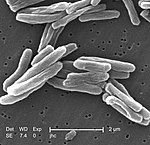
Tuberculosis
| Tuberculosis | |
|---|---|
| Specialty | Infectious diseases, pulmonology |
| Frequency | 0.043—0.045% (Suriname), 0.00033—0.00053% (Iceland), 0.077—0.079% (Ecuador), -0.99—1.01% (Norway), -0.00088—0.00112% (France), 0.0029% (United States of America), 0.0028% |
Tuberculosis, MTB, or TB (short for tubercle bacillus) is a common, and in many cases lethal, infectious disease caused by various strains of mycobacteria, usually Mycobacterium tuberculosis.[1] Tuberculosis typically attacks the lungs but can also affect other parts of the body. It is spread through the air when people who have an active TB infection cough, sneeze, or otherwise transmit their saliva through the air.[2] Most infections are asymptomatic and latent, but about one in ten latent infections eventually progresses to active disease which, if left untreated, kills more than 50% of those so infected.
The classic symptoms of active TB infection are a chronic cough with blood-tinged sputum, fever, night sweats, and weight loss (the latter giving rise to the formerly prevalent term "consumption"). Infection of other organs causes a wide range of symptoms. Diagnosis of active TB relies on radiology (commonly chest X-rays) as well as microscopic examination and microbiological culture of body fluids. Diagnosis of latent TB relies on the tuberculin skin test (TST) and/or blood tests. Treatment is difficult and requires administration of multiple antibiotics over a long period of time. Social contacts are also screened and treated if necessary. Antibiotic resistance is a growing problem in multiple drug-resistant tuberculosis (MDR-TB) infections. Prevention relies on screening programs and vaccination with the bacillus Calmette–Guérin vaccine.
One third of the world's population is thought to have been infected with M. tuberculosis,[3] with new infections occurring at a rate of about one per second.[3] In 2007, there were an estimated 13.7 million chronic active cases globally,[4] while in 2010 there were an estimated 8.8 million new cases and 1.5 million associated deaths, mostly occurring in developing countries.[5] The absolute number of tuberculosis cases has been decreasing since 2006, and new cases have decreased since 2002.[5] The distribution of tuberculosis is not uniform across the globe; about 80% of the population in many Asian and African countries test positive in tuberculin tests, while only 5–10% of the United States population tests positive.[1] More people in the developing world contract tuberculosis because of compromised immunity, largely due to high rates of HIV infection and the corresponding development of AIDS.[6]
Signs and symptoms
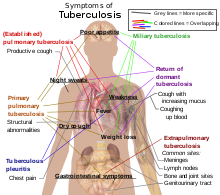
About 5–10% of those without HIV, infected with tuberculosis, develop active disease during their lifetimes.[8] In contrast, 30% of those co-infected with HIV develop active disease.[8] Tuberculosis may infect any part of the body, but most commonly occurs in the lungs (known as pulmonary tuberculosis).[9] Extrapulmonary TB occurs when tuberculosis develops outside of the lungs. Extrapulmonary TB may co-exist with pulmonary TB as well.[9] General signs and symptoms include fever, chills, night sweats, loss of appetite, weight loss, and fatigue,[9] and significant finger clubbing may also occur.[8]
Pulmonary
If a tuberculosis infection does become active, it most commonly involves the lungs (in about 90% of cases).[6][10] Symptoms may include chest pain and a prolonged cough producing sputum. About 25% of people may not have any symptoms (i.e. they remain "asymptomatic").[6] Occasionally, people may cough up blood in small amounts, and in very rare cases the infection may erode into the pulmonary artery, resulting in massive bleeding (Rasmussen's aneurysm). Tuberculosis may become a chronic illness and cause extensive scarring in the upper lobes of the lungs. The upper lung lobes are more frequently affected by tuberculosis than the lower ones.[9] The reason for this difference is not entirely clear.[1] It may be due either to better air flow,[1] or to poor lymph drainage within the upper lungs.[9]
Extrapulmonary
In 15–20% of active cases, the infection spreads outside the respiratory organs, causing other kinds of TB.[11] These are collectively denoted as "extrapulmonary tuberculosis".[12] Extrapulmonary TB occurs more commonly in immunosuppressed persons and young children. In those with HIV this occurs in more than 50% of cases.[12] Notable extrapulmonary infection sites include the pleura (in tuberculous pleurisy), the central nervous system (in tuberculous meningitis), the lymphatic system (in scrofula of the neck), the genitourinary system (in urogenital tuberculosis), and the bones and joints (in Pott's disease of the spine), among others. When it spreads to the bones, it is also known as "osseous tuberculosis".[13] a form of osteomyelitis.[1] A potentially more serious, widespread form of TB is called "disseminated" TB, commonly known as miliary tuberculosis.[9] Miliary TB makes up about 10% of extra pulmonary cases.[14]
Causes
Mycobacteria

The main cause of TB is Mycobacterium tuberculosis, a small, aerobic nonmotile bacillus.[9] The high lipid content of this pathogen accounts for many of its unique clinical characteristics.[15] It divides every 16 to 20 hours, which is an extremely slow rate compared with other bacteria, which usually divide in less than an hour.[16] Mycobacteria have an outer membrane lipid bilayer.[17] If a Gram stain is performed, MTB either stains very weakly "Gram-positive" or does not retain dye as a result of the high lipid and mycolic acid content of its cell wall.[18] MTB can withstand weak disinfectants and survive in a dry state for weeks. In nature, the bacterium can grow only within the cells of a host organism, but M. tuberculosis can be cultured in the laboratory.[19]
Using histological stains on expectorated samples from phlegm (also called "sputum"), scientists can identify MTB under a regular (light) microscope. Since MTB retains certain stains even after being treated with acidic solution, it is classified as an acid-fast bacillus (AFB).[1][18] The most common acid-fast staining techniques are the Ziehl–Neelsen stain, which dyes AFBs a bright red that stands out clearly against a blue background,[20] and the auramine-rhodamine stain followed by fluorescence microscopy.[21]
The M. tuberculosis complex (MTBC) includes four other TB-causing mycobacteria: M. bovis, M. africanum, M. canetti, and M. microti.[22] M. africanum is not widespread, but it is a significant cause of tuberculosis in parts of Africa.[23][24] M. bovis was once a common cause of tuberculosis, but the introduction of pasteurized milk has largely eliminated this as a public health problem in developed countries.[1][25] M. canetti is rare and seems to be limited to the Horn of Africa, although a few cases have been seen in African emigrants.[26][27] M. microti is also rare and is mostly seen in immunodeficient people, although the prevalence of this pathogen has possibly been significantly underestimated.[28]
Other known pathogenic mycobacteria include M. leprae, M. avium, and M. kansasii. The latter two species are classified as "nontuberculous mycobacteria" (NTM). NTM cause neither TB nor leprosy, but they do cause pulmonary diseases that resemble TB.[29]
Risk factors
