Dipeptidyl peptidase-4 inhibitor
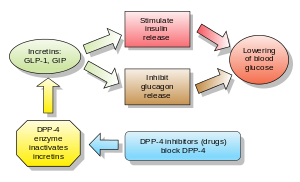
Inhibitors of dipeptidyl peptidase 4 (DPP-4 inhibitors or gliptins) are a class of oral hypoglycemics that block the enzyme dipeptidyl peptidase-4 (DPP-4). They can be used to treat diabetes mellitus type 2.
The first agent of the class – sitagliptin – was approved by the FDA in 2006.[1]
Glucagon increases blood glucose levels, and DPP-4 inhibitors reduce glucagon and blood glucose levels. The mechanism of DPP-4 inhibitors is to increase incretin levels (GLP-1 and GIP),[2][3][4] which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying, and decreases blood glucose levels.
A 2018 meta-analysis found no favorable effect of DPP-4 inhibitors on all-cause mortality, cardiovascular mortality, myocardial infarction or stroke in patients with type 2 diabetes.[5]
Examples
[edit]Drugs belonging to this class are:
- Sitagliptin[6] (FDA approved 2006, marketed by Merck & Co. as Januvia)
- Vildagliptin[7] (EU approved 2007, marketed in the EU by Novartis as Galvus)
- Saxagliptin (FDA approved in 2009, marketed as Onglyza)
- Linagliptin (FDA approved in 2011, marketed as Tradjenta by Eli Lilly and Company and Boehringer Ingelheim)[8]
- Gemigliptin (approved in Korea in 2012, marketed by LG Life Sciences)[9] Marketed as Zemiglo
- Anagliptin (approved in Japan as Suiny in 2012, marketed by Sanwa Kagaku Kenkyusho Co., Ltd. and Kowa Company, Ltd.)[10]
- Teneligliptin (approved in Japan as Tenelia in 2012[11])
- Alogliptin (FDA approved 2013 as Nesina/ Vipidia, marketed by Takeda Pharmaceutical Company)
- Trelagliptin (approved for use in Japan as Zafatek/ Wedica in 2015)
- Omarigliptin (MK-3102) (approved as Marizev in Japan in 2015,[12] developed by Merck & Co.; research showed that omarigliptin can be used as once-weekly treatment and generally well tolerated throughout the base and extension studies[13])
- Evogliptin (approved as Suganon/ Evodine for use in South Korea[14])
- Gosogliptin (approved as Saterex for use in Russia[15])
- Dutogliptin (PHX- 1149 free base, being developed by Phenomix Corporation), Phase III[16]
- Neogliptin[17]
- Retagliptin (SP-2086), approved in China.
- Denagliptin
- Cofrogliptin (HSK- 7653, compound 2)
- Fotagliptin
- Prusogliptin
Other chemicals which may inhibit DPP-4 include:
- Berberine, an alkaloid found in plants of the genus Berberis, inhibits dipeptidyl peptidase-4 which may at least partly explains its antihyperglycemic activity.[18]
Adverse effects
[edit]In those already taking sulphonylureas, there is an increased risk of low blood sugar when taking a medicine in the DPP-4 drug class.[19]
Adverse effects include nasopharyngitis, headache, nausea, heart failure, hypersensitivity and skin reactions.
The U.S. Food and Drug Administration (FDA) is warning that the type 2 diabetes medicines like sitagliptin, saxagliptin, linagliptin, and alogliptin may cause joint pain that can be severe and disabling. FDA has added a new Warning and Precaution about this risk to the labels of all medicines in this drug class, called dipeptidyl peptidase-4 (DPP-4) inhibitors.[20] However, studies assessing risk of rheumatoid arthritis among DPP-4 inhibitor users have been inconclusive.[21]
A 2014 review found increased risk of heart failure with saxagliptin and alogliptin, prompting the FDA in 2016 to add warnings to the relevant drug labels.[22]
A 2018 meta analysis showed that use of DPP-4 inhibitors was associated with a 58% increased risk of developing acute pancreatitis compared with placebo or no treatment.[23]
A 2018 observational study suggested an elevated risk of developing inflammatory bowel disease (specifically, ulcerative colitis), reaching a peak after three to four years of use and decreasing after more than four years of use.[24]
A 2020 Cochrane systematic review did not find enough evidence of reduction of all-cause mortality, serious adverse events, cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke or end-stage renal disease when comparing metformin monotherapy to dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes.[25]
Cancer
[edit]In response to a report of precancerous changes in the pancreases of rats and organ donors treated with the DPP-4 inhibitor sitagliptin,[26][27] the United States FDA and the European Medicines Agency each undertook independent reviews of all clinical and preclinical data related to the possible association of DPP-4 inhibitors with pancreatic cancer. In a joint letter to the New England Journal of Medicine, the agencies stated that they had not yet reached a final conclusion regarding a possible causative relationship.[28]
A 2014 meta-analysis found no evidence for increased pancreatic cancer risk in people treated with DPP-4 inhibitors, but owing to the modest amount of data available, was not able to completely exclude possible risk.[29]
Combination drugs
[edit]Some DPP-4 inhibitor drugs have received approval from the FDA to be used with metformin concomitantly with additive effect to increase the level of glucagon-like peptide 1 (GLP-1) which also decreases hepatic glucose production.[citation needed]
See also
[edit]References
[edit]- ^ "FDA Approves New Treatment for Diabetes" (Press release). U.S. Food and Drug Administration. October 17, 2006. Retrieved 2006-10-17.
- ^ McIntosh CH, Demuth HU, Pospisilik JA, Pederson R (June 2005). "Dipeptidyl peptidase IV inhibitors: how do they work as new antidiabetic agents?". Regulatory Peptides. 128 (2): 159–65. doi:10.1016/j.regpep.2004.06.001. PMID 15780435. S2CID 9151210.
- ^ Behme MT, Dupré J, McDonald TJ (April 2003). "Glucagon-like peptide 1 improved glycemic control in type 1 diabetes". BMC Endocrine Disorders. 3 (1): 3. doi:10.1186/1472-6823-3-3. PMC 154101. PMID 12697069.
- ^ Dupre J, Behme MT, Hramiak IM, McFarlane P, Williamson MP, Zabel P, McDonald TJ (June 1995). "Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM". Diabetes. 44 (6): 626–30. doi:10.2337/diabetes.44.6.626. PMID 7789625.
- ^ Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, Meeran K (April 2018). "Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis". JAMA. 319 (15): 1580–1591. doi:10.1001/jama.2018.3024. PMC 5933330. PMID 29677303.
- ^ Banting and Best Diabetes Centre at UT sitagliptin
- ^ Banting and Best Diabetes Centre at UT vildagliptin
- ^ "FDA approves new treatment for Type 2 diabetes". Fda.gov. 2011-05-02. Retrieved 2013-04-15.
- ^ "LG Life Science". Lgls.com. Archived from the original on 2013-09-06. Retrieved 2013-04-15.
- ^ "New Drugs Approved in FY 2012" (PDF). Archived from the original (PDF) on 2013-07-18. Retrieved 2013-08-07.
- ^ Bronson J, Black A, Dhar TM, Ellsworth BA, Merritt JR (2012). Teneligliptin (Antidiabetic). To Market, To Market. Vol. 48. pp. 523–524. doi:10.1016/b978-0-12-417150-3.00028-4. ISBN 9780124171503.
{{cite book}}:|journal=ignored (help) - ^ "Merck MARIZEV Once-Weekly DPP-4 Inhibitor For Type2 Diabetes Approved In Japan". NASDAQ. 28 September 2015. Retrieved 29 September 2015.
- ^ Sheu WH, Gantz I, Chen M, Suryawanshi S, Mirza A, Goldstein BJ, et al. (November 2015). "Safety and Efficacy of Omarigliptin (MK-3102), a Novel Once-Weekly DPP-4 Inhibitor for the Treatment of Patients With Type 2 Diabetes". Diabetes Care. 38 (11): 2106–14. doi:10.2337/dc15-0109. PMID 26310692.
