Pheochromocytoma: Difference between revisions
| Line 388: | Line 388: | ||
# [[Hyperglycemia|Hyperglycemia:]] Catecholamines prevent the secretion of [[insulin]] - a hormone responsible for lowering the body's [[Blood sugar level|blood glucose]] (sugar). Blood glucose levels should be checked frequently in the perioperative period and insulin should be given as needed if levels are elevated. Following resection, tumor-related hyperglycemia is likely to resolve. |
# [[Hyperglycemia|Hyperglycemia:]] Catecholamines prevent the secretion of [[insulin]] - a hormone responsible for lowering the body's [[Blood sugar level|blood glucose]] (sugar). Blood glucose levels should be checked frequently in the perioperative period and insulin should be given as needed if levels are elevated. Following resection, tumor-related hyperglycemia is likely to resolve. |
||
# [[Hypoglycemia]]: After the tumor is removed, insulin is no longer inhibited, which can bring the blood glucose dangerously low. Symptoms include [[tremor]], [[anxiety]], [[palpitations]], [[Perspiration|sweating]], [[Altered level of consciousness|altered mental status]] (confusion), [[dizziness]], and [[blurred vision]].<ref>{{Cite journal|last=Iqbal|first=Ahmed|last2=Heller|first2=Simon|date=06 2016|title=Managing hypoglycaemia|url=https://pubmed.ncbi.nlm.nih.gov/27432075/|journal=Best Practice & Research. Clinical Endocrinology & Metabolism|volume=30|issue=3|pages=413–430|doi=10.1016/j.beem.2016.06.004|issn=1878-1594|pmid=27432075}}</ref> It is important to note that patients on a [[beta blocker]] are more prone to hypoglycemia and may not experience these symptoms, which could delay the diagnosis.<ref>{{Cite journal|last=Dungan|first=Kathleen|last2=Merrill|first2=Jennifer|last3=Long|first3=Clarine|last4=Binkley|first4=Philip|date=11 27, 2019|title=Effect of beta blocker use and type on hypoglycemia risk among hospitalized insulin requiring patients|url=https://pubmed.ncbi.nlm.nih.gov/31775749/|journal=Cardiovascular Diabetology|volume=18|issue=1|pages=163|doi=10.1186/s12933-019-0967-1|issn=1475-2840|pmc=6882013|pmid=31775749}}</ref> Glucose levels should be check regularly and the hypoglycemia protocol at your institution should be followed for treatment of low levels |
|||
== Prognosis == |
== Prognosis == |
||
Revision as of 15:11, 15 August 2020
| Pheochromocytoma | |
|---|---|
| Other names | Phaeochromocytoma, Adrenal Medullary Tumor, Chromaffin Cell Tumors, Paraganglioma |
 | |
| Normal remnant adrenal gland (left) with a pheochromocytoma (right) involving the adrenal medulla | |
| Pronunciation |
|
| Specialty | Endocrinology, Oncology |
| Symptoms | Hypertension, Tachycardia, Sweating, Headache, Pallor |
| Complications | Hypertensive Crisis |
| Diagnostic method | Elevated plasma free metanephrines, plasma catecholamines, or urinary catecholamines |
| Treatment | The only curative option is surgery. Treating metastatic disease involves the addition of chemotherapy, radiation, and new pharmacologic agents |
| Frequency | 0.8 per 100,000 person-years [1] |
Pheochromocytoma (PHEO or PCC) is a rare, chromaffin cell tumor of the adrenal medulla.[2] When a tumor composed of the same cells as a pheochromocytoma develops outside the adrenal gland, it is referred to as a paraganglioma.[3] These neuroendocrine tumors are capable of producing and releasing massive amounts of catecholamines, metanephrines, or methoxytyramine, which result in the most common symptoms, including hypertension (high blood pressure), tachycardia (fast heart rate), and diaphoresis (sweating).[4] However, not all of these tumors will secrete catecholamines. Those that do not are referred to as biochemically silent, and a predominately located in the head and neck.[5] While patients with biochemically silent disease will not suffer from the typical disease manifestations described above, the tumors grow and compress the surrounding structures of the head and neck, and can result in pulsatile tinnitus (ringing of the ear), hearing loss, aural fullness, dyspnea (difficulty breathing), and hoarseness.[6] While tumors of the head and neck are parasympathetic, their sympathetic counterparts are predominantly located in the abdomen and pelvis, particularly concentrated at the Organ of Zuckerkandl.[7]
Signs and symptoms
The signs and symptoms of a pheochromocytoma are those related to sympathetic nervous system hyperactivity.[8] The classic triad includes headaches (likely related to elevated blood pressure, or hypertension), tachycardia/elevated heart rate, and diaphoresis (excessive sweating, particularly at night). However, patients are unlikely to experience continuous symptoms. Due to the paroxysmal nature of catecholamine synthesis and release, patients may experience "attacks" or "spells" where they are suddenly overwhelmed with signs and symptoms of their tumor.[9] Attacks can occur spontaneously (without warning) or may be triggered by a variety of pharmaceutical agents, foods, intraoperative tumor manipulation, intubation, or during anesthetic induction.[10]
| Lifestyle | Medications | Diet |
|---|---|---|
| Physical Exertion | Histamine | Cheese |
| Anxiety/Stress | Metoclopramide | Fermented wine/beer |
| Trauma/Pain | Glucagon | Tomatoes |
| Micturition | ACTH | Coffee/Beans |
While the above symptoms are classic, other common clinical manifestations have been reported and include (in no particular order)[4][10]
- Pallor
- Heat intolerance
- Weight loss
- Chest and/or Abdominal Discomfort
- Nausea/Vomiting
- Constipation
- Orthostatic Hypotension
- Medically defined as a decrease in systolic blood pressure (top number) of 20 mm Hg or diastolic blood pressure (bottom number) of 10 mm Hg after a change in position from lying down or sitting to a standing position[12]
- Feeling of becoming light-headed or dizzy after swiftly changing positions
- Psychological Manifestations
- Anxiety, Panic Attacks, Nervousness, Tremulousness
- Hyperglycemia (high blood sugar)
Complications
While the symptoms of a pheochromocytoma are quite common, the disease is often referred to as "the great mimic." [13] Literature reports that just 0.1% of patients with hypertension are diagnosed with this rare endocrine disorder and symptomatic patients are often mistaken for much more common diseases.[14] As symptoms are often paroxysmal (episodic/sporadic), patients may not immediately seek treatment as the problem "disappears on its own." Furthermore, when pictured in the ideal clinical scenario (an older woman in her mid-50s), the spontaneous attacks of flushing, sweating, and a racing heart may be mistaken for pre-menopausal related hot-flashes. Unmanaged pheochromocytoma is dangerous and can lead to serious complications, including death.[15] The cardiovascular system is the most commonly involved.[16][17][18]
Cardiovascular system
- Hypertensive Crisis: Pheochromocytoma-related hypertensive emergencies are one of the most feared clinical manifestations. Attacks are random and may occur secondary to a trigger (see Signs and Symptoms above) or spontaneously after a catecholamine surge.[17] The prevailing symptom is elevated systolic blood pressure (> 200 mmHg) that is unresponsive to traditional treatment regimens and threatens end-organ damage.[16] Patients require immediate, life-saving treatment to prevent further damage to other organs and/or death.
- Myocardial Ischemia/Infarction: A heart attack is often caused by a significant build-up of plaque (atherosclerosis) in the coronary vessels. Patients with pheochromocytoma present with myocardial infarctions despite an overall lack of plaque build-up, indicating a different mechanism for the myocardial infarction. Current research hypothesizes that the tumor secretes massive amounts of catecholamines, which directly interact with myocardial (heart) tissue and exert negative effects including oxygen deprivation, leading to accelerated scarring and cell death.[16]
- Toxic Myocarditis: Even in patients without myocardial damage, excessive catecholamines can result in abnormal ST changes on an ECG. Norepinephrine (a catecholamine) is hypothesized to resulted in damaged cardiac tissue by inhibiting coronary blood flow and depriving cells of oxygen, thus resulting in ischemic tissue.[18] Fortunately, following tumor excision and the subsequent quelling of catecholamines, the damage has been proven reversible.
- Cardiomyopathy: Pheochromocytoma's have been implicated in various types of cardiomyopathy, including (myocarditis, see above), dilated cardiomyopathy, and stress-induced or Takotsubo cardiomyopathy.[19] As with the other cardiovascular-related complications, excess catecholamines are responsible for the increased myocardial burden and significant physiologic stress.[20] Current literature indicates that most of the catecholamine-induced damage is reversible, thereby strengthening the argument for early and accurate diagnosis in order to allow for cardiac remodeling and prevent further destruction.[19][20]
- Arrhythmias: Sinus tachycardia is the most common abnormal heart rhythm associated with a pheochromocytoma and is experienced by patients as the feeling of a "fluttering heart" or palpitations.[16] Many other tachyarrhythmias (fast heart rate) have also been reported.
Nervous system
- Cerebrovascular Accident (Stroke): Multiple reports have detailed transient ischemic attacks or strokes in patients with a pheochromocytoma.[21][22][23][24][25][26][27] In a study of 130 patients with pheochromocytoma, 7 patients were diagnosed with a transient ischemic attack (the neurologic deficit completely resolved) and 3 patients experienced a stroke with persistent symptoms.[28]
- Headache: Headaches are one of the core clinical manifestations of a pheochromocytoma and can result in debilitating pain. The majority of studied patients report their pain began and ended abruptly without warning and described the pain as a severe, bilateral throbbing (although the scale of severity was not published). While 71% of the studied patients reported headaches, just over 20% of the affected patients endorsed associated nausea, vomiting, photophobia, or phonophobia, which are typically associated with migraines.[29]
Urinary system
- Acute Renal Failure: Several reports have detailed rhabdomyolysis (rapid skeletal muscle breakdown) leading to acute kidney injury and the need for transient dialysis in the undiagnosed pheochromocytoma patient as their primary presenting symptom.[30][31][32][33] Kidney failure is brought about by catecholamine-induced muscle injury. Norepinephrine causes vessels to narrow, thereby limiting blood flow and inducing ischemia.[30]
Multiple Organ Dysfunction System (MODS)[34]: Caused by an elevated inflammatory response, multiple organ dysfunction is a severe, life-threatening emergency with increasing mortality based on the number of systems involved.[35] Pheochromocytoma-related MODS is associated with multiple organ failure, hyperthermia > 40 degrees Celsius, neurologic manifestations, and cardiovascular instability resulting in either hypo or hypertension.[36] In contrast to a hypertensive crisis, pheochromocytoma-associated MODS may not respond to traditional alpha-receptor agents and may require emergent surgical excision if clinical stability is not achieved.
