Atherosclerosis: Difference between revisions
removed redlink and dubious comment |
removing unsourced and syntheses; see Wikipedia_talk:WikiProject_Medicine#The_Hemorheologic-Hemodynamic_Theory_in_Atherosclerosis_looks_like_spam |
||
| Line 357: | Line 357: | ||
The immunomodulation approaches mentioned above, because they deal with innate responses of the host to promote atherosclerosis, have far greater prospects for success. |
The immunomodulation approaches mentioned above, because they deal with innate responses of the host to promote atherosclerosis, have far greater prospects for success. |
||
==The Hemorheologic- |
==The Hemorheologic-hemodynamic theory== |
||
| ⚫ | |||
The '''hemorheologic–hemodynamic theory''' holds that atherosclerosis is a disease of stasis of blood, which promotes the organization of a [[thrombus]] into an [[atherosclerotic plaque]].<ref name="Sloop"/> Stasis of blood predisposes to thrombosis, as described in [[Virchow's triad]]. Risk factors for atherosclerosis create larger areas of decreased shