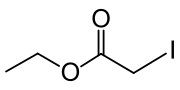
Ethyl iodoacetate
| |
| |
| Names | |
|---|---|
| IUPAC name
ethyl 2-iodoacetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.009.816 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H7IO2 | |
| Molar mass | 214.002 g·mol−1 |
| Density | 1.808 g/mL |
| Boiling point | 179-180 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ethyl iodoacetate is a chemical compound that is a derivative of ethyl acetate. It is a lachrymatory agent.
Like many alkyl iodides, ethyl iodoacetate is an alkylating agent, which makes it useful in organic synthesis, yet toxic.