Genetics
Current estimates predict that upwards of 40% of all pheochromocytomas are related to an inherited germline susceptibility mutation.[37] Of the remaining 60% of tumors, more than 30% are associated with a somatic mutation.[38] Given the high association with genetic inheritance, the United States Endocrine Society recommends that all patients diagnosed with a pheochromocytoma undergo an evaluation with a genetic counselor to consider genetic testing.[39] The most recent data indicates that there are 25 pheochromocytoma susceptibility genes; however, just 12 are recognized as part of a well-known syndrome.[7] Determining the genetic status of a pheochromocytoma patient is crucial - each gene is inherited in a different pattern, associated with specific disease characteristics, and may respond more favorably to certain treatment options. Furthermore, early identification can guide physicians on screening recommendations for first degree relatives of patients with pheochromocytoma.[40] There is no current consensus for how and when asymptomatic carriers (individual who has a genetic variant associated with pheochromocytoma, but no current evidence of disease) should be evaluated. Conversations should occur at an individual level with the patient and their provider to develop a personalized screening plan that alternates between a biochemical (blood work) evaluation and whole-body imaging to monitor disease progression.[41]
Pediatric considerations
When pursuing genetic evaluation in a child, it is important to consider several additional guidelines to maintain the emotional and psychological well-being of the minor. Proper screening includes a multidisciplinary team (endocrinologist, oncologist, psychologist, geneticist, parent, and child) where the primary focus is ensuring the pediatric patient is supported during this time.[42]
- If at all possible, avoid testing around family observed days of celebration; a positive result may mask the happiness associated with these events in the future
- If at all possible, avoid testing siblings at the same time. While convenient, each child may receive a different result. Testing one pediatric patient at a time allows the family to narrow their focus when results are returned and support each sibling individually
- Allow each child the opportunity to ask questions and process the results. Consider that even a negative result may be upsetting if their sibling was positive and they are struggling to understand the difference and what this means for their futures
Hereditary syndromes
The following table(s) detail the clinical characteristics of the well-known hereditary pheochromocytoma gene variants[43][44][45][40][38][37][46]
| Gene | Inheritance | Penetrance | Metastatic Potential | 1o Disease Characteristics | |
|---|---|---|---|---|---|
| MEN2 | RET | Autosomal Dominant | 40-50% | <5% | Medullary thyroid carcinoma, hyperparathyroidism, marfanoid habitus, pheochromocytoma |
| VHL | VHL | 10-30% | 5% | Renal cell carcinoma, pancreatic NET, retinal and CNS hemangioblastoma, pheochromocytoma | |
| NF1 | NF1 | 1-5% | 12% | Neurofibromas, cafe-au-lait macules, lisch nodules, cognitive impairment, pheochromocytoma |
MEN2 (Multiple Endocrine Neoplasia-2); VHL (von-Hippel Lindau); NF1 (Neurofibromatosis-1); NET (Neuroendocrine Tumor); CNS (Central Nervous System)
| Gene | Inheritance | Penetrance | Metastatic Potential | 1o Disease Characteristics | |
|---|---|---|---|---|---|
| PGL1 | SDHD | Autosomal Dominant | 90% | <5% | Head and neck paraganglioma, pheochromocytoma, gastrointestinal stromal tumor |
| PGL2 | SDHAF2 | 100% | Low | Head and neck paraganglioma | |
| PGL3 | SDHC | Autosomal Dominant | Inconsistent | Inconsistent | Pheochromocytoma, head and neck paraganglioma, gastrointestinal stromal tumor |
| PGL4 | SDHB | 30-50% | 30-70% | Head and neck paraganglioma, pheochromocytoma, gastrointestinal stromal tumor | |
| PGL5 | SDHA | 10-15% | Low | Pheochromocytoma, head and neck paraganglioma, gastrointestinal stromal tumor |
SDHx (Succinate Dehydrogenase Subunit x)
| Inheritance | Penetrance | Metastatic Potential | 1o Disease Characteristics | |
|---|---|---|---|---|
| MAX | Autosomal Dominant | Inconsistent | <5% | Bilateral pheochromocytoma |
| TMEM127 | Inconsistent | Low | Pheochromocytoma, head and neck paraganglioma |
MAX (MYC Associated Factor X); TMEM127 (Transmembrane Protein 127)
Other gene variants
There have been several published reports of other, rare pheochromocytoma-associated susceptibility genes:
- Pacak-Zhuang Syndrome[47][48][49][50][51]
- Hypoxia-inducible factor 2 alpha (HIF2A)
- Polycythemia
- Duodenal somatostatinoma
- Retinal and choroidal vascular changes
- Paraganglioma/Pheochromocytoma
- Pheochromocytoma and Giant Cell Tumor of Bone[52]
- H3 histone, family 3A (H3F3A), post-zygotic G34W
- Pheochromocytoma/Paraganglioma
- Carney Triad[53]
- Gastrointestinal stromal tumor
- Pulmonary chondroma
- Paraganglioma
- Carney-Stratakis Syndrome[54]
- Gastrointestinal stromal tumor
- Paraganglioma
Several additional gene variants have been described, but the provided information is inconsistent and a consensus has not been reached in the community if these mutations are truly pheochromocytoma susceptibility genes.
Diagnosis
Differential
If a patient has the characteristic signs and symptoms of a pheochromocytoma and the decision is made to pursue additional biochemical (blood work) evaluation, the differential diagnosis is important as it is more likely to be something other than a pheochromocytoma given the relative frequency of 0.8 per 100,000 person-years.[1]
| Endocrine | Cardiovascular | Neurologic | Psychiatric | Other |
|---|---|---|---|---|
| Hyperthyroidism | Heart Failure | Migraine | Anxiety | Porphyria |
| Carcinoid Syndrome | Arrhythmias | Stroke | Panic Disorder | Medications3 |
| Hypoglycemia | Ischemic Heart Disease | Epilepsy | Substance Use1 | |
| Menopausal Syndrome | Baroreflex Failure | Meningioma | Factious Disorder2 | |
| Medullary Thyroid Carcinoma | - | POTS | - |
Adopted from Lenders et al., Phaeochromocytoma. The Lancet. 366(9486; 665-675.
1Cocaine; 2Misuse of over-the-counter medications like Pseudoephedrine (Sudafed®) that are sympathomimetics; 3Monoamine Oxidase Inhibitors, Clonidine Withdrawal
Biochemical evaluation
Gold standard
Elevated plasma free metanephrines is considered the gold standard diagnosis for pheochromocytoma.[55] Over 10 studies have confirmed that the sensitivity and specificity of this test is 97% and 93% respectively; however, there is still concern for false positive results in the correct clinical scenario.[56] When interpreting a biochemical analysis for pheochromocytoma, the provider must pay close attention to the (1) conditions of the collection, (2) all medications the patient is taking, and (3) their diet.[57]
- Conditions of Collection: Unlike many routine laboratory tests that can be drawn at a moments notice, there are several recommendations that should be followed to ensure the ideal conditions and an accurate sample. Current research indicates that blood work should only be drawn after a patient has been resting supine (flat on their back) for 30 minutes before collection.[58][59] Specific supine reference values should be used in this scenario. Ensuring these conditions is difficult and may be cost-prohibitive at most institutions. In these cases, a rested, supine draw can be repeated following a positive result in a seated position to eliminate false-positive results.[57]
- Pharmaceutical Interference: Many prescription, over-the-counter, and illicit substances can interfere with the proper collection of plasma metanephrines and lead to false-positive results. Providers should review a patient's medication list in-detail and have a discussion if temporarily discontinuing any of the interfering medications is possible. The most reported medications to result in falsely elevated metanephrines include: β-adrenoceptor blockers, phenoxybenzamine, tricyclic antidepressants, monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors (SNRI), and methyldopa.[60][57] As the majority of these medications are commonly prescribed for psychiatric conditions, a conversation with the prescriber may be necessary fo facilitate alternative therapeutic options while the patient is undergoing evaluation for a pheochromocytoma.[60] After any possible prescription medications have been held, it is important to review any over-the-counter medications/supplements as well as the commonly used acetaminophen and pseudoephedrine cause false elevations in metanephrine levels.[57][60] Finally, it is important to have open, non-judgemental discussions about the patient's recreational substance use. Amphetamines, nicotine, and cocaine can result in marked plasma norepinephrine levels.
- Lifestyle and Diet: As with most lab work, the patient should refrain from eating (fasting) after midnight the night prior to their collection. However, there are further recommendations specific to a metanephrines collection, including abstaining from nicotine, alcohol, and exercise for at least 12 hours prior to their lab draw.[7] Patient's should also avoid catecholamine-containing foods (fruits, fruit drinks, chocolate, caffeine, tomatoes, beans, nuts, and potatoes) for a minimum of 24 hours prior to collection.[61][62]
While the above (3) conditions are likely to contribute to false-positive results if not controlled for, any value greater than 3 to 4 times the upper reference limit of normal should be considered diagnostic for a pheochromocytoma.[39][63]
Alternative Tests
Twenty-four hour urinary metanephrines are an acceptable alternative if the plasma test is unavailable.[64] Other additional biomarkers can be helpful to aid in the diagnosis of pheochromocytoma as well, most notable is Chromogranin A. In comparison to the specificity of elevated catecholamines in the pheochromocytoma patient, chromogranin A is a non-specific polypeptide that is high in a variety of neuroendocrine tumors.[65] However, a 2006 report from Italy found that over 90% of studied pheochromocytoma patients demonstrated elevated chromogranin A levels.[66] If metanephrine values are equivocal, chromogranin A can be used as an adjunct marker to predict the presence of a tumor.
Borderline elevated metanephrines present a diagnostic challenge to the physician - the first step is to repeat the labs, taking extra precautions to follow the gold standard diagnosis described above, including the conditions of collection, pharmaceutical interference, and any potential diet and lifestyle habits that could alter the results. If the offending medications cannot be discontinued or repeated labs remained the same, consider administering a clonidine suppression test.[67] In the 1970s, the drug clonidine hydrocloride swept the market as a novel agent for hypertension; however, the reported side-effects (nausea, vomiting, drowsiness, dryness of the eyes and mouth, constipation, and generalized weakness) limit compliance and have vastly diminished prescriptions.[68] While the adverse side-effects with clonidine are inconvenient, the most dangerous aspect of clonidine is withdrawal rebound hypertension - that is, when the medicine is abruptly discontinued, blood pressure may rapidly return or surpass the original value.[69][70][71] However, a one-time, weight-based dose can be utilized in limited settings to help determine disease status.[57] After fasting overnight, patient's will present to their testing site for a baseline metanephrines blood draw and clonidine administration. They will remain supine for (3) hours and a repeat blood draw will be taken. A positive result (indicating a pheochromocytoma) will occur if the plasma metanephrine levels remain elevated after clonidine is given. If the results are the same or fall, the test is negative and the patient does not have a pheochromocytoma.[57] It is important to note that if a patient does not have a pheochromocytoma, they may become extremely hypotensive following clonidine. Patient's should not depend on themselves for transport following this test.