A number of factors make people more susceptible to TB infections. The most important risk factor globally is HIV; 13% of all TB cases are infected by the virus.[5] This is a particular problem in sub-Saharan Africa, where rates of HIV are high.[30][31] Tuberculosis is closely linked to both overcrowding and malnutrition, making it one of the principal diseases of poverty.[6] Those at high risk thus include: people who inject illicit drugs, inhabitants and employees of locales where vulnerable people gather (e.g. prisons and homeless shelters), medically underprivileged and resource-poor communities, high-risk ethnic minorities, children in close contact with high-risk category patients and health care providers serving these clients.[32] Chronic lung disease is another significant risk factor - with silicosis increasing the risk about 30-fold.[33] Those who smoke cigarettes have nearly twice the risk of TB than non-smokers.[34] Other disease states can also increase the risk of developing tuberculosis, including alcoholism[6] and diabetes mellitus (threefold increase).[35] Certain medications, such as corticosteroids and infliximab (an anti-αTNF monoclonal antibody) are becoming increasingly important risk factors, especially in the developed world.[6] There is also a genetic susceptibility[36] for which overall importance is still undefined.[6]
Mechanism

Transmission
When people with active pulmonary TB cough, sneeze, speak, sing, or spit, they expel infectious aerosol droplets 0.5 to 5 µm in diameter. A single sneeze can release up to 40,000 droplets.[37] Each one of these droplets may transmit the disease, since the infectious dose of tuberculosis is very low (the inhalation of fewer than 10 bacteria may cause an infection).[38]
People with prolonged, frequent, or close contact with people with TB are at particularly high risk of becoming infected, with an estimated 22% infection rate.[39] A person with active but untreated tuberculosis may infect 10–15 (or more) other people per year.[3] Transmission should only occur from people with active TB - those with latent infection are not thought to be contagious.[1] The probability of transmission from one person to another depends upon several factors, including the number of infectious droplets expelled by the carrier, the effectiveness of ventilation, the duration of exposure, the virulence of the M. tuberculosis strain, the level of immunity in the uninfected person, and others.[40] The cascade of person-to-person spread can be circumvented by effectively segregating those with active ("overt") TB and putting them on anti-TB drug regimens. After about two weeks of effective treatment, subjects with non-resistant active infections generally do not remain contagious to others.[39] If someone does become infected, it typically takes three to four weeks before the newly infected person becomes infectious enough to transmit the disease to others.[41]
Pathogenesis
About 90% of those infected with M. tuberculosis have asymptomatic, latent TB infections (sometimes called LTBI),[42] with only a 10% lifetime chance that the latent infection will progress to overt, active tuberculous disease.[43] In those with HIV, the risk of developing active TB increases to nearly 10% a year.[43] If effective treatment is not given, the death rate for active TB cases is up to 66%.[3]
TB infection begins when the mycobacteria reach the pulmonary alveoli, where they invade and replicate within endosomes of alveolar macrophages.[1][44] The primary site of infection in the lungs, known as the "Ghon focus", is generally located in either the upper part of the lower lobe, or the lower part of the upper lobe.[1] Tuberculosis of the lungs may also occur via infection from the blood stream. This is known as a Simon focus and is typically found in the top of the lung.[45] This hematogenous transmission can also spread infection to more distant sites such as peripheral lymph nodes, the kidneys, the brain, and the bones.[1][46] All parts of the body can be affected by the disease, though for unknown reasons it rarely affects the heart, skeletal muscles, pancreas, or thyroid.[47]
Tuberculosis is classified as one of the granulomatous inflammatory diseases. Macrophages, T lymphocytes, B lymphocytes, and fibroblasts are among the cells that aggregate to form granulomas, with lymphocytes surrounding the infected macrophages. The granuloma prevents dissemination of the mycobacteria and provides a local environment for interaction of cells of the immune system. Bacteria inside the granuloma can become dormant, resulting in latent infection. Another feature of the granulomas is the development of abnormal cell death (necrosis) in the center of tubercles. To the naked eye, this has the texture of soft, white cheese and is termed caseous necrosis.[48]
If TB bacteria gain entry to the bloodstream from an area of damaged tissue, they can spread throughout the body and set up many foci of infection, all appearing as tiny, white tubercles in the tissues.[49] This severe form of TB disease, most common in young children and those with HIV, is called miliary tuberculosis.[50] People with this disseminated TB have a high fatality rate even with treatment (about 30%).[14][51]
In many people, the infection waxes and wanes. Tissue destruction and necrosis are often balanced by healing and fibrosis.[48] Affected tissue is replaced by scarring and cavities filled with caseous necrotic material. During active disease, some of these cavities are joined to the air passages bronchi and this material can be coughed up. It contains living bacteria, and so can spread the infection. Treatment with appropriate antibiotics kills bacteria and allows healing to take place. Upon cure, affected areas are eventually replaced by scar tissue.[48]
Diagnosis
Active tuberculosis
Diagnosing active tuberculosis based merely on signs and symptoms is difficult,[52] as is diagnosing the disease in those who are immunosuppressed.[53] A diagnosis of TB should, however, be considered in those with signs of lung disease or constitutional symptoms lasting longer than two weeks.[53] A chest X-ray and multiple sputum cultures for acid-fast bacilli are typically part of the initial evaluation.[53] Interferon-γ release assays and tuberculin skin tests are of little use in the developing world.[54][55] IGRA have similar limitations in those with HIV.[56][57]
A definitive diagnosis of TB is made by identifying M. tuberculosis in a clinical sample (e.g. sputum, pus, or a tissue biopsy). However, the difficult culture process for this slow-growing organism can take two to six weeks for blood or sputum culture.[58] Thus, treatment is often begun before cultures are confirmed.[59]
Nucleic acid amplification tests and adenosine deaminase testing may allow rapid diagnosis of TB.[52] These tests, however, are not routinely recommended, as they rarely alter how a person is treated.[59] Blood tests to detect antibodies are not specific or sensitive, so they are not recommended.[60]
Latent tuberculosis

The Mantoux tuberculin skin test is often used to screen people at high risk for TB.[53] Those who have been previously immunized may have a false-positive test result.[61] The test may be falsely negative in those with sarcoidosis, Hodgkin's lymphoma, malnutrition, or most notably, in those who truly do have active tuberculosis.[1] Interferon gamma release assays (IGRAs), on a blood sample, are recommended in those who are positive to the Mantoux test.[59] These are not affected by immunization or most environmental mycobacteria, so they generate fewer false-positive results.[62] However they are affected by M. szulgai, M. marinum and M. kansasii.[63] IGRAs may increase sensitivity when used in addition to the skin test but may be less sensitive than the skin test when used alone.[64]
Prevention
Tuberculosis prevention and control efforts primarily rely on the vaccination of infants and the detection and appropriate treatment of active cases.[6] The World Health Organization has achieved some success with improved treatment regimens, and a small decrease in case numbers.[6]
Vaccines
The only currently available vaccine as of 2011 is bacillus Calmette–Guérin (BCG) which, while it is effective against disseminated disease in childhood, confers inconsistent protection against contracting pulmonary TB.[65] Nevertheless, it is the most widely used vaccine worldwide, with more than 90% of all children being vaccinated.[6] However, the immunity it induces decreases after about ten years.[6] As tuberculosis is uncommon in most of Canada, the United Kingdom, and the United States, BCG is only administered to people at high risk.[66][67][68] Part of the reasoning arguing against the use of the vaccine is that it makes the tuberculin skin test falsely positive, and therefore, of no use in screening.[68] A number of new vaccines are currently in development.[6]