- ^ "Dong-A ST's DPP4 inhibitor, SUGANON, got approved for type 2 diabetes in Korea". pipelinereview.com. October 2, 2015.
- ^ "SatRx LLC Announces First Registration in Russia of SatRx (gosogliptin), an Innovative Drug for Treatment of Type 2 Diabetes" (Press release). SatRx LLC.
- ^ "Forest Splits With Phenomix", San Diego Business Journal, Tuesday, April 20, 2010 http://www.sdbj.com/news/2010/apr/20/forest-splits-phenomix/
- ^ Maslov IO, Zinevich TV, Kirichenko OG, Trukhan MV, Shorshnev SV, Tuaeva NO, Gureev MA, Dahlén AD, Porozov YB, Schiöth HB, Trukhan VM (February 2022). "Design, Synthesis and Biological Evaluation of Neogliptin, a Novel 2-Azabicyclo[2.2.1]heptane-Based Inhibitor of Dipeptidyl Peptidase-4 (DPP-4)". Pharmaceuticals. 15 (3): 273. doi:10.3390/ph15030273. PMC 8949241. PMID 35337071.
- ^ Al-masri IM, Mohammad MK, Tahaa MO (October 2009). "Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine". Journal of Enzyme Inhibition and Medicinal Chemistry. 24 (5): 1061–6. doi:10.1080/14756360802610761. PMID 19640223. S2CID 25517996.
- ^ Salvo F, Moore N, Arnaud M, Robinson P, Raschi E, De Ponti F, et al. (May 2016). "Addition of dipeptidyl peptidase-4 inhibitors to sulphonylureas and risk of hypoglycaemia: systematic review and meta-analysis". BMJ. 353: i2231. doi:10.1136/bmj.i2231. PMC 4854021. PMID 27142267.
- ^ "DPP-4 Inhibitors for Type 2 Diabetes: Drug Safety Communication - May Cause Severe Joint Pain". FDA. 2015-08-28. Retrieved 1 September 2015.
- ^ Kathe N, Shah A, Said Q, Painter JT (February 2018). "DPP-4 Inhibitor-Induced Rheumatoid Arthritis Among Diabetics: A Nested Case-Control Study". Diabetes Therapy. 9 (1): 141–151. doi:10.1007/s13300-017-0353-5. PMC 5801239. PMID 29236221.
- ^ "Diabetes Meds Containing Saxagliptin and Alogliptin Linked to Increased HF". Pharmacy Practice News. April 2016.
- ^ Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, Meeran K (April 2018). "Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis". JAMA. 319 (15): 1580–1591. doi:10.1001/jama.2018.3024. PMC 5933330. PMID 29677303.
- ^ Abrahami D, Douros A, Yin H, Yu OH, Renoux C, Bitton A, Azoulay L (March 2018). "Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study". BMJ. 360: k872. doi:10.1136/bmj.k872. PMC 5861502. PMID 29563098.
- ^ Gnesin F, Thuesen AC, Kähler LK, Madsbad S, Hemmingsen B, et al. (Cochrane Metabolic and Endocrine Disorders Group) (June 2020). "Metformin monotherapy for adults with type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews. 2020 (6): CD012906. doi:10.1002/14651858.CD012906.pub2. PMC 7386876. PMID 32501595.
- ^ Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, Galasso R, et al. (July 2009). "Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin". Diabetes. 58 (7): 1604–15. doi:10.2337/db09-0058. PMC 2699878. PMID 19403868.
- ^ Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC (July 2013). "Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors". Diabetes. 62 (7): 2595–604. doi:10.2337/db12-1686. PMC 3712065. PMID 23524641.
- ^ Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C (February 2014). "Pancreatic safety of incretin-based drugs--FDA and EMA assessment". The New England Journal of Medicine. 370 (9): 794–7. doi:10.1056/NEJMp1314078. PMID 24571751.
- ^ Monami M, Dicembrini I, Mannucci E (January 2014). "Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: a meta-analysis of randomized clinical trials". Diabetes, Obesity & Metabolism. 16 (1): 48–56. doi:10.1111/dom.12176. PMID 23837679. S2CID 7642027.