Plasma methoxytyramine is a breakdown product of the catecholamine, dopamine. Paragangliomas of the head and neck commonly secrete dopamine, but are referred to as "biochemically silent" because they do not cause the characteristic symptoms associated with a pheochromocytoma. However, methoxytyramine can be utilized to detect the tumors of the head and neck.[72] Further research indicates that the biomarker is also a useful indicator of metastatic disease - which is the only current biochemical evidence of metastases to date.[73]
Biochemical phenotypes
While diagnostic, laboratory values can also provide physician's with important information about the type, location, size, and associated tumor genotype.[63] There are (3) major, well-recognized biochemical phenotypes that can be used by heath care providers to direct patient care.[74]
- Adrenergic (Epinephrine and metanephrine)
- More likely to indicate an adrenal tumor[75]
- When plasma metanephrine levels were elevated to greater than 15% of the combined levels of normetanephrine and metanephrine, an adrenal tumor or a recurrence of an adrenal tumor that had already been excised can be predicted
- Patients are more likely to present with the classic, paroxysmal (episodic) symptoms described above[63]
- Noradrengeric (Norepinephrine and normetanephrine)
- More likely to indicate an extra-adrenal tumor[75]
- Patients are more likely to present with continuous, persistent pheochromocytoma-related symptoms (hypertension and tachycardia) compared to those that are classically episode with an adrenergic phenotype[63]
- Common in patients with von-Hippel Lindau and succinate dehydrogenase subunit X genetic variants[63]
- Dopaminergic (Dopamine and 3-methoxytyramine)
- More likely to indicate an extra-adrenal tumor of the head and neck[74]
- Patients are more likely to be asymptomatic; however, they may present with non-specific signs of nausea, vomiting, abdominal pain, diarrhea, and weight loss secondary to the stimulation of dopamine receptors throughout the gastrointestinal tract[63]
- Particularly prevalent in patients with succinate dehydrogenase subunit B genetic variants [63]
Across both an adrenergic and a noradrenergic phenotype, the greater the sum of plasma or urinary concentrations of metanephrine and normetanephrine, the larger the expected tumor diameter.[75]
Tumor Localization
Anatomic Imaging
Anatomic imaging refers to computed tomography (CT) [CAT scan] or magnetic resonance imaging (MR) scans. These imaging modalities serve to initially locate the tumor and provide detailed information about size, morphology, and structural relation to adjacent internal structures.[76] Traditionally, a patient presents to their physician for symptoms concerning for a pheochromocytoma, which prompts a biochemical evaluation. If the results are positive, the patient is referred for anatomic imaging with a CT or MR scan. However, as anatomic imaging becomes more readily available, patients are referred to an endocrinologist after an incidental (unanticipated finding) adrenal nodule is found on a scan ordered for another reason.[77] For example, "Patient M" presents to his local emergency room for abdominal pain and a CT is ordered to rule-out appendicitis; however, the radiologist notes there is a 3.5 centimeter right adrenal mass.
While there has not been a consensus on if CT or MR is the preferred imaging modality in pheochromocytoma, each method has its associated strengths and weaknesses. As CT expose the patient to ionizing radiation, MR is preferred in children and pregnant women.[78] Furthermore, the intravenous contrast used in CT can cause kidney damage and should therefore be avoided in patients with pre-existing damage.[79] However, patients who struggle with being in confined spaces for extended periods of time (claustrophobia) cannot often tolerate an MR as the machine is close-ended compared to the open-ended design of a CT.[80] When patients become anxious and begin to move in the machine, this causes motion artifact, which occurs less in CT-based images.[81]
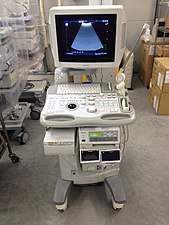
Compared to CT and MR, ultrasound is not a preferred imaging modality and should be avoided in the pheochromocytoma patient. However, in specific patient populations where avoid ionizing radiation is the top priority (children, pregnant women), ultrasound can be used as an adjunct method when MR may be unavailable or the patient is unable to complete the scan. Furthermore, if an acute adrenal hemorrhage is suspected in a pheochromocytoma patient, ultrasound is a quick, painless, radiation-less, and cheap modality for a "first-pass" before the above imaging modalities or surgery is used to confirm the diagnosis.[82]
- Anatomic Imaging Modalities
-
A
-
B
-
C
A: Computed Tomography. Patient lies on the table (screen left) and is maneuvered through the machine (screen left) to obtain images. The machine is shaped like a donut, with the patient passing through the center. The machine is "open-ended," which increases patient comfort.
B: Magnetic Resonance. Patient lies on the table and is completely inserted into the machine. The machine is shaped like a tube and is not open-ended, which makes some patients feel claustrophobic.
C: Ultrasound. The ultrasound probe is covered in jelly and placed directly on the patient. Images are immediately available and cause no discomfort to the patient.
Functional Imaging
The imaging modalities discussed below are for tumor characterization, confirmation of metastatic disease, and treatment planning - they are not used to discern tumor location or help the surgical team prepare for excision.[83] For most pheochromocytoma patients, functional imaging will follow a CT or MR. If anatomic imaging only demonstrates an adrenal tumor without evidence of disease anywhere else in the body and the metanephrine levels are overtly elevated, functional imaging can be foregone in favor of prompt surgical excision.[78] Over the last decade, there have been four functional techniques used to evaluate the pheochromocytoma patient (1) 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET), commonly referred to as the PET scan, (2) iodine-123 meta-iodobenzylguanadine (123I-MIBG), (3) 18F-flurodihydroxyphenylalanine (18F-FDOPA), and (4) 68Ga-DOTA coupled somatostatin analogs (68Ga-DOTA). From this point forward, these imaging modalities will be referred to in their abbreviated names found in parentheses.

The first functional imaging technique utilized in pheochromocytoma patients was 123I-MIBG scintigraphy (Image Right). Given the compounds similar structure to the catecholamine norepinephrine (secreted by pheochromocytomas), MIBG was well-suited for uptake by most neuroendocrine tumors.[84] Furthermore, if a patient was found to be positive on an MIBG scan, they were eligible for MIBG treatment, offering additional avenues for those suffering from widespread metastatic disease.[85] However, further investigation revealed that while MIBG excelled with adrenal lesions, it was far less superior in patients with extra-adrenal paragangliomas, particularly with specific genetic variants like succinate dehydrogenase subunit X (SDHx).[73] As the positron emission tomography scans were developed, MIBG has slowly loss its favor for the pheochromocytoma patient.[73]

Of the four above mentioned modalities, 18F-FDG PET is the most common and readily available functional imaging technique at most hospital systems, but the least-specific to neuroendocrine tumors (Image Left). In 2012, over 200 patients participated in a trial that compared the current gold standard of the time (MIBG/CT/MRI) to the novel FDG PET. Compared to its functional counterpart, FDG outperformed MIBG in detecting soft-tissue and bone metastases with a higher specificity in patients with biochemically active tumors.[73]
Following the development of FDG-PET, neuroendocrine-specific PET scans began to emerge. One of the first favorable imaging modalities was 18F-FDOPA, which demonstrated a high sensitivity in detecting head and neck paragangliomas as well as non-metastatic disease outside of the head and neck.[73][86] Unfortunately, in cases of metastatic disease, particularly related to succinate dehydrogenase subunit B (SDHB) mutations, 18F-FDOPA fell inferior to the traditional FDG-PET.[87] However, for patients with genetic variants in other pheochromocytoma-susceptibility genes (NF1, VHL, RET) 18F-FDOPA has become the preferred radiopharmaceutical agent.[88]
The newest PET modality involves somatostatin receptor type two receptor imaging with 68Ga-DOTA analogues.[81] Over the last decade, further research continues to indicate the superiority of this functional imaging modality in a wide-range of clinical scenarios, even surpassing anatomic imaging (CT/MR) in pediatric patients with succinate dehydrogenase (SDHx) mutations.[89] While FDOPA inconsistently detected metastatic disease, 68Ga-DOTA analogues have demonstrated superior localization of metastatic pheochromocytoma.[90] When directly compared in a head-to-head study, 68Ga-DOTA analogues outperformed FDOPA, particularly in the detection of metastatic bone lesions.[91] An additional benefit of the DOTA analogues is the ability for treatment with peptide receptor radionuclide therapy, which will be discussed in the treatment section below.[92]
Management
Surgery
Surgical resection remains the only curative option for pheochromocytoma.[93] A successful excision is a multidisciplinary effort involving the endocrinologist and the patient pre-operatively (discussed below) and the surgical team and anesthesiologist intraoperatively. Without frequent and adequate communication between all of the above mentioned teams, a favorable outcome is much more difficult.[93] The United States Endocrine Society Clinical Practice Guideline for pheochromocytoma currently recommends a laparoscopic adrenalectomy (minimally invasive technique) for most adrenal tumors, unless they are invasive or are larger than 6.0 centimeters.[94] It is important to note that larger tumors can be attempted with a minimally invasive approach, but the team should be prepared to convert to an open procedure if necessary.[95] An open procedure (traditional surgical technique) is currently preferred for extra-adrenal disease, unless the tumor is small, non-invasive, and in an easy to maneuver location. While previous data indicated the need for a minimally invasive approach with malignant and/or metastatic disease, current research indicates a successful operation is feasible and results in a shorter hospital stay.[96] Literature within the last decade has also demonstrated that the robotic technique can successfully be utilized for adrenal tumors.[97] In 2013, a surgical team at the Cleveland Clinic were the first to directly compare laparoscopic to robotic adrenalectomy techniques and reported that the robotic approach resulted in lower morbidity, less reported postoperative pain, and a shorter duration to discharge.[98]
Typically, a complete or total adrenalectomy is performed; however, a technique referred to as "cortical-sparing" can leave a remnant (piece) of the adrenal gland in hopes of avoiding life-long steroid replacement if the left and right adrenal glands need to be removed.[99] The issue is particularly important in patients with MEN and VHL-related disease, which has a higher chance of bilateral pheochromocytomas.[100] Of course, the risk of leaving adrenal tissue is recurrent disease (tumor comes back). A 2019 cohort study reported that despite a 13% recurrent rate in patients who underwent a cortical-sparing adrenalectomy for pheochromocytoma, there was no decreased survival compared to their total adrenalectomy counterparts.[99]
- A Path to Pheochromocytoma Excision
-
A
-
B
-
C
-
D
-
E
A: Axial computed tomography (CT) scan of the abdomen. White arrow (screen left) indicates the left adrenal gland with a pheochromocytoma
B: Minimally invasive laparoscopic surgery technique. The surgical team (screen left) work with instruments that are placed through several ports. One of the instruments is a camera, which projects the intra-abdominal images onto a screen within the operating room (screen right)
C: Robotic surgery technique. The lead surgeon (screen left) sits at the consul, which is completely removed from the patient. The surgeon controls several joysticks and foot pedals that are connected to the robotic arms (screen right). The robotic arms are assisted by other surgical staff
D: Gross resection of a bilateral adrenalectomy for MEN2A-related pheochromocytoma
E: Pathological pheochromocytoma surgical resection
Pre-Operative Management
Arguably, the most important part of a pheochromocytoma surgical plan is an adequate pre-operative blockade. Excess catecholamines have been described as a dormant volcano, ready to erupt at any time, wreaking catastrophic havoc on the body.[101] While the an eruption can occur at any time, two of the most common triggers are anesthesia and direct tumor manipulation, making surgery one of the most dangerous times for a pheochromocytoma patient if not properly prepared.[102] In order to help circumvent a catecholamine-crisis, the United States Endocrine Society recommends that all patients with functional (hormonally active) tumors be started on an pre-operative alpha-adrenoceptor blockade a minimum of seven days prior to surgery.[94] There are several medication options depending on the clinical scenario, each with their own associated strengths and weaknesses.