Public health
The World Health Organization declared TB a "global health emergency" in 1993,[6] and in 2006, the Stop TB Partnership developed a Global Plan to Stop Tuberculosis that aims to save 14 million lives between its launch and 2015.[69] A number of targets they have set are not likely to be achieved by 2015, mostly due to the increase in HIV-associated tuberculosis and the emergence of multiple drug-resistant tuberculosis (MDR-TB).[6] A tuberculosis classification system developed by the American Thoracic Society is used primarily in public health programs.[70]
Management
Treatment of TB uses antibiotics to kill the bacteria. Effective TB treatment is difficult, due to the unusual structure and chemical composition of the mycobacterial cell wall, which hinders the entry of drugs and makes many antibiotics ineffective.[71] The two antibiotics most commonly used are isoniazid and rifampicin, and treatments can be prolonged (months).[40] Latent TB treatment usually employs a single antibiotic,[72] while active TB disease is best treated with combinations of several antibiotics to reduce the risk of the bacteria developing antibiotic resistance.[6] People with latent infections are also treated to prevent them from progressing to active TB disease later in life.[72] Directly observed therapy, i.e. having a health care provider watch the person take their medications, is recommended by the WHO in an effort to reduce the number of people not appropriately taking antibiotics.[73] The evidence to support this practice over people simply taking their medications independently is poor.[74] Methods to remind people of the importance of treatment do however appear effective.[75]
New onset
The recommended treatment of new-onset pulmonary tuberculosis, as of 2010, is six months of a combination of antibiotics containing rifampicin, isoniazid, pyrazinamide and ethambutol for the first two months, and only rifampicin and isoniazid for the last four months.[6] Where resistance to isoniazid is high, ethambutol may be added for the last four months as an alternative.[6]
Recurrent disease
If tuberculosis recurs, testing to determine to which antibiotics it is sensitive is important before determining treatment.[6] If multiple drug-resistant TB (MDR-TB) is detected, treatment with at least four effective antibiotics for 18 to 24 months is recommended.[6]
Medication resistance
Primary resistance occurs when a person becomes infected with a resistant strain of TB. A person with fully susceptible TB may develop secondary (acquired) resistance during therapy because of inadequate treatment, not taking the prescribed regimen appropriately (lack of compliance), or using low-quality medication.[76] Drug-resistant TB is a serious public health issue in many developing countries, as its treatment is longer and requires more expensive drugs. MDR-TB is defined as resistance to the two most effective first-line TB drugs: rifampicin and isoniazid. Extensively drug-resistant TB is also resistant to three or more of the six classes of second-line drugs.[77] Totally drug-resistant TB, which was first observed in 2003 in Italy but not widely reported until 2012, is resistant to all currently used drugs.[78]
Prognosis
Progression from TB infection to overt TB disease occurs when the bacilli overcome the immune system defenses and begin to multiply. In primary TB disease (some 1–5% of cases) this occurs soon after the initial infection.[1] However, in the majority of cases, a latent infection occurs with no obvious symptoms.[1] These dormant bacilli produce active tuberculosis in 5–10% of these latent cases, often many years after infection.[8]
The risk of reactivation increases with immunosuppression, such as that caused by infection with HIV. In people co-infected with M. tuberculosis and HIV, the risk of reactivation increases to 10% per year.[1] Studies using DNA fingerprinting of M. tuberculosis strains have shown that reinfection contributes more substantially to recurrent TB than previously thought,[79] with estimates that it might account for more than 50% of reactivated cases in areas where TB is common.[80] The chance of death from a case of tuberculosis is about 4% as of 2008, down from 8% in 1995.[6]
Epidemiology
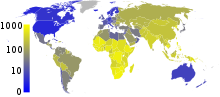
Roughly one third of the world's population has been infected with M. tuberculosis, and new infections occur at a rate of one per second on a global scale.[3] However, most infections with M. tuberculosis do not cause TB disease,[82] and 90–95% of infections remain asymptomatic.[42] In 2007, there were an estimated 13.7 million chronic active cases.[4] In 2010, there were 8.8 million new cases of TB diagnosed, and 1.45 million deaths, most of these occurring in developing countries.[5] Of these 1.45 million deaths, about 0.35 million occur in those coinfected with HIV.[83]
Tuberculosis is the second most common cause of death from infectious disease (after those due to HIV/AIDS).[9] The absolute number of tuberculosis cases ("prevalence") has been decreasing since 2005, while new cases ("incidence") have decreased since 2002.[5] China has achieved particularly dramatic progress, with an approximate 80% reduction in its TB mortality rate between 1990 and 2010.[83] Tuberculosis is more common in developing countries; about 80% of the population in many Asian and African countries test positive in tuberculin tests, while only 5–10% of the US population test positive.[1] Hopes of totally controlling the disease have been dramatically dampened because of a number of factors, including the difficulty of developing an effective vaccine, the expensive and time-consuming diagnostic process, the necessity of many months of treatment, the increase in HIV-associated tuberculosis, and the emergence of drug-resistant cases in the 1980s.[6]

In 2007, the country with the highest estimated incidence rate of TB was Swaziland, with 1,200 cases per 100,000 people. India had the largest total incidence, with an estimated 2.0 million new cases.[4] In developed countries, tuberculosis is less common and is found mainly in urban areas. Rates per 100,000 people in different areas of the world where: globally 178, Africa 332, the Americas 36, Eastern Mediterranean 173, Europe 63, South East Asia 278, and Western Pacific 139 in 2010.[83] In Canada and Australia, tuberculosis is many times more common among the aboriginal peoples, especially in remote areas.[85][86] In the United States the Aborigines have a five fold greater mortality from TB.[87]
The incidence of TB varies with age. In Africa, it primarily affects adolescents and young adults.[88] However, in countries where incidence rates have declined dramatically (such as the United States), TB is mainly a disease of older people and the immunocompromised.[1][89]
History

Tuberculosis has been present in humans since antiquity.[6] The earliest unambiguous detection of M. tuberculosis involves evidence of the disease in the remains of bison dated to approximately 17,000 years ago.[90] However, whether tuberculosis originated in bovines, then was transferred to humans, or whether it diverged from a common ancestor, is currently unclear.[91] A comparison of the genes of M. tuberculosis complex (MTBC) in humans to MTBC in animals suggests that humans did not acquire MTBC from animals during animal domestication, as was previously believed. Both strains of the tuberculosis bacteria share a common ancestor, which could have infected humans as early as the Neolithic Revolution.[92] Skeletal remains show prehistoric humans (4000 BC) had TB, and researchers have found tubercular decay in the spines of Egyptian mummies dating from 3000–2400 BC.[93] "Phthisis" is a Greek word for consumption, an old term for pulmonary tuberculosis;[94] around 460 BC, Hippocrates identified phthisis as the most widespread disease of the times. It was said to involve fever and the coughing up of blood, which was almost always fatal.[95] Genetic studies suggest TB was present in the Americas from about the year 100 AD.[96]
