Main-group element
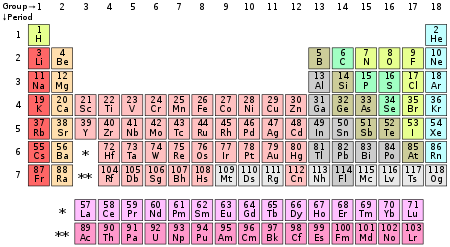
In chemistry and atomic physics, the main group is the group of elements whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon as arranged in the periodic table of the elements. The main group includes the elements (except hydrogen) in groups 1 and 2 (s-block), and groups 13 to 18 (p-block). Group 12 elements are usually considered to be transition metals; however, zinc (Zn), cadmium (Cd), and mercury (Hg) share some properties of both groups, and some scientists believe they should be included in the main group.[1][2]
In older nomenclature the main-group elements are groups IA and IIA, and groups IIIB to 0 (CAS groups IIIA to VIIIA). Group 12 is labelled as group IIB in both systems.
Main-group elements (with some of the lighter transition metals) are the most abundant elements on Earth, in the Solar System, and in the Universe. They are sometimes also called the representative elements.
References
- ^ "Nomenclature of Inorganic Chemistry". International Union of Pure and Applied Chemistry. Retrieved 27 September 2011.
- ^ Jensen, William B. (2003). "The Place of Zinc, Cadmium, and Mercury in the Periodic Table" (PDF). Journal of Chemical Education. 80 (8): 952–561. Bibcode:2003JChEd..80..952J. doi:10.1021/ed080p952.
Bibliography
- Ralf Steudel, "Chemie der Nichtmetalle" (Chemistry of the nonmetals), 2nd Edition. Walter deGruyter, Berlin 1998. ISBN 3-11-012322-3