Alpha Blockade
If the patient's blood pressure is moderately elevated, a selective, short-acting alpha-1 adrenoceptor antagonist (doxazosin, prazosin, terazosin) is the preferred agent.[101] However, the patient should be warned about the potential side-effect known as "the first-dose phenomenon." When patients are initially exposed to one of the above agents, they may become lightheaded, dizzy, and nauseous, particularly when transferring from a seated to standing position due to a rapid decrease in blood pressure.[103] These effects will decrease with time, but providers can try to avoid them by starting at a low-dose and slowly increasing until they reach their desired amount. In patient's with uncontrolled hypertension, the non-selective alpha-1 and 2 adrenoceptor antagonist (phenoxybenzamine) should be utilized.[101] Unfortunately, compared to the selective agents listed above, phenoxybenzamine is much more expensive and may not be readily available to some patients. Common side-effects include dry mouth, nasal congestion, and impaired male ejaculation, all of which do not cease with time and may limit patient compliance.[104] While uncommon, patients may have a hormonally-active pheochromocytoma and a normal blood pressure. If this is the case, a small dose of a calcium-channel blocker (amlodipine) may be used pre-operatively.[105] This will not drastically lower the patients blood pressure and make them hypotensive, but it will assist the surgical and anesthesia teams if there is hemodynamic instability during the operation.
Beta Blockade
An elevated heart rate (tachycardia) and the feeling of a racing heart (palpitations) may follow after initiating an alpha-adrenoceptor antagonist. If that is the case, a beta-adrenoceptor antagonist is then prescribed to control the heart rate.[101] Just as with the alpha antagonists, there are selective (beta-1) and non-selective (beta-1 and beta-2) adrenoceptor antagonists. The selective agents (atenolol, metoprolol, propranolol) are preferred to the non-selective agents.[101] There are several (labetalol, carvedilol) combined alpha-beta-adrenoceptor antagonists. These agents should be avoided whenever possible as there is upwards of seven times more beta-adrenoceptor antagonism than alpha, which can worsen hypertension and lead to a catecholamine crisis.[106]
Complications
It is important that a beta-adrenoceptor antagonist is never given alone in a pheochromocytoma patient - this can lead to catastrophic consequences.[107] In 1995, a team of physicians from London described the unfortunate death of a recently diagnosed pheochromocytoma patient after initiation of propranolol, a non-selective beta blocker. She quickly developed a hypertensive crisis leading to shock, myocardial infarction, heart failure, and dense right hemiplegia. Despite the team's best resuscitative efforts, she never regained consciousness and eventually died several days later.[108] This complication is related to the impact that alpha and beta-adrenoceptor antagonists have on blood vessels combined with the actions of catecholamines. The normal blood vessel is open, allowing for adequate blood flow. When catecholamines activate the alpha receptor, the vessel constricts (gets smaller), which results in hypertension.[109] However, when catecholamines active the beta receptor, the blood vessel dilates (gets larger) and allows for increased blood flow, reducing the blood pressure.[110] If a pheochromocytoma patient is only started on a beta-adrenoceptor antagonist, this reverses the protective vasodilation and worsens the patients hypertension.
Controversy
While the pre-operative alpha and beta blockade discussed above is overwhelmingly recognized as the standard of care, particularly in the United States, there has been discussion at the international level if a blockade is necessary. In 2017, a team of researchers from Germany published an observational case series that called into question the current recommendations for a blockade.[111] Their study looked at the intraoperative maximal systolic arterial pressure in patients with and without an alpha-adrenoceptor blockade and found no difference in complications between the two groups.[111] The following year, a group from France published an article of a similar spirit and warned that waiting an entire week to begin a blockade could put the patient at risk. They called for immediate surgical intervention with an experienced anesthesiology team and felt this would mitigate any intraoperative catecholamine crisis.[112] These articles resulted in rebuttals[102][113] from research teams in the United States, but an international consensus has not yet been reached.
Perioperative Fluid Status
Excess catecholamines cause a decrease in the total blood volume, making a patient vulnerable to hypotension during the operation.[114] Therefore, a high-sodium diet with adequate fluid intake should be encouraged prior to surgery.[115] Some institutions in the United States will even admit patients the night prior to surgery for intravenous fluid replacement starting at midnight until the time of the operation.[101] However, one research study reported no difference in mortality in patients treated with preoperative intravenous fluids compared to those who did not.[116]
In a 2010 report from Cedars-Sinai Medical Center in Los Angelos, California, nearly all of the forty surveyed endocrinologists indicated the importance of preoperative volume resuscitation (having the patient take in plenty of fluids prior to surgery). However, after reviewing their patient data, over 60% of the same physicians failed to discuss salt-loading and adequate hydration.[117] Interestingly, when the patients were stratified by age, those that were younger received the advice to hydrate, but older patients did not. It was hypothesized that the providers chose to forego volume repletion in the older patient population for fear of their potential comorbidities (heart failure) where excess fluid is dangerous.[117] While there is still no recognized consensus or gold standard, providers should individualize the decision based on the patient's perceived nutritional standing, volume status, comorbidities, and ability to self-hydrate.
Post-Operative Management
Following surgery, there are several important potential complications that all anesthesiologists, surgeons, and endocrinologists need to be aware of and know how to manage. The section below will detail (by system) the most common possible complications, likely etiologies (cause), and treatment options.[118][119]
- Hypertension: In the pheochromocytoma patient, postoperative hypertension could indicate incomplete tumor resection or another tumor of unknown location. However, the traditional, non-specific causes of postoperative hypertension including pain, fluid overload, and essential hypertension must also be considered. A perioperative hypertensive crisis is first treated with a 5.0 milligram (mg) intravenous bolus of phentolamine, with additional 5.0 mg dose every ten minutes until the blood pressure falls within an acceptable range.[120] If the blood pressure is only minimally elevated, the patient can resume their alpha and beta-adrenoceptor antagonist from prior to surgery.[118] Remember to appropriately treat patient reported as well - this is a known cause of high blood pressure and could be a contributing factor.[121]
- Hypotension: There are several reasons a patient may have low blood pressure in the post-operative period. First and foremost, the tumor (and its abundance of catecholamines causing high blood pressure) has been removed. Furthermore, the patient may still experience the effects of their alpha-adrenoceptor antagonist, which causes lower blood pressure.[119] First-line treatment for postoperative hypotension is aggressive fluid resuscitation, which is why ensuring the patient is well-hydrated (see above) prior to surgery is so imperative.[118] Vasopressors may be needed if the blood pressure does not respond to fluids.
- Hyperglycemia: Catecholamines prevent the secretion of insulin - a hormone responsible for lowering the body's blood glucose (sugar). Blood glucose levels should be checked frequently in the perioperative period and insulin should be given as needed if levels are elevated. Following resection, tumor-related hyperglycemia is likely to resolve.
- Hypoglycemia: After the tumor is removed, insulin is no longer inhibited, which can bring the blood glucose dangerously low. Symptoms include tremor, anxiety, palpitations, sweating, altered mental status (confusion), dizziness, and blurred vision.[122] It is important to note that patients on a beta blocker are more prone to hypoglycemia and may not experience these symptoms, which could delay the diagnosis.[123] Glucose levels should be check regularly and the hypoglycemia protocol at your institution should be followed for treatment of low levels
Prognosis
The overall 5-year survival in people with pheochromocytoma is estimated to be about 90%.[124] There is increased life-time risk of secondary cancers (relative risk 3.63), with a slightly increased mortality risk (1.21) according to a 2004 Swedish study of 481 patients.[125]
Epidemiology

Pheochromocytoma is seen in between two and eight in 1,000,000, with approximately 1000 cases diagnosed in United States yearly. It mostly occurs in young or middle age adults, though it presents earlier in hereditary cases.
- About 10% of adrenal cases are bilateral (suggesting hereditary disease)
- About 10% of adrenal cases occur in children (also suggesting hereditary disease)
- About 15% are extra-adrenal (located in any orthosympathetic tissue): Of these 9% are in the abdomen, and 1% are located elsewhere. Some extra-adrenal pheochromocytomas are probably actually paragangliomas, but the distinction can only be drawn after surgical resection.
- About 11.1% of adrenal cases are malignant, but this rises to 30% for extra-adrenal cases
- About 15–20% are hereditary[127]: 1470
- About 5% are caused by VHL disease
- About 3% recur after being resected
- About 14% of affected individuals do not have arterial hypertension (Campbell's Urology)
History
Charles Surgue of Cork (1775-1816). In 1886, Felix Fränkel made the first description of a patient with pheochromocytoma. The term "pheochromocytoma" was first coined by Ludwig Pick, a pathologist, in 1912. In 1926, César Roux (in Switzerland) and Charles Horace Mayo (in the US) were the first surgeons to successfully remove pheochromocytomas.