Before the Industrial Revolution, folklore often associated tuberculosis with vampires. When one member of a family died from it, the other infected members would lose their health slowly. People believed this was caused by the original person with TB draining the life from the other family members.[97]
Although the pulmonary form associated with tubercles was established as a pathology by Dr Richard Morton in 1689,[98][99] due to the variety of its symptoms, TB was not identified as a single disease until the 1820s, and was not named tuberculosis until 1839 by J. L. Schönlein.[100] During the years 1838–1845, Dr. John Croghan, the owner of Mammoth Cave, brought a number of people with tuberculosis into the cave in the hope of curing the disease with the constant temperature and purity of the cave air: they died within a year.[101] Hermann Brehmer opened the first TB sanatorium in 1859 in Sokołowsko, Poland.[102]

The bacillus causing tuberculosis, Mycobacterium tuberculosis, was identified and described on 24 March 1882 by Robert Koch. He received the Nobel Prize in physiology or medicine in 1905 for this discovery.[103] Koch did not believe the bovine (cattle) and human tuberculosis diseases were similar, which delayed the recognition of infected milk as a source of infection. Later, the risk of transmission from this source was dramatically reduced by the invention of the pasteurization process. Koch announced a glycerine extract of the tubercle bacilli as a "remedy" for tuberculosis in 1890, calling it 'tuberculin'. While it was not effective, it was later successfully adapted as a screening test for the presence of presymptomatic tuberculosis.[104]
Albert Calmette and Camille Guérin achieved the first genuine success in immunization against tuberculosis in 1906, using attenuated bovine-strain tuberculosis. It was called BCG (bacillus of Calmette and Guérin). The BCG vaccine was first used on humans in 1921 in France,[105] but only received widespread acceptance in the USA, Great Britain, and Germany after World War II.[106]
Tuberculosis caused the most widespread public concern in the 19th and early 20th centuries as an endemic disease of the urban poor. In 1815, one in four deaths in England was due to "consumption". By 1918, one in six deaths in France was still caused by TB. After determining that the disease was contagious in the 1880s, TB was put on a notifiable disease list in Britain, campaigns were started to stop people from spitting in public places, and the infected poor were "encouraged" to enter sanatoria that resembled prisons (the sanatoria for the middle and upper classes offered excellent care and constant medical attention).[102] Whatever the (purported) benefits of the "fresh air" and labor in the sanatoria, even under the best conditions, 50% of those who entered died within five years (ca. 1916).[102]
In Europe, rates of tuberculosis began to rise in the early 1600s to a peak level in the 1800s, when it caused nearly 25% of all deaths.[107] Mortality then decreased nearly 90% by the 1950s.[108] Improvements in public health began significantly reducing rates of tuberculosis even before the arrival of streptomycin and other antibiotics, although the disease remained a significant threat to public health such that when the Medical Research Council was formed in Britain in 1913, its initial focus was tuberculosis research.[109]
In 1946, the development of the antibiotic streptomycin made effective treatment and cure of TB a reality. Prior to the introduction of this drug, the only treatment (except sanatoria) was surgical intervention, including the "pneumothorax technique", which involved collapsing an infected lung to "rest" it and allow tuberculous lesions to heal.[110] The emergence of MDR-TB has again introduced surgery as an option within the generally accepted standard of care in treating TB infections. Current surgical interventions involve removal of pathological chest cavities ("bullae") in the lungs to reduce the number of bacteria and to increase the exposure of the remaining bacteria to drugs in the bloodstream, thereby simultaneously reducing the total bacterial load and increasing the effectiveness of systemic antibiotic therapy.[111] Hopes of completely eliminating TB (cf. smallpox) were dashed after the rise of drug-resistant strains in the 1980s. The subsequent resurgence of tuberculosis resulted in the declaration of a global health emergency by the World Health Organization in 1993.[112]
Society and culture
The World Health Organization and the Bill and Melinda Gates Foundation are subsidizing a new fast-acting diagnostic test for use in low- and middle-income countries.[113][114] Many resource-poor places as of 2011 still only have access to sputum microscopy.[115]
India had the highest total number of TB cases worldwide in 2010, in part due to poor disease management within the private health care sector. Programs such as the Revised National Tuberculosis Control Program are helping to reduce TB levels amongst people receiving public health care.[116][117]
Research
The BCG vaccine has limitations, and research to develop new TB vaccines is ongoing.[118] A number of potential candidates are currently in phase I and II clinical trials.[118] Two main approaches are being used to attempt to improve the efficacy of available vaccines. One approach involves adding a subunit vaccine to BCG, while the other strategy is attempting to create new and better live vaccines.[118]MVA85A, an example of a subunit vaccine which is currently in trials in South Africa, is based on a genetically modified vaccinia virus.[119] Vaccines are hoped to play a significant role in treatment of both latent and active disease.[120]
To encourage further discovery, researchers and policymakers are promoting new economic models of vaccine development, including prizes, tax incentives, and advance market commitments.[121][122] A number of groups, including the Stop TB Partnership,[123] the South African Tuberculosis Vaccine Initiative, and the Aeras Global TB Vaccine Foundation, are involved with research.[124] Among these, the Aeras Global TB Vaccine Foundation received a gift of more than $280 million (US) from the Bill and Melinda Gates Foundation to develop and license an improved vaccine against tuberculosis for use in high burden countries.[125][126]
In other animals
Mycobacteria infect many different animals, including birds,[127] rodents,[128] and reptiles.[129] The subspecies Mycobacterium tuberculosis, though, is rarely present in wild animals.[130] An effort to eradicate bovine tuberculosis caused by Mycobacterium bovis from the cattle and deer herds of New Zealand has been relatively successful.[131] Efforts in Great Britain have been less successful.[132][133]
References
- ^ a b c d e f g h i j k l m n o p q Kumar V, Abbas AK, Fausto N, Mitchell RN (2007). Robbins Basic Pathology (8th ed.). Saunders Elsevier. pp. 516–522. ISBN 978-1-4160-2973-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Konstantinos A (2010). "Testing for tuberculosis". Australian Prescriber. 33 (1): 12–18.
- ^ a b c d e "Tuberculosis Fact sheet N°104". World Health Organization. November 2010. Retrieved 26 July 2011.
- ^ a b c World Health Organization (2009). "Epidemiology". Global tuberculosis control: epidemiology, strategy, financing. pp. 6–33. ISBN 978-92-4-156380-2.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ a b c d e World Health Organization (2011). "The sixteenth global report on tuberculosis" (PDF).
