covid-19 en ″el Perú [Covid-19 in Peru] (in Spanish). 19 November 2022.
- ^ "COVID-19 Tracker". Department of Health (Philippines). 7 January 2023.
- ^ "COVID-19 Tracker". Department of Health (Philippines). 16 April 2021.
- ^ "diagnostyka pod kątem koronawirusa". Official Twitter account of the Ministerstwo Zdrowia [Ministry of Health] (in Polish). 27 April 2022.
- ^ "Ponto de Situação Atual em Portugal". COVID-19 (in Portuguese). Ministry of Health. 5 January 2022.
- ^ "COVID19 Home". Ministry of Public Health. 12 November 2022.
- ^ "Buletin informativ". Ministerul Sănătăţii [Ministry of Health] (in Romanian). 29 January 2021.
- ^ Информационный бюллетень о ситуации и принимаемых мерах по недопущению распространения заболеваний, вызванных новым коронавирусом. Федеральная служба по надзору в сфере защиты прав потребителей и благополучия человека (Роспотребнадзор) [Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)] (in Russian). 7 June 2022.
- ^ стопкоронавирус. Оперативные данные [Stop Coronavirus] (in Russian). 4 June 2022.
- ^ "Amakuru Mashya | Update". Twitter account of the Ministry of Health-Rwanda. 6 October 2021.
- ^ "COVID-19 Updates". Government of St. Kitts and Nevis. 27 August 2021.
- ^ "Saint Lucia's COVID-19 Dashboard". Ministry of Health and Wellness. 7 October 2022.
- ^ "COVID-19 Report". Ministry of Health, Wellness and the Environment (St. Vincent and the Grenadines). 30 January 2023.
- ^ "Aggiornamento Epidemia COVID-19". Istituto per la Sicurezza Sociale [Institute for Social Security] (in Italian). 30 January 2023.
- ^ "COVID 19 Dashboard: Saudi Arabia". Ministry of Health. 26 April 2022.
- ^ "Riposte à l'épidémie du nouveau coronavirus COVID-19, Sénégal" (PDF). Ministère de la Santé et l'Action sociale [Ministry of Health and Social Action] (in French). 12 July 2021.
- ^ "Coronavirus COVID-19". Ministry of Health of the Republic of Serbia. 3 February 2023.
- ^ "Updates on COVID-19 (Coronavirus Disease 2019) Local Situation". Ministry of Health. 3 August 2021.
- ^ "COVID-19 Situation Report". Ministry of Health. 2 March 2020.
- ^ "Covid-19 in graphs". korona.gov.sk. Office of the Deputy Prime Minister of the Slovak Republic for Investments and Informatization. 3 February 2023.
- ^ "Dnevno spremljanje okužb s SARS-CoV-2 (COVID-19)". Nacionalni inštitut za javno zdravje [National Institute of Public Health] (in Slovenian). 2 February 2023.
- ^ "COVID-19 South African coronavirus news and information". South African Government. 24 May 2021.
- ^ "COVID-19 statistics in South Africa". South Africa Health Twitter Account. 24 May 2021.
- ^ 코로나바이러스감염증-19(COVID-19). 코로나바이러스감염증-19(COVID-19) [Coronavirus infection-19 (COVID-19)] (in Korean). Ministry of Health and Welfare. 1 March 2021.
- ^ "Update on COVID-19 Response". Ministry of Health - South Sudan. 26 May 2021.
- ^ "La pandemia del coronavirus, en datos, mapas y gráficos". RTVE ( Radio y Televisión Española) [RTVE ( Spanish Radio and Television)] (in Spanish). 1 July 2021.
- ^ "Resumen de la situación - Pruebas de laboratorio". Ministerio de Sanidad, Consumo y Bienestar Social [Ministry of Health, Consumption and Social Welfare] (in Spanish). 5 July 2021.
- ^ "COVID-19 Situation Report". Health Promotion Bureau, Sri Lanka. 31 March 2021.
- ^ "COVID-19 : Live Situational Analysis Dashboard of Sri Lanka". Health Promotion Bureau, Sri Lanka. 31 March 2021.
- ^ "Veckorapport om covid-19, vecka 20" (PDF). folkhalsomyndigheten.se (in Swedish). Public Health Agency of Sweden. 28 May 2021. p. 18.
- ^ "Folkhalsomyndigheten Antal fall av Covid-19". folkhalsomyndigheten.se (in Swedish). Public Health Agency of Sweden. 1 February 2021.
- ^ "COVID-19 Switzerland". Federal Office of Public Health FOPH. 8 November 2022.
- ^ "Taiwan Centers for Disease Control". Taiwan Centers for Disease Control. 4 February 2023.
- ^ รายงานสถานการณ์โรคติดเชื้อไวรัสโคโรนา 2019 ฉบับที่ 426 วันที่ 4 มีนาคม 2564 (PDF). Department of Disease Control (in Thai). 4 March 2021.
- ^ "Coronavirus Au Togo". Government of Togo (in French). 7 January 2023.
- ^ "COVID-19 Update Trinidad and Tobago". Ministry of Health. 3 January 2022.
- ^ الأرقام الرئيسيّة المسجّلة بتاريخ 03 فيفري 2021 #كوفيد_19. Official Facebook account of the Ministére de la Santé وزارة الصحة [Ministry of Health, Tunisia] (in Arabic and French). 24 August 2021.
- ^ "Türkıye COVID-19 Hasta Tablosu". Türkiye Cumhuriyeti Sağlık Bakanlığı [Republic of Turkey Ministry of Health] (in Turkish). 2 July 2021.
- ^ "COVID-19 Daily Updates". Facebook page of the Ministry of Health - Uganda. 12 February 2021.
- ^ "COVID-19 pandemic in Ukraine". COVID-19 pandemic in Ukraine. Cabinet of Ministers of Ukraine. 24 November 2021.
- ^ "COVID-19 Updates – Ministry of Health and Prevention – UAE". Ministry of Health & Prevention. 2 February 2023.
- ^ "Coronavirus (COVID-19) in the UK". GOV.UK. 19 May 2022.
- ^ "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University". coronavirus.jhu.edu. 9 August 2021.
- ^ "COVID Data Tracker Weekly Review". Centers for Disease Control and Prevention. 30 July 2022. Retrieved 3 August 2022.
- ^ "Visualizador de casos coronavirus COVID-19 en Uruguay". Sistema Nacional de Emergencias [National Emergency System] (in Spanish). 16 April 2022.
- ^ Дневной прирост случаев COVID-19 продолжает увеличиваться. Gazeta.uz Газета.uz (in Russian). 11 September 2020.
- ^ "Día 353 de la lucha contra la COVID-19". COVID-19 Patria (in Spanish). 30 March 2021.
- ^ "COVID-19 in Viet Nam Situation Report 32". WHO. 30 August 2022. Retrieved 1 September 2022.
- ^ "Daily #COVID19 update". Official Twitter account of the Zambia National Public Health Institute. 10 March 2022.
- ^ "COVID-19 update". Official Twitter account of the Ministry of Health and Child Care (Zimbabwe). 16 October 2022.
External links
- Data on COVID-19 testing at Our World in Data (31 March 2020)
- COVID-19 Testing (at least) – now Free for all? (CDC; US Congress; CSPAN video/6:00; March 12, 2020)
- Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR; NCBI-PMC, DOI (23 January 2020)
- "EUA Authorized Serology Test Performance". U.S. Food and Drug Administration (FDA).