In the 1970s, Greene and Tischler derived a line of cells, called the PC12 cell line, from a rat pheochromocytoma.[128]
References
- ^ a b Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT (December 1983). "Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979". Mayo Clinic Proceedings. 58 (12): 802–4. PMID 6645626.
- ^ a b Lenders JW, Eisenhofer G, Mannelli M, Pacak K (20–26 August 2005). "Phaeochromocytoma". Lancet. 366 (9486): 665–75. doi:10.1016/S0140-6736(05)67139-5. PMID 16112304. S2CID 208788653.
- ^ Oyasu, Ryoichi; Yang, Ximing J.; Yoshida, Osamu, eds. (2008). "What is the difference between pheochromocytoma and paraganglioma? What are the familial syndromes that have pheochromocytoma as a component? What are the pathologic features of pheochromocytoma indicating malignancy?". Questions in Daily Urologic Practice. Tokyo: Springer Japan. pp. 280–284. doi:10.1007/978-4-431-72819-1_49. ISBN 978-4-431-72819-1.
{{cite book}}:|work=ignored (help); Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. (March 2002). "Biochemical diagnosis of pheochromocytoma: which test is best?". JAMA. 287 (11): 1427–34. doi:10.1001/jama.287.11.1427. PMID 11903030.
- ^ Moore MG, Netterville JL, Mendenhall WM, Isaacson B, Nussenbaum B (April 2016). "Head and Neck Paragangliomas: An Update on Evaluation and Management". Otolaryngology--Head and Neck Surgery. 154 (4): 597–605. doi:10.1177/0194599815627667. PMID 26861230. S2CID 23547346.
- ^ Williams MD (September 2017). "Paragangliomas of the Head and Neck: An Overview from Diagnosis to Genetics". Head and Neck Pathology. 11 (3): 278–287. doi:10.1007/s12105-017-0803-4. PMC 5550402. PMID 28321772.
- ^ a b c Kellerman RD, Rakel D (2020). Conn's Current Therapy. Elsevier–Health Science. ISBN 978-0-323-79006-2. OCLC 1145315791.
- ^ Tevosian SG, Ghayee HK (December 2019). "Pheochromocytomas and Paragangliomas". Endocrinology and Metabolism Clinics of North America. 48 (4): 727–750. doi:10.1016/j.ecl.2019.08.006. PMID 31655773.
- ^ Zuber SM, Kantorovich V, Pacak K (June 2011). "Hypertension in pheochromocytoma: characteristics and treatment". Endocrinology and Metabolism Clinics of North America. 40 (2): 295–311, vii. doi:10.1016/j.ecl.2011.02.002. PMC 3094542. PMID 21565668.
- ^ a b c Manger WM (August 2006). "An overview of pheochromocytoma: history, current concepts, vagaries, and diagnostic challenges". Annals of the New York Academy of Sciences. 1073 (1): 1–20. Bibcode:2006NYASA1073....1M. doi:10.1196/annals.1353.001. PMID 17102067.
- ^ Hosseinnezhad A, Black RM, Aeddula NR, Adhikari D, Trivedi N (2011). "Glucagon-induced pheochromocytoma crisis". Endocrine Practice. 17 (3): e51-4. doi:10.4158/EP10388.CR. PMID 21324811.
- ^ Lanier JB, Mote MB, Clay EC (September 2011). "Evaluation and management of orthostatic hypotension". American Family Physician. 84 (5): 527–36. PMID 21888303.
- ^ Mitchell L, Bellis F (September 2007). "Phaeochromocytoma--"the great mimic": an unusual presentation". Emergency Medicine Journal. 24 (9): 672–3. doi:10.1136/emj.2007.049569. PMC 2464664. PMID 17711956.
- ^ Harrison's principles of internal medicine. Braunwald, Eugene, 1929- (15th ed.). New York: McGraw-Hill. 2001. ISBN 0-07-913686-9. OCLC 44860874.
{{cite book}}: CS1 maint: others (link) - ^ Riester A, Weismann D, Quinkler M, Lichtenauer UD, Sommerey S, Halbritter R, et al. (December 2015). "Life-threatening events in patients with pheochromocytoma". European Journal of Endocrinology. 173 (6): 757–64. doi:10.1530/EJE-15-0483. PMID 26346138.
- ^ a b c d Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A (November 2011). "Cardiovascular manifestations of phaeochromocytoma". Journal of Hypertension. 29 (11): 2049–60. doi:10.1097/HJH.0b013e32834a4ce9. PMID 21826022. S2CID 23444609.
- ^ a b Young WF (December 2007). "Adrenal causes of hypertension: pheochromocytoma and primary aldosteronism". Reviews in Endocrine & Metabolic Disorders. 8 (4): 309–20. doi:10.1007/s11154-007-9055-z. PMID 17914676. S2CID 6009557.
- ^ a b Liao WB, Liu CF, Chiang CW, Kung CT, Lee CW (September 2000). "Cardiovascular manifestations of pheochromocytoma". The American Journal of Emergency Medicine. 18 (5): 622–5. doi:10.1053/ajem.2000.7341. PMID 10999582.
- ^ a b Zhang R, Gupta D, Albert SG (December 2017). "Pheochromocytoma as a reversible cause of cardiomyopathy: Analysis and review of the literature". International Journal of Cardiology. 249: 319–323. doi:10.1016/j.ijcard.2017.07.014. PMID 29121733.
- ^ a b Agrawal S, Shirani J, Garg L, Singh A, Longo S, Longo A, et al. (March 2017). "Pheochromocytoma and stress cardiomyopathy: Insight into pathogenesis". World Journal of Cardiology. 9 (3): 255–260. doi:10.4330/wjc.v9.i3.255. PMC 5368675. PMID 28400922.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Van YH, Wang HS, Lai CH, Lin JN, Lo FS (November 2002). "Pheochromocytoma presenting as stroke in two Taiwanese children". Journal of Pediatric Endocrinology & Metabolism. 15 (9): 1563–7. doi:10.1515/jpem.2002.15.9.1563. PMID 12503867. S2CID 37955071.
- ^ Abourazzak S, Atmani S, Arqam LE, Chaouki S, Labib S, Harrandou M, et al. (May 2010). "Cerebral ischaemic stroke and bilateral pheochromocytoma". BMJ Case Reports. 2010: bcr1220092535. doi:10.1136/bcr.12.2009.2535. PMC 3047554. PMID 22736758.
- ^ Dagartzikas MI, Sprague K, Carter G, Tobias JD (February 2002). "Cerebrovascular event, dilated cardiomyopathy, and pheochromocytoma". Pediatric Emergency Care. 18 (1): 33–5. doi:10.1097/00006565-200202000-00011. PMID 11862137. S2CID 44533238.
- ^ Cohen JK, Cisco RM, Scholten A, Mitmaker E, Duh QY (April 2014). "Pheochromocytoma crisis resulting in acute heart failure and cardioembolic stroke in a 37-year-old man". Surgery. 155 (4): 726–7. doi:10.1016/j.surg.2012.11.013. PMID 23305592.
- ^ Lin PC, Hsu JT, Chung CM, Chang ST (2007). "Pheochromocytoma Underlying Hypertension, Stroke, and Dilated Cardiomyopathy". Texas Heart Institute Journal. 34 (2): 244–6. OCLC 679006463. PMC 1894695. PMID 17622380.
- ^ Buchbinder NA, Yu R, Rosenbloom BE, Sherman CT, Silberman AW (December 2009). "Left ventricular thrombus and embolic stroke caused by a functional paraganglioma". Journal of Clinical Hypertension. 11 (12): 734–7. doi:10.1111/j.1751-7176.2009.00182.x. PMID 20021531.
- ^ Luiz HV, da Silva TN, Pereira BD, Santos JG, Gonçalves D, Manita I, Portugal J (December 2013). "Malignant paraganglioma presenting with hemorrhagic stroke in a child". Pediatrics. 132 (6): e1709-14. doi:10.1542/peds.2013-0492. PMID 24276837. S2CID 7618637.
- ^ Potapova G, Chazova I, Kuznetsov N, Sitina V, Popov E, Gavrilov I (June 2011). "Pheochromocytoma and Stroke". Journal of Hypertension. 29: e505. doi:10.1097/00004872-201106001-01534.
- ^ Anderson NE, Chung K, Willoughby E, Croxson MS (April 2013). "Neurological manifestations of phaeochromocytomas and secretory paragangliomas: a reappraisal". Journal of Neurology, Neurosurgery, and Psychiatry. 84 (4): 452–7. doi:10.1136/jnnp-2012-303028. PMID 23204473. S2CID 207005321.
- ^ a b Shemin D, Cohn PS, Zipin SB (November 1990). "Pheochromocytoma presenting as rhabdomyolysis and acute myoglobinuric renal failure". Archives of Internal Medicine. 150 (11): 2384–5. doi:10.1001/archinte.1990.00390220118024. PMID 2241450.
- ^ Hamada N, Akamatsu A, Joh T (January 1993). "A case of pheochromocytoma complicated with acute renal failure and cardiomyopathy". Japanese Circulation Journal. 57 (1): 84–90. doi:10.1253/jcj.57.84. PMID 8437346.
- ^ Celik H, Celik O, Guldiken S, Inal V, Puyan FO, Tugrul A (February 2014). "Pheochromocytoma presenting with rhabdomyolysis and acute renal failure: a case report". Renal Failure. 36 (1): 104–7. doi:10.3109/0886022X.2013.832856. PMID 24059440. S2CID 2062065.
- ^ Takabatake T, Kawabata M, Ohta H, Yamamoto Y, Ishida Y, Hara H, Hattori N (July 1985). "Acute renal failure and transient, massive proteinuria in a case of pheochromocytoma". Clinical Nephrology. 24 (1): 47–9. PMID 4017298.
- ^ Lorz W, Cottier C, Imhof E, Gyr N (1993). "Multiple organ failure and coma as initial presentation of pheochromocytoma in a patient with multiple endocrine neoplasia (MEN) type II A". Intensive Care Medicine. 19 (4): 235–8. doi:10.1007/BF01694777. PMC 7095150. PMID 8103532.