- ^ a b c d e f g h i j k l m n o p q r s t u v Lawn, SD (2 July 2011). "Tuberculosis". Lancet. 378 (9785): 57–72. doi:10.1016/S0140-6736(10)62173-3. PMID 21420161.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Schiffman G (15 January 2009). "Tuberculosis Symptoms". eMedicineHealth.
- ^ a b c d al.], edited by Peter G. Gibson ; section editors, Michael Abramson ... [et (2005). Evidence-based respiratory medicine (1. publ. ed.). Oxford: Blackwell. p. 321. ISBN 978-0-7279-1605-1.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h Dolin, [edited by] Gerald L. Mandell, John E. Bennett, Raphael (2010). Mandell, Douglas, and Bennett's principles and practice of infectious diseases (7th ed.). Philadelphia, PA: Churchill Livingstone/Elsevier. pp. Chapter 250. ISBN 978-0-443-06839-3.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Behera, D. (2010). Textbook of pulmonary medicine (2nd ed. ed.). New Delhi: Jaypee Brothers Medical Pub. p. 457. ISBN 978-81-8448-749-7.
{{cite book}}:|edition=has extra text (help) - ^ Jindal, editor-in-chief SK. Textbook of pulmonary and critical care medicine. New Delhi: Jaypee Brothers Medical Publishers. p. 549. ISBN 978-93-5025-073-0.
{{cite book}}:|first=has generic name (help) - ^ a b Golden MP, Vikram HR (2005). "Extrapulmonary tuberculosis: an overview". American Family Physician. 72 (9): 1761–8. PMID 16300038.
- ^ Kabra, [edited by] Vimlesh Seth, S.K. (2006). Essentials of tuberculosis in children (3rd ed. ed.). New Delhi: Jaypee Bros. Medical Publishers. p. 249. ISBN 978-81-8061-709-6.
{{cite book}}:|edition=has extra text (help);|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ a b Ghosh, editors-in-chief, Thomas M. Habermann, Amit K. (2008). Mayo Clinic internal medicine : concise textbook. Rochester, MN: Mayo Clinic Scientific Press. p. 789. ISBN 978-1-4200-6749-1.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Southwick F (10 December 2007). "Chapter 4: Pulmonary Infections". Infectious Diseases: A Clinical Short Course, 2nd ed. McGraw-Hill Medical Publishing Division. p. 104. ISBN 0-07-147722-5.
{{cite book}}: More than one of|pages=and|page=specified (help) - ^ Jindal, editor-in-chief SK. Textbook of pulmonary and critical care medicine. New Delhi: Jaypee Brothers Medical Publishers. p. 525. ISBN 978-93-5025-073-0.
{{cite book}}:|first=has generic name (help) - ^ Niederweis M, Danilchanka O, Huff J, Hoffmann C, Engelhardt H (2010). "Mycobacterial outer membranes: in search of proteins". Trends in Microbiology. 18 (3): 109–16. doi:10.1016/j.tim.2009.12.005. PMC 2931330. PMID 20060722.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Madison B (2001). "Application of stains in clinical microbiology". Biotechnic & Histochemistry. 76 (3): 119–25. doi:10.1080/714028138. PMID 11475314.
- ^ Parish T, Stoker N (1999). "Mycobacteria: bugs and bugbears (two steps forward and one step back)". Molecular Biotechnology. 13 (3): 191–200. doi:10.1385/MB:13:3:191. PMID 10934532.
- ^ Medical Laboratory Science: Theory and Practice. New Delhi: Tata McGraw-Hill. 2000. p. 473. ISBN 0-07-463223-X.
- ^ Piot, editors, Richard D. Semba, Martin W. Bloem ; foreword by Peter (2008). Nutrition and health in developing countries (2nd ed. ed.). Totowa, NJ: Humana Press. p. 291. ISBN 978-1-934115-24-4.
{{cite book}}:|edition=has extra text (help);|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ van Soolingen D; et al. (1997). "A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa". International Journal of Systematic Bacteriology. 47 (4): 1236–45. doi:10.1099/00207713-47-4-1236. PMID 9336935.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Niemann S; et al. (2002). "Mycobacterium africanum Subtype II Is Associated with Two Distinct Genotypes and Is a Major Cause of Human Tuberculosis in Kampala, Uganda". Journal of Clinical Microbiology. 40 (9): 3398–405. doi:10.1128/JCM.40.9.3398-3405.2002. PMC 130701. PMID 12202584.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Niobe-Eyangoh SN; et al. (2003). "Genetic Biodiversity of Mycobacterium tuberculosis Complex Strains from Patients with Pulmonary Tuberculosis in Cameroon". Journal of Clinical Microbiology. 41 (6): 2547–53. doi:10.1128/JCM.41.6.2547-2553.2003. PMC 156567. PMID 12791879.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Thoen C, Lobue P, de Kantor I (2006). "The importance of Mycobacterium bovis as a zoonosis". Vet. Microbiol. 112 (2–4): 339–45. doi:10.1016/j.vetmic.2005.11.047. PMID 16387455.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Acton, Q. Ashton (2011). Mycobacterium Infections: New Insights for the Healthcare Professional. ScholarlyEditions. p. 1968. ISBN 978-1-4649-0122-5.
- ^ Pfyffer, GE (1998 Oct-Dec). "Mycobacterium canettii, the smooth variant of M. tuberculosis, isolated from a Swiss patient exposed in Africa". Emerging infectious diseases. 4 (4): 631–4. PMID 9866740.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Panteix, G (2010 Aug). "Pulmonary tuberculosis due to Mycobacterium microti: a study of six recent cases in France". Journal of medical microbiology. 59 (Pt 8): 984–9. PMID 20488936.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ American Thoracic Society (1997). "Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association". Am J Respir Crit Care Med. 156 (2 Pt 2): S1–25. PMID 9279284.
- ^ World Health Organization. "Global tuberculosis control–surveillance, planning, financing WHO Report 2006". Retrieved 13 October 2006.
- ^ Chaisson, RE (13 March 2008). "Tuberculosis in Africa--combating an HIV-driven crisis". The New England Journal of Medicine. 358 (11): 1089–92. doi:10.1056/NEJMp0800809. PMID 18337598.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Griffith D, Kerr C (1996). "Tuberculosis: disease of the past, disease of the present". J Perianesth Nurs. 11 (4): 240–5. doi:10.1016/S1089-9472(96)80023-2. PMID 8964016.
- ^ ATS/CDC Statement Committee on Latent Tuberculosis Infection (200). "Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society". MMWR Recomm Rep. 49 (RR–6): 1–51. PMID 10881762.
{{cite journal}}:|author1=has generic name (help); Unknown parameter|month=ignored (help) - ^ van Zyl Smit, RN (2010 Jan). "Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD". The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 35 (1): 27–33. PMID 20044459.
These analyses indicate that smokers are almost twice as likely to be infected with TB and to progress to active disease (RR of ∼1.5 for latent TB infection (LTBI) and RR of ∼2.0 for TB disease). Smokers are also twice as likely to die from TB (RR of ∼2.0 for TB mortality), but data are difficult to interpret because of heterogeneity in the results across studies.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Restrepo, BI (15 August 2007). "Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances". Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 45 (4): 436–8. doi:10.1086/519939. PMC 2900315. PMID 17638190.
- ^ Möller, M (2010 Mar). "Current findings, challenges and novel approaches in human genetic susceptibility to tuberculosis". Tuberculosis (Edinburgh, Scotland). 90 (2): 71–83. doi:10.1016/j.tube.2010.02.002. PMID 20206579.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cole E, Cook C (1998). "Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies". Am J Infect Control. 26 (4): 453–64. doi:10.1016/S0196-6553(98)70046-X. PMID 9721404.
- ^ Nicas M, Nazaroff WW, Hubbard A (2005). "Toward understanding the risk of secondary airborne infection: emission of respirable pathogens". J Occup Environ Hyg. 2 (3): 143–54. doi:10.1080/15459620590918466. PMID 15764538.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Ahmed N, Hasnain S (2011). "Molecular epidemiology of tuberculosis in India: Moving forward with a systems biology approach". Tuberculosis. 91 (5): 407–3. doi:10.1016/j.tube.2011.03.006. PMID 21514230.
- ^ a b "Core Curriculum on Tuberculosis: What the Clinician Should Know" (4th ed.). Centers for Disease Control and Prevention (CDC), Division of Tuberculosis Elimination. 2000, updated August 2003.
{{cite web}}: Check date values in:|year=(help)CS1 maint: year (link) - ^ "Causes of Tuberculosis". Mayo Clinic. 21 December 2006. Retrieved 19 October 2007.
- ^ a b Skolnik, Richard (2011). Global health 101 (2nd ed. ed.). Burlington, MA: Jones & Bartlett Learning. p. 253. ISBN 978-0-7637-9751-5.
{{cite book}}:|edition=has extra text (help) - ^ a b editors, Arch G. Mainous III, Claire Pomeroy, (2009). Management of antimicrobials in infectious diseases : impact of antibiotic resistance (2nd rev. ed. ed.). Totowa, N.J.: Humana. p. 74. ISBN 978-1-60327-238-4.
{{cite book}}:|edition=has extra text (help);|last=has generic name (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Houben E, Nguyen L, Pieters J (2006). "Interaction of pathogenic mycobacteria with the host immune system". Curr Opin Microbiol. 9 (1): 76–85. doi:10.1016/j.mib.2005.12.014. PMID 16406837.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Khan (2011). Essence Of Paediatrics. Elsevier India. p. 401. ISBN 978-81-312-2804-3.
- ^ Herrmann J, Lagrange P (2005). "Dendritic cells and Mycobacterium tuberculosis: which is the Trojan horse?". Pathol Biol (Paris). 53 (1): 35–40. doi:10.1016/j.patbio.2004.01.004. PMID 15620608.
- ^ Agarwal R, Malhotra P, Awasthi A, Kakkar N, Gupta D (2005). "Tuberculous dilated cardiomyopathy: an under-recognized entity?". BMC Infect Dis. 5 (1): 29. doi:10.1186/1471-2334-5-29. PMC 1090580. PMID 15857515.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b c Grosset J (2003). "Mycobacterium tuberculosis in the Extracellular Compartment: an Underestimated Adversary". Antimicrob Agents Chemother. 47 (3): 833–6. doi:10.1128/AAC.47.3.833-836.2003. PMC 149338. PMID 12604509.
- ^ Crowley, Leonard V. (2010). An introduction to human disease : pathology and pathophysiology correlations (8th ed. ed.). Sudbury, Mass.: Jones and Bartlett. p. 374. ISBN 978-0-7637-6591-0.
{{cite book}}:|edition=has extra text (help) - ^ Anthony, Harries (2005). TB/HIV a Clinical Manual (2nd ed.). Geneva: World Health Organization. p. 75. ISBN 978-92-4-154634-8.
- ^ Jacob, JT (2009 Jan). "Acute forms of tuberculosis in adults". The American journal of medicine. 122 (1): 12–7. PMID 19114163.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Bento, J (2011 Jan-Feb). "[Diagnostic tools in tuberculosis]". Acta medica portuguesa. 24 (1): 145–54. PMID 21672452.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d Escalante, P (2009 Jun 2). "In the clinic. Tuberculosis". Annals of internal medicine. 150 (11): ITC61-614, quiz ITV616. PMID 19487708.
{{cite journal}}: Check date values in:|date=(help) - ^ Metcalfe, JZ (2011 Nov 15). "Interferon-γ release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis". The Journal of infectious diseases. 204 Suppl 4: S1120-9. PMID 21996694.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sester, M (2011 Jan). "Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis". The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 37 (1): 100–11. PMID 20847080.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Chen, J (2011). "Interferon-gamma release assays for the diagnosis of active tuberculosis in HIV-infected patients: a systematic review and meta-analysis". PloS one. 6 (11): e26827. PMID 22069472.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sester, M (2011 Jan). "Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis". The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 37 (1): 100–11. PMID 20847080.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Diseases, Special Programme for Research & Training in Tropical (2006). Diagnostics for tuberculosis : global demand and market potential. Geneva: World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases. p. 36. ISBN 978-92-4-156330-7.
- ^ a b c National Institute for Health and Clinical Excellence. Clinical guideline 117: Tuberculosis. London, 2011.
- ^ Steingart, KR (2011 Aug). "Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis". PLoS medicine. 8 (8): e1001062. doi:10.1371/journal.pmed.1001062. PMC 3153457. PMID 21857806.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Rothel J, Andersen P (2005). "Diagnosis of latent Mycobacterium tuberculosis infection: is the demise of the Mantoux test imminent?". Expert Rev Anti Infect Ther. 3 (6): 981–93. doi:10.1586/14787210.3.6.981. PMID 16307510.
- ^ Pai M, Zwerling A, Menzies D (2008). "Systematic Review: T-Cell–based Assays for the Diagnosis of Latent Tuberculosis Infection: An Update". Ann. Intern. Med. 149 (3): 1–9. PMC 2951987. PMID 18593687.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jindal, editor-in-chief SK. Textbook of pulmonary and critical care medicine. New Delhi: Jaypee Brothers Medical Publishers. p. 544. ISBN 978-93-5025-073-0.
{{cite book}}:|first=has generic name (help) - ^ Amicosante, M (2010 Apr). "Rational use of immunodiagnostic tools for tuberculosis infection: guidelines and cost effectiveness studies". The new microbiologica. 33 (2): 93–107. PMID 20518271.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ McShane, H (12 October 2011). "Tuberculosis vaccines: beyond bacille Calmette–Guérin". Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 366 (1579): 2782–9. doi:10.1098/rstb.2011.0097. PMC 3146779. PMID 21893541.