- ^ Marshall JC (2001). "The multiple organ dysfunction syndrome". In Holzheimer RG, Mannick JA (eds.). Surgical Treatment: Evidence-Based and Problem-Oriented. Munich: Zuckschwerdt. ISBN 978-3-88603-714-8.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Newell KA, Prinz RA, Pickleman J, Braithwaite S, Brooks M, Karson TH, Glisson S (August 1988). "Pheochromocytoma multisystem crisis. A surgical emergency". Archives of Surgery. 123 (8): 956–9. doi:10.1001/archsurg.1988.01400320042007. PMID 2899426.
- ^ a b Fishbein L (February 2016). "Pheochromocytoma and Paraganglioma: Genetics, Diagnosis, and Treatment". Hematology/Oncology Clinics of North America. 30 (1): 135–50. doi:10.1016/j.hoc.2015.09.006. PMID 26614373.
- ^ a b Mercado-Asis LB, Wolf KI, Jochmanova I, Taïeb D (January 2018). "Pheochromocytoma: A Genetic and Diagnostic Update" (PDF). Endocrine Practice. 24 (1): 78–90. doi:10.4158/EP-2017-0057. PMID 29144820.
- ^ a b Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. (June 2014). "Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline". The Journal of Clinical Endocrinology and Metabolism. 99 (6): 1915–42. doi:10.1210/jc.2014-1498. PMID 24893135.
- ^ a b Kavinga Gunawardane PT, Grossman A (October 2017). "The clinical genetics of phaeochromocytoma and paraganglioma". Archives of Endocrinology and Metabolism. 61 (5): 490–500. doi:10.1590/2359-3997000000299. PMID 29166454.
- ^ Jochmanova I, Wolf KI, King KS, Nambuba J, Wesley R, Martucci V, et al. (August 2017). "SDHB-related pheochromocytoma and paraganglioma penetrance and genotype-phenotype correlations". Journal of Cancer Research and Clinical Oncology. 143 (8): 1421–1435. doi:10.1007/s00432-017-2397-3. PMC 5505780. PMID 28374168.
- ^ Lahlou-Laforêt K, Consoli SM, Jeunemaitre X, Gimenez-Roqueplo AP (May 2012). "Presymptomatic genetic testing in minors at risk of paraganglioma and pheochromocytoma: our experience of oncogenetic multidisciplinary consultation". Hormone and Metabolic Research = Hormon- und Stoffwechselforschung = Hormones et Metabolisme. 44 (5): 354–8. doi:10.1055/s-0032-1311568. PMID 22517555.
- ^ Neumann HP, Young WF, Krauss T, Bayley JP, Schiavi F, Opocher G, et al. (August 2018). "65 YEARS OF THE DOUBLE HELIX: Genetics informs precision practice in the diagnosis and management of pheochromocytoma". Endocrine-Related Cancer. 25 (8): T201–T219. doi:10.1530/ERC-18-0085. PMID 29794110.
- ^ Favier J, Amar L, Gimenez-Roqueplo AP (February 2015). "Paraganglioma and phaeochromocytoma: from genetics to personalized medicine". Nature Reviews. Endocrinology. 11 (2): 101–11. doi:10.1038/nrendo.2014.188. PMID 25385035. S2CID 26205361.
- ^ Dahia PL (February 2014). "Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity". Nature Reviews. Cancer. 14 (2): 108–19. doi:10.1038/nrc3648. PMID 24442145. S2CID 31457232.
- ^ Jochmanova I, Pacak K (January 2018). "Genomic Landscape of Pheochromocytoma and Paraganglioma". Trends in Cancer. 4 (1): 6–9. doi:10.1016/j.trecan.2017.11.001. PMC 5819363. PMID 29413423.
- ^ Taïeb D, Yang C, Delenne B, Zhuang Z, Barlier A, Sebag F, Pacak K (May 2013). "First report of bilateral pheochromocytoma in the clinical spectrum of HIF2A-related polycythemia-paraganglioma syndrome". The Journal of Clinical Endocrinology and Metabolism. 98 (5): E908-13. doi:10.1210/jc.2013-1217. PMC 3644612. PMID 23539726.
- ^ Yang C, Sun MG, Matro J, Huynh TT, Rahimpour S, Prchal JT, et al. (March 2013). "Novel HIF2A mutations disrupt oxygen sensing, leading to polycythemia, paragangliomas, and somatostatinomas". Blood. 121 (13): 2563–6. doi:10.1182/blood-2012-10-460972. PMC 3612863. PMID 23361906.
- ^ Pacak K, Jochmanova I, Prodanov T, Yang C, Merino MJ, Fojo T, et al. (May 2013). "New syndrome of paraganglioma and somatostatinoma associated with polycythemia". Journal of Clinical Oncology. 31 (13): 1690–8. doi:10.1200/JCO.2012.47.1912. PMC 3807138. PMID 23509317.
- ^ Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, et al. (September 2012). "Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia". The New England Journal of Medicine. 367 (10): 922–30. doi:10.1056/NEJMoa1205119. PMC 3432945. PMID 22931260.
- ^ Dmitriev PM, Wang H, Rosenblum JS, Prodanov T, Cui J, Pappo AS, et al. (December 2019). "Vascular Changes in the Retina and Choroid of Patients With EPAS1 Gain-of-Function Mutation Syndrome". JAMA Ophthalmology. 138 (2): 148. doi:10.1001/jamaophthalmol.2019.5244. PMC 7042897. PMID 31876943.
- ^ Toledo RA, Qin Y, Cheng ZM, Gao Q, Iwata S, Silva GM, et al. (May 2016). "Recurrent Mutations of Chromatin-Remodeling Genes and Kinase Receptors in Pheochromocytomas and Paragangliomas". Clinical Cancer Research. 22 (9): 2301–10. doi:10.1158/1078-0432.CCR-15-1841. PMC 4854762. PMID 26700204.
- ^ Carney JA (2013). "Carney triad". Frontiers of Hormone Research. 41: 92–110. doi:10.1159/000345672. ISBN 978-3-318-02330-5. PMID 23652673.
- ^ Stratakis CA, Carney JA (July 2009). "The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications". Journal of Internal Medicine. 266 (1): 43–52. doi:10.1111/j.1365-2796.2009.02110.x. PMC 3129547. PMID 19522824.
- ^ Neumann HP, Young WF, Eng C (August 2019). "Pheochromocytoma and Paraganglioma". The New England Journal of Medicine. 381 (6): 552–565. doi:10.1056/NEJMra1806651. PMID 31390501.
- ^ Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. (March 2002). "Biochemical diagnosis of pheochromocytoma: which test is best?". Jama. 287 (11): 1427–34. doi:10.1001/jama.287.11.1427. PMID 11903030.
- ^ a b c d e f Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K (June 2003). "Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results". The Journal of Clinical Endocrinology and Metabolism. 88 (6): 2656–66. doi:10.1210/jc.2002-030005. PMID 12788870.
- ^ Griffin TP, Casey R, Wall D, Bell M, O'Shea PM (August 2016). "Evaluating the optimum rest period prior to blood collection for fractionated plasma free metanephrines analysis". Practical Laboratory Medicine. 5: 39–46. doi:10.1016/j.plabm.2016.05.001. PMC 5574516. PMID 28856203.
- ^ Lenders JW, Willemsen JJ, Eisenhofer G, Ross HA, Pacak K, Timmers HJ, Sweep CG (February 2007). "Is supine rest necessary before blood sampling for plasma metanephrines?". Clinical Chemistry. 53 (2): 352–4. doi:10.1373/clinchem.2006.076489. PMID 17200132.
- ^ a b c Neary NM, King KS, Pacak K (June 2011). "Drugs and pheochromocytoma--don't be fooled by every elevated metanephrine". The New England Journal of Medicine. 364 (23): 2268–70. doi:10.1056/NEJMc1101502#SA1. PMC 4724800. PMID 21651412.
- ^ de Jong WH, Post WJ, Kerstens MN, de Vries EG, Kema IP (June 2010). "Elevated urinary free and deconjugated catecholamines after consumption of a catecholamine-rich diet". The Journal of Clinical Endocrinology and Metabolism. 95 (6): 2851–5. doi:10.1210/jc.2009-2589. PMID 20382681.
- ^ de Jong WH, Eisenhofer G, Post WJ, Muskiet FA, de Vries EG, Kema IP (August 2009). "Dietary influences on plasma and urinary metanephrines: implications for diagnosis of catecholamine-producing tumors". The Journal of Clinical Endocrinology and Metabolism. 94 (8): 2841–9. doi:10.1210/jc.2009-0303. PMID 19567530.
- ^ a b c d e f g Alrezk R, Suarez A, Tena I, Pacak K (2018-11-27). "Update of Pheochromocytoma Syndromes: Genetics, Biochemical Evaluation, and Imaging". Frontiers in Endocrinology. 9: 515. doi:10.3389/fendo.2018.00515. PMID 30538672.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Martucci VL, Pacak K (January 2014). "Pheochromocytoma and paraganglioma: diagnosis, genetics, management, and treatment". Current Problems in Cancer. 38 (1): 7–41. doi:10.1016/j.currproblcancer.2014.01.001. PMID 24636754.
- ^ d'Herbomez M, Do Cao C, Vezzosi D, Borzon-Chasot F, Baudin E (September 2010). "Chromogranin A assay in clinical practice". Annales d'Endocrinologie. 71 (4): 274–80. doi:10.1016/j.ando.2010.04.004. PMID 20538257.
- ^ Grossrubatscher E, Dalino P, Vignati F, Gambacorta M, Pugliese R, Boniardi M, et al. (September 2006). "The role of chromogranin A in the management of patients with phaeochromocytoma". Clinical Endocrinology. 65 (3): 287–93. doi:10.1111/j.1365-2265.2006.02591.x. PMID 16918946.
- ^ Därr R, Lenders JW, Stange K, Kindel B, Hofbauer LC, Bornstein SR, Eisenhofer G (January 2013). "[Diagnosis of pheochromocytoma and paraganglioma: the clonidine suppression test in patients with borderline elevations of plasma free normetanephrine]". Deutsche Medizinische Wochenschrift. 138 (3): 76–81. doi:10.1055/s-0032-1327395. PMID 23299341.
- ^ Kosman ME (July 1975). "Evaluation of clonidine hydrochloride (Catapres). A new antihypertensive agent". JAMA. 233 (2): 174–6. doi:10.1001/jama.1975.03260020060030. PMID 1173448.
- ^ England JF (May 1977). "Clonidine rebound hypertension". The Medical Journal of Australia. 1 (20): 756–7. PMID 875850.
- ^ Geyskes GG, Boer P, Dorhout Mees EJ (January 1979). "Clonidine withdrawal. Mechanism and frequency of rebound hypertension". British Journal of Clinical Pharmacology. 7 (1): 55–62. doi:10.1111/j.1365-2125.1979.tb00897.x. PMC 1429594. PMID 760743.
- ^ Malaty J, Malaty IA (October 2014). "Hypertensive urgency: an important aetiology of rebound hypertension". BMJ Case Reports. 2014. doi:10.1136/bcr-2014-206022. PMC 4208112. PMID 25336552.
- ^ Rao D, Peitzsch M, Prejbisz A, Hanus K, Fassnacht M, Beuschlein F, et al. (August 2017). "Plasma methoxytyramine: clinical utility with metanephrines for diagnosis of pheochromocytoma and paraganglioma". European Journal of Endocrinology. 177 (2): 103–113. doi:10.1530/EJE-17-0077. PMC 5488393. PMID 28476870.
- ^ a b c d e Lenders JW, Eisenhofer G (June 2017). "Update on Modern Management of Pheochromocytoma and Paraganglioma". Endocrinology and Metabolism. 32 (2): 152–161. doi:10.3803/EnM.2017.32.2.152. PMC 5503859. PMID 28685506.
- ^ a b Gupta G, Pacak K (June 2017). "Precision Medicine: An Update on Genotype/Biochemical Phenotype Relationships in Pheochromocytoma/Paraganglioma Patients". Endocrine Practice. 23 (6): 690–704. doi:10.4158/EP161718.RA. PMID 28332883.
- ^ a b c Eisenhofer G, Lenders JW, Goldstein DS, Mannelli M, Csako G, Walther MM, et al. (April 2005). "Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines". Clinical Chemistry. 51 (4): 735–44. doi:10.1373/clinchem.2004.045484. PMID 15718487.
- ^ Histed SN, Lindenberg ML, Mena E, Turkbey B, Choyke PL, Kurdziel KA (April 2012). "Review of functional/anatomical imaging in oncology". Nuclear Medicine Communications. 33 (4): 349–61. doi:10.1097/MNM.0b013e32834ec8a5. PMC 3295905. PMID 22314804.
- ^ Neumann HP, Young WF, Eng C (August 2019). Longo DL (ed.). "Pheochromocytoma and Paraganglioma". The New England Journal of Medicine. 381 (6): 552–565. doi:10.1056/NEJMra1806651. PMID 31390501.
- ^ a b Timmers HJ, Taieb D, Pacak K (May 2012). "Current and future anatomical and functional imaging approaches to pheochromocytoma and paraganglioma". Hormone and Metabolic Research = Hormon- und Stoffwechselforschung = Hormones et Metabolisme. 44 (5): 367–72. doi:10.1055/s-0031-1299712. PMC 4714588. PMID 22399235.
- ^ McCullough PA, Choi JP, Feghali GA, Schussler JM, Stoler RM, Vallabahn RC, Mehta A (September 2016). "Contrast-Induced Acute Kidney Injury". Journal of the American College of Cardiology. 68 (13): 1465–1473. doi:10.1016/j.jacc.2016.05.099. PMID 27659469.
- ^ Caraiani C, Dong Y, Rudd AG, Dietrich CF (December 2018). "Reasons for inadequate or incomplete imaging techniques". Medical Ultrasonography. 20 (4): 498–507. doi:10.11152/mu-1736. PMID 30534659.
- ^ a b Castinetti F, Kroiss A, Kumar R, Pacak K, Taieb D (August 2015). "15 YEARS OF PARAGANGLIOMA: Imaging and imaging-based treatment of pheochromocytoma and paraganglioma". Endocrine-Related Cancer. 22 (4): T135-45. doi:10.1530/ERC-15-0175. PMID 26045470.
- ^ Leung K, Stamm M, Raja A, Low G (February 2013). "Pheochromocytoma: the range of appearances on ultrasound, CT, MRI, and functional imaging". AJR. American Journal of Roentgenology. 200 (2): 370–8. doi:10.2214/AJR.12.9126. PMID 23345359.
- ^ Chaudhary V, Bano S (September 2012). "Anatomical and functional imaging in endocrine hypertension". Indian Journal of Endocrinology and Metabolism. 16 (5): 713–21. doi:10.4103/2230-8210.100659. PMC 3475894. PMID 23087854.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rufini V, Treglia G, Perotti G, Giordano A (January 2013). "The evolution in the use of MIBG scintigraphy in pheochromocytomas and paragangliomas". Hormones. 12 (1): 58–68. doi:10.1007/bf03401287. PMID 23624132. S2CID 4716903.
- ^ van Hulsteijn LT, Niemeijer ND, Dekkers OM, Corssmit EP (April 2014). "(131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis". Clinical Endocrinology. 80 (4): 487–501. doi:10.1111/cen.12341. PMID 24118038.
- ^ Santhanam P, Taïeb D (December 2014). "Role of (18) F-FDOPA PET/CT imaging in endocrinology". Clinical Endocrinology. 81 (6): 789–98. doi:10.1111/cen.12566. PMID 25056984.
- ^ Taïeb D, Tessonnier L, Sebag F, Niccoli-Sire P, Morange I, Colavolpe C, et al. The role of 18F-FDOPA and 18F-FDG-PET in the management of malignant and multifocal phaeochromocytomas. OCLC 798350389.
- ^ Taïeb D, Hicks RJ, Hindié E, Guillet BA, Avram A, Ghedini P, et al. (September 2019). "European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma". European Journal of Nuclear Medicine and Molecular Imaging. 46 (10): 2112–2137. doi:10.1007/s00259-019-04398-1. PMID 31254038. S2CID 195738862.
- ^ Jha A, Ling A, Millo C, Gupta G, Viana B, Lin FI, et al. (May 2018). "18F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx )-related pheochromocytoma and paraganglioma in the pediatric population". European Journal of Nuclear Medicine and Molecular Imaging. 45 (5): 787–797. doi:10.1007/s00259-017-3896-9. PMC 6707509. PMID 29204718.
- ^ Janssen I, Chen CC, Millo CM, Ling A, Taieb D, Lin FI, et al. (September 2016). "PET/CT comparing (68)Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma". European Journal of Nuclear Medicine and Molecular Imaging. 43 (10): 1784–91. doi:10.1007/s00259-016-3357-x. PMID 26996779. S2CID 23005709.
- ^ Kroiss AS, Uprimny C, Shulkin BL, Gruber L, Frech A, Jazbec T, et al. (March 2019). "18F-DOPA PET/CT". Revista Espanola de Medicina Nuclear e Imagen Molecular. 38 (2): 94–99. doi:10.1016/j.remn.2018.09.004. PMID 30630744.
- ^ Mak IY, Hayes AR, Khoo B, Grossman A (2019). "Peptide Receptor Radionuclide Therapy as a Novel Treatment for Metastatic and Invasive Phaeochromocytoma and Paraganglioma". Neuroendocrinology. 109 (4): 287–298. doi:10.1159/000499497. PMID 30856620. S2CID 75140335.
- ^ a b Wiseman, Douglas; Lakis, Mustapha El; Nilubol, Naris (July 2019). "Precision Surgery for Pheochromocytomas and Paragangliomas". Hormone and Metabolic Research = Hormon- Und Stoffwechselforschung = Hormones Et Metabolisme. 51 (7): 470–482. doi:10.1055/a-0926-3618. ISSN 1439-4286. PMID 31307109.
- ^ a b Lenders, Jacques W. M.; Duh, Quan-Yang; Eisenhofer, Graeme; Gimenez-Roqueplo, Anne-Paule; Grebe, Stefan K. G.; Murad, Mohammad Hassan; Naruse, Mitsuhide; Pacak, Karel; Young, William F. (2014-06-01). "Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline". The Journal of Clinical Endocrinology & Metabolism. 99 (6): 1915–1942. doi:10.1210/jc.2014-1498. ISSN 0021-972X.
- ^ Aggeli, Chrysanthi; Nixon, Alexander M.; Parianos, Christos; Vletsis, Georgios; Papanastasiou, Labrini; Markou, Athina; Kounadi, Theodora; Piaditis, Georgrios; Zografos, Georgios N. (October 2017). "Surgery for pheochromocytoma: A 20-year experience of a single institution". Hormones (Athens, Greece). 16 (4): 388–395. doi:10.14310/horm.2002.1759. ISSN 2520-8721. PMID 29518759.
- ^ Goffredo, P.; Adam, M. A.; Thomas, S. M.; Scheri, R. P.; Sosa, J. A.; Roman, S. A. (August 2015). "Patterns of Use and Short-Term Outcomes of Minimally Invasive Surgery for Malignant Pheochromocytoma: A Population-Level Study". World Journal of Surgery. 39 (8): 1966–1973. doi:10.1007/s00268-015-3040-6. ISSN 1432-2323. PMID 25821949.
- ^ Berber, Eren; Mitchell, Jamie; Milas, Mira; Siperstein, Allan (August 2010). "Robotic posterior retroperitoneal adrenalectomy: operative technique". Archives of Surgery (Chicago, Ill.: 1960). 145 (8): 781–784. doi:10.1001/archsurg.2010.148. ISSN 1538-3644. PMID 20713932.
- ^ Aliyev, Shamil; Karabulut, Koray; Agcaoglu, Orhan; Wolf, Katherine; Mitchell, Jamie; Siperstein, Allan; Berber, Eren (December 2013). "Robotic versus laparoscopic adrenalectomy for pheochromocytoma". Annals of Surgical Oncology. 20 (13): 4190–4194. doi:10.1245/s10434-013-3134-z. ISSN 1534-4681. PMID 23864309.
- ^ a b Neumann, Hartmut P. H.; Tsoy, Uliana; Bancos, Irina; Amodru, Vincent; Walz, Martin K.; Tirosh, Amit; Kaur, Ravinder Jeet; McKenzie, Travis; Qi, Xiaoping; Bandgar, Tushar; Petrov, Roman (2 August 2019). "Comparison of Pheochromocytoma-Specific Morbidity and Mortality Among Adults With Bilateral Pheochromocytomas Undergoing Total Adrenalectomy vs Cortical-Sparing Adrenalectomy". JAMA network open. 2 (8): e198898. doi:10.1001/jamanetworkopen.2019.8898. ISSN 2574-3805. PMC 6692838. PMID 31397861.
- ^ Lee, Jeffrey E.; Curley, Steven A.; Gagel, Robert F.; Evans, Douglas B.; Hickey, Robert C. (1996-12-01). "Cortical-sparing adrenalectomy for patients with bilateral pheochromocytoma". Surgery. 120 (6): 1064–1071. doi:10.1016/S0039-6060(96)80056-0. ISSN 0039-6060. PMID 8957496.