- ^ "Vaccine and Immunizations: TB Vaccine (BCG)". Centers for Disease Control and Prevention. 2011. Retrieved 26 July 2011.
- ^ "BCG Vaccine Usage in Canada - Current and Historical". Public Health Agency of Canada. 2010. Retrieved 30 December 2011.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ a b Teo, SS (2006 Jun). "Does BCG have a role in tuberculosis control and prevention in the United Kingdom?". Archives of Disease in Childhood. 91 (6): 529–31. doi:10.1136/adc.2005.085043. PMC 2082765. PMID 16714729.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "The Global Plan to Stop TB". World Health Organization. 2011. Retrieved 13 June 2011.
- ^ Warrell, ed. by D. J. Weatherall ... [4. + 5. ed.] ed. by David A. (2005). Sections 1 - 10 (4. ed., paperback. ed.). Oxford [u.a.]: Oxford Univ. Press. p. 560. ISBN 978-0-19-857014-1.
{{cite book}}:|first=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ Brennan PJ, Nikaido H (1995). "The envelope of mycobacteria". Annu. Rev. Biochem. 64: 29–63. doi:10.1146/annurev.bi.64.070195.000333. PMID 7574484.
- ^ a b Menzies, D (2011 Mar). "Recent developments in treatment of latent tuberculosis infection". The Indian journal of medical research. 133: 257–66. PMID 21441678.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Arch G., III Mainous (2010). Management of Antimicrobials in Infectious Diseases: Impact of Antibiotic Resistance. Humana Pr. p. 69. ISBN 1-60327-238-0.
- ^ Volmink J, Garner P (2007). "Directly observed therapy for treating tuberculosis". Cochrane Database Syst Rev (4): CD003343. doi:10.1002/14651858.CD003343.pub3. PMID 17943789.
- ^ Liu, Q (2008 Oct 8). "Reminder systems and late patient tracers in the diagnosis and management of tuberculosis". Cochrane database of systematic reviews (Online) (4): CD006594. PMID 18843723.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ O'Brien R (1994). "Drug-resistant tuberculosis: etiology, management and prevention". Semin Respir Infect. 9 (2): 104–12. PMID 7973169.
- ^ Centers for Disease Control and Prevention (CDC) (2006). "Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000–2004". MMWR Morb Mortal Wkly Rep. 55 (11): 301–5. PMID 16557213.
- ^ Maryn McKenna (12 January 2012). "Totally Resistant TB: Earliest Cases in Italy". Wired. Retrieved 12 January 2012.
- ^ Lambert M; et al. (2003). "Recurrence in tuberculosis: relapse or reinfection?". Lancet Infect Dis. 3 (5): 282. doi:10.1016/S1473-3099(03)00607-8. PMID 12726976.
{{cite journal}}: More than one of|pages=and|page=specified (help); Unknown parameter|author-separator=ignored (help) - ^ Wang, JY (15 July 2007). "Prediction of the tuberculosis reinfection proportion from the local incidence". The Journal of infectious diseases. 196 (2): 281–8. doi:10.1086/518898. PMID 17570116.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ World Health Organization (2009). "The Stop TB Strategy, case reports, treatment outcomes and estimates of TB burden". Global tuberculosis control: epidemiology, strategy, financing. pp. 187–300. ISBN 978-92-4-156380-2.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ "Fact Sheets: The Difference Between Latent TB Infection and Active TB Disease". Centers for Disease Control. 20 June 2011. Retrieved 26 July 2011.
- ^ a b c "Global Tuberculosis Control 2011" (PDF). World Health Organization. Retrieved 15 April 2012.
- ^ World Health Organization. "WHO report 2008: Global tuberculosis control". Retrieved 13 April 2009.
- ^ FitzGerald, JM (2000 Feb 8). "Tuberculosis: 13. Control of the disease among aboriginal people in Canada". CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 162 (3): 351–5. PMID 10693593.
{{cite journal}}: Check date values in:|date=(help); Missing pipe in:|journal=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Quah, Stella R.; Carrin, Guy; Buse, Kent; Kristian Heggenhougen (2009). Health Systems Policy, Finance, and Organization. Boston: Academic Press. p. 424. ISBN 0-12-375087-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Anne-Emanuelle Birn (2009). Textbook of International Health: Global Health in a Dynamic World. p. 261. ISBN 9780199885213.
- ^ World Health Organization. "Global Tuberculosis Control Report, 2006 – Annex 1 Profiles of high-burden countries" (PDF). Retrieved 13 October 2006.
- ^ Centers for Disease Control and Prevention (12 September 2006). "2005 Surveillance Slide Set". Retrieved 13 October 2006.
- ^ Rothschild BM; Martin LD; Lev G; et al. (2001). "Mycobacterium tuberculosis complex DNA from an extinct bison dated 17,000 years before the present". Clin. Infect. Dis. 33 (3): 305–11. doi:10.1086/321886. PMID 11438894.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Pearce-Duvet J (2006). "The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease". Biol Rev Camb Philos Soc. 81 (3): 369–82. doi:10.1017/S1464793106007020. PMID 16672105.
- ^ Comas, I (2009 Oct). "The past and future of tuberculosis research". PLoS pathogens. 5 (10): e1000600. PMID 19855821.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zink A, Sola C, Reischl U, Grabner W, Rastogi N, Wolf H, Nerlich A (2003). "Characterization of Mycobacterium tuberculosis Complex DNAs from Egyptian Mummies by Spoligotyping". J Clin Microbiol. 41 (1): 359–67. doi:10.1128/JCM.41.1.359-367.2003. PMC 149558. PMID 12517873.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ The Chambers Dictionary. New Delhi: Allied Chambers India Ltd. 1998. p. 352. ISBN 978-81-86062-25-8.
- ^ Hippocrates. Aphorisms. Accessed 7 October 2006.
- ^ Konomi N, Lebwohl E, Mowbray K, Tattersall I, Zhang D (2002). "Detection of Mycobacterial DNA in Andean Mummies". J Clin Microbiol. 40 (12): 4738–40. doi:10.1128/JCM.40.12.4738-4740.2002. PMC 154635. PMID 12454182.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sledzik, Paul S. (1994). "Bioarcheological and biocultural evidence for the New England vampire folk belief" (PDF). American Journal of Physical Anthropology. 94 (2): 269–274. doi:10.1002/ajpa.1330940210. ISSN 0002-9483. PMID 8085617.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Léon Charles Albert Calmette at Who Named It?
- ^ Trail RR (1970). "Richard Morton (1637-1698)". Med Hist. 14 (2): 166–74. PMC 1034037. PMID 4914685.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Zur Pathogenie der Impetigines. Auszug aus einer brieflichen Mitteilung an den Herausgeber. [Müller’s] Archiv für Anatomie, Physiologie und wissenschaftliche Medicin. 1839, page 82.
- ^ Kentucky: Mammoth Cave long on history. CNN. 27 February 2004. Accessed 8 October 2006.