- ^ a b c d e f Pacak, Karel (November 2007). "Preoperative management of the pheochromocytoma patient". The Journal of Clinical Endocrinology and Metabolism. 92 (11): 4069–4079. doi:10.1210/jc.2007-1720. ISSN 0021-972X. PMID 17989126.
- ^ a b Wolf, Katherine I.; Santos, Jenn Rachelle U.; Pacak, Karel (January 2019). "WHY TAKE THE RISK? WE ONLY LIVE ONCE: THE DANGERS ASSOCIATED WITH NEGLECTING A PRE-OPERATIVE ALPHA ADRENOCEPTOR BLOCKADE IN PHEOCHROMOCYTOMA PATIENTS". Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 25 (1): 106–108. doi:10.4158/EP-2018-0455. ISSN 1530-891X. PMC 6478021. PMID 30289301.
- ^ Graham, R. M.; Thornell, I. R.; Gain, J. M.; Bagnoli, C.; Oates, H. F.; Stokes, G. S. (1976-11-27). "Prazosin: the first-dose phenomenon". British Medical Journal. 2 (6047): 1293–1294. doi:10.1136/bmj.2.6047.1293. ISSN 0007-1447. PMC 1689975. PMID 793676.
- ^ Kleeman, F. J. (June 1977). "Phenoxybenzamine". The Journal of Urology. 117 (6): 814. doi:10.1016/s0022-5347(17)58643-7. ISSN 0022-5347. PMID 875171.
- ^ Brunaud, Laurent; Boutami, Myriam; Nguyen-Thi, Phi-Linh; Finnerty, Brendan; Germain, Adeline; Weryha, Georges; Fahey, Thomas J.; Mirallie, Eric; Bresler, Laurent; Zarnegar, Rasa (December 2014). "Both preoperative alpha and calcium channel blockade impact intraoperative hemodynamic stability similarly in the management of pheochromocytoma". Surgery. 156 (6): 1410–1417, discussion1417–1418. doi:10.1016/j.surg.2014.08.022. ISSN 1532-7361. PMID 25456922.
- ^ Clark, Barbara K. (1992-05-01). "Beta-adrenergic Blocking Agents: Their Current Status". AACN Advanced Critical Care. 3 (2): 447–460. doi:10.4037/15597768-1992-2016. ISSN 1559-7768.
- ^ Luiz, Henrique Vara; Tanchee, Mary Jane; Pavlatou, Maria G.; Yu, Run; Nambuba, Joan; Wolf, Katherine; Prodanov, Tamara; Wesley, Robert; Adams, Karen; Fojo, Tito; Pacak, Karel (July 2016). "Are patients with hormonally functional phaeochromocytoma and paraganglioma initially receiving a proper adrenoceptor blockade? A retrospective cohort study". Clinical Endocrinology. 85 (1): 62–69. doi:10.1111/cen.13066. ISSN 1365-2265. PMC 4899243. PMID 26998836.
- ^ Sheaves, R.; Chew, S. L.; Grossman, A. B. (January 1995). "The dangers of unopposed beta-adrenergic blockade in phaeochromocytoma". Postgraduate Medical Journal. 71 (831): 58–59. doi:10.1136/pgmj.71.831.58-a. ISSN 0032-5473. PMC 2397901. PMID 7708599.
- ^ van Brummelen, P.; Jie, K.; van Zwieten, P. A. (1986). "α-Adrenergic receptors in human blood vessels". British Journal of Clinical Pharmacology. 21 (Suppl 1): 33S–39S. ISSN 0306-5251. PMC 1400759. PMID 2871855.
- ^ Chruscinski, A.; Brede, M. E.; Meinel, L.; Lohse, M. J.; Kobilka, B. K.; Hein, L. (November 2001). "Differential distribution of beta-adrenergic receptor subtypes in blood vessels of knockout mice lacking beta(1)- or beta(2)-adrenergic receptors". Molecular Pharmacology. 60 (5): 955–962. doi:10.1124/mol.60.5.955. ISSN 0026-895X. PMID 11641423.
- ^ a b Groeben, H.; Nottebaum, B. J.; Alesina, P. F.; Traut, A.; Neumann, H. P.; Walz, M. K. (February 2017). "Perioperative α-receptor blockade in phaeochromocytoma surgery: an observational case series". British Journal of Anaesthesia. 118 (2): 182–189. doi:10.1093/bja/aew392. ISSN 1471-6771. PMID 28100521.
- ^ Lentschener, Claude; Baillard, Christophe; Dousset, Bertrand; Gaujoux, Sebastien (February 2019). "DOGMA IS MADE TO BE BROKEN. WHY ARE WE POSTPONING CURATIVE SURGERY TO ADMINISTER INEFFECTIVE ALPHA ADRENORECEPTOR BLOCKADE IN MOST PATIENTS UNDERGOING PHEOCHROMOCYTOMA REMOVAL?". Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 25 (2): 199. doi:10.4158/1934-2403-25.2.199. ISSN 1530-891X. PMID 30817194.
- ^ Santos, Jenn Rachelle U.; Wolf, Katherine I.; Pacak, Karel (February 2019). "A NECESSITY, NOT A SECOND THOUGHT: PRE-OPERATIVE ALPHA-ADRENOCEPTOR BLOCKADE IN PHEOCHROMOCYTOMA PATIENTS". Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 25 (2): 200–201. doi:10.4158/1934-2403-25.2.200. ISSN 1530-891X. PMID 30817195.
- ^ Challis, B. G.; Casey, R. T.; Simpson, H. L.; Gurnell, M. (February 2017). "Is there an optimal preoperative management strategy for phaeochromocytoma/paraganglioma?". Clinical Endocrinology. 86 (2): 163–167. doi:10.1111/cen.13252. ISSN 1365-2265. PMID 27696513.
- ^ Jiang, Minchun; Ding, Huanyu; Liang, Ying; Tang, Juying; Lin, Ying; Xiang, Kexu; Guo, Ying; Zhang, Shaoling (March 2018). "Preoperative risk factors for haemodynamic instability during pheochromocytoma surgery in Chinese patients". Clinical Endocrinology. 88 (3): 498–505. doi:10.1111/cen.13544. ISSN 1365-2265. PMID 29292527.
- ^ LENTSCHENER, C.; GAUJOUX, S.; THILLOIS, J. M.; DUBOC, D.; BERTHERAT, J.; OZIER, Y.; DOUSSET, B. (April 2009). "Increased arterial pressure is not predictive of haemodynamic instability in patients undergoing adrenalectomy for phaeochromocytoma". Acta Anaesthesiologica Scandinavica. 53 (4): 522–527. doi:10.1111/j.1399-6576.2008.01894.x. ISSN 0001-5172.
- ^ a b Wong, C.; Yu, R. (July 2010). "Preoperative preparation for pheochromocytoma resection: physician survey and clinical practice". Experimental and Clinical Endocrinology & Diabetes: Official Journal, German Society of Endocrinology [and] German Diabetes Association. 118 (7): 400–404. doi:10.1055/s-0029-1225339. ISSN 1439-3646. PMID 19609840.
- ^ a b c Mamilla, Divya; Araque, Katherine A.; Brofferio, Alessandra; Gonzales, Melissa K.; Sullivan, James N.; Nilubol, Naris; Pacak, Karel (07 03, 2019). "Postoperative Management in Patients with Pheochromocytoma and Paraganglioma". Cancers. 11 (7). doi:10.3390/cancers11070936. ISSN 2072-6694. PMC 6678461. PMID 31277296.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ a b Naranjo, Julian; Dodd, Sarah; Martin, Yvette N. (2017-08). "Perioperative Management of Pheochromocytoma". Journal of Cardiothoracic and Vascular Anesthesia. 31 (4): 1427–1439. doi:10.1053/j.jvca.2017.02.023. ISSN 1532-8422. PMID 28392094.
{{cite journal}}: Check date values in:|date=(help) - ^ Aronow, Wilbert S. (2017-05). "Treatment of hypertensive emergencies". Annals of Translational Medicine. 5 (S1): S5–S5. doi:10.21037/atm.2017.03.34. PMC 5440310. PMID 28567387.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Chiang, Han-Liang; Huang, Yu-Chi; Lin, Huey-Shyan; Chan, Min-Ho; Chia, Yuan-Yi (2019). "Hypertension and Postoperative Pain: A Prospective Observational Study". Pain Research & Management. 2019: 8946195. doi:10.1155/2019/8946195. ISSN 1918-1523. PMC 6343159. PMID 30728877.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Iqbal, Ahmed; Heller, Simon (06 2016). "Managing hypoglycaemia". Best Practice & Research. Clinical Endocrinology & Metabolism. 30 (3): 413–430. doi:10.1016/j.beem.2016.06.004. ISSN 1878-1594. PMID 27432075.
{{cite journal}}: Check date values in:|date=(help) - ^ Dungan, Kathleen; Merrill, Jennifer; Long, Clarine; Binkley, Philip (11 27, 2019). "Effect of beta blocker use and type on hypoglycemia risk among hospitalized insulin requiring patients". Cardiovascular Diabetology. 18 (1): 163. doi:10.1186/s12933-019-0967-1. ISSN 1475-2840. PMC 6882013. PMID 31775749.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ Noshiro T, Shimizu K, Watanabe T, Akama H, Shibukawa S, Miura W, et al. (January 2000). "Changes in clinical features and long-term prognosis in patients with pheochromocytoma". American Journal of Hypertension. 13 (1 Pt 1): 35–43. doi:10.1016/S0895-7061(99)00139-9. PMID 10678269.
- ^ Khorram-Manesh A, Ahlman H, Nilsson O, Odén A, Jansson S (June 2004). "Mortality associated with pheochromocytoma in a large Swedish cohort". European Journal of Surgical Oncology. 30 (5): 556–9. doi:10.1016/j.ejso.2004.03.006. PMID 15135486.
- ^ Data and references for pie chart are located at file description page in Wikimedia Commons.
- ^ Goldman, Lee (2011). Goldman's Cecil Medicine (24th ed.). Philadelphia: Elsevier Saunders. pp. 1362. ISBN 978-1437727883.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Greene LA, Tischler AS (July 1976). "Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor". Proceedings of the National Academy of Sciences of the United States of America. 73 (7): 2424–8. Bibcode:1976PNAS...73.2424G. doi:10.1073/pnas.73.7.2424. PMC 430592. PMID 1065897.