- ^ a b c McCarthy OR (2001). "The key to the sanatoria". J R Soc Med. 94 (8): 413–7. PMC 1281640. PMID 11461990.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Nobel Foundation. The Nobel Prize in Physiology or Medicine 1905. Accessed 7 October 2006.
- ^ Waddington K (2004). "To stamp out "So Terrible a Malady": bovine tuberculosis and tuberculin testing in Britain, 1890–1939". Med Hist. 48 (1): 29–48. PMC 546294. PMID 14968644.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bonah C (2005). "The 'experimental stable' of the BCG vaccine: safety, efficacy, proof, and standards, 1921–1933". Stud Hist Philos Biol Biomed Sci. 36 (4): 696–721. doi:10.1016/j.shpsc.2005.09.003. PMID 16337557.
- ^ Comstock G (1994). "The International Tuberculosis Campaign: a pioneering venture in mass vaccination and research". Clin Infect Dis. 19 (3): 528–40. doi:10.1093/clinids/19.3.528. PMID 7811874.
- ^ Bloom, editor, Barry R. (1994). Tuberculosis : pathogenesis, protection, and control. Washington, D.C.: ASM Press. ISBN 978-1-55581-072-6.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Persson, Sheryl (2010). Smallpox, Syphilis and Salvation: Medical Breakthroughs That Changed the World. ReadHowYouWant.com. p. 141. ISBN 978-1-4587-6712-7.
- ^ editor, Caroline Hannaway, (2008). Biomedicine in the twentieth century: practices, policies, and politics. Amsterdam: IOS Press. p. 233. ISBN 978-1-58603-832-8.
{{cite book}}:|last=has generic name (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Shields, Thomas (2009). General thoracic surgery (7th ed. ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 792. ISBN 978-0-7817-7982-1.
{{cite book}}:|edition=has extra text (help) - ^ Lalloo UG, Naidoo R, Ambaram A (2006). "Recent advances in the medical and surgical treatment of multi-drug resistant tuberculosis". Curr Opin Pulm Med. 12 (3): 179–85. doi:10.1097/01.mcp.0000219266.27439.52. PMID 16582672.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Frequently asked questions about TB and HIV". World Health Organization. Retrieved 15 April 2012.
- ^ Lawn, SD (2011 Sep). "Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance". Future microbiology. 6 (9): 1067–82. PMID 21958145.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "WHO says Cepheid rapid test will transform TB care". Reuters. 8 December 2010.
- ^ Lienhardt, C (2011 Nov). "What research is needed to stop TB? Introducing the TB Research Movement". PLoS medicine. 8 (11): e1001135. doi:10.1371/journal.pmed.1001135. PMC 3226454. PMID 22140369.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Anurag Bhargava, Lancelot Pinto, Madhukar Pai (2011). "Mismanagement of tuberculosis in India: Causes, consequences, and the way forward". Hypothesis. 9 (1): e7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Amdekar, Y (2009 Jul). "Changes in the management of tuberculosis". Indian journal of pediatrics. 76 (7): 739–42. PMID 19693453.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Martín Montañés, C (2011 Mar). "New tuberculosis vaccines". Enfermedades infecciosas y microbiologia clinica. 29 Suppl 1: 57–62. doi:10.1016/S0213-005X(11)70019-2. PMID 21420568.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ibanga H, Brookes R, Hill P, Owiafe P, Fletcher H, Lienhardt C, Hill A, Adegbola R, McShane H (2006). "Early clinical trials with a new tuberculosis vaccine, MVA85A, in tuberculosis-endemic countries: issues in study design". Lancet Infect Dis. 6 (8): 522–8. doi:10.1016/S1473-3099(06)70552-7. PMID 16870530.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kaufmann SH (2010). "Future vaccination strategies against tuberculosis: Thinking outside the box". Immunity. 33 (4): 567–77. doi:10.1016/j.immuni.2010.09.015. PMID 21029966.
- ^ Webber D, Kremer M (2001). "Stimulating Industrial R&D for Neglected Infectious Diseases: Economic Perspectives" (PDF). Bulletin of the World Health Organization. 79 (8): 693–801.
- ^ Barder O, Kremer M, Williams H (2006). "Advance Market Commitments: A Policy to Stimulate Investment in Vaccines for Neglected Diseases". The Economists' Voice. 3 (3). doi:10.2202/1553-3832.1144.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Economic, Department of (2009). Achieving the global public health agenda : dialogues at the Economic and Social Council. New York: United Nations. p. 103. ISBN 978-92-1-104596-3.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jong, [edited by] Jane N. Zuckerman, Elaine C. (2010). Travelers' vaccines (2nd ed. ed.). Shelton, CT: People's Medical Pub. House. p. 319. ISBN 978-1-60795-045-5.
{{cite book}}:|edition=has extra text (help);|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Bill and Melinda Gates Foundation Announcement (12 February 2004). "Gates Foundation Commits $82.9 Million to Develop New Tuberculosis Vaccines".
- ^ Nightingale, Katherine (19 September 2007). "Gates foundation gives US$280 million to fight TB".
- ^ Shivaprasad, HL (2012 Jan). "Pathology of mycobacteriosis in birds". The veterinary clinics of North America. Exotic animal practice. 15 (1): 41–55, v–vi. PMID 22244112.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Reavill, DR (2012 Jan). "Mycobacterial lesions in fish, amphibians, reptiles, rodents, lagomorphs, and ferrets with reference to animal models". The veterinary clinics of North America. Exotic animal practice. 15 (1): 25–40, v. PMID 22244111.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Mitchell, MA (2012 Jan). "Mycobacterial infections in reptiles". The veterinary clinics of North America. Exotic animal practice. 15 (1): 101–11, vii. PMID 22244116.
{{cite journal}}: Check date values in:|date=(help) - ^ Wobeser, Gary A. (2006). Essentials of disease in wild animals (1st ed. ed.). Ames, Iowa [u.a.]: Blackwell Publ. p. 170. ISBN 978-0-8138-0589-4.
{{cite book}}:|edition=has extra text (help) - ^ Ryan, TJ (2006 Feb 25). "Advances in understanding disease epidemiology and implications for control and eradication of tuberculosis in livestock: the experience from New Zealand". Veterinary microbiology. 112 (2–4): 211–9. PMID 16330161.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ White, PC (2008 Sep). "Control of bovine tuberculosis in British livestock: there is no 'silver bullet'". Trends in microbiology. 16 (9): 420–7. PMID 18706814.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ward, AI (2010 Jan 1). "Farm husbandry and badger behaviour: opportunities to manage badger to cattle transmission of Mycobacterium bovis?". Preventive veterinary medicine. 93 (1): 2–10. PMID 19846226.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)
External links
- Template:Dmoz
- "Tuberculosis (TB)". Centers for Disease Control.
- "Tuberculosis (TB)". UK Health Protection Agency.
Template:Link GA Template:Link FA Template:Link FA Template:Link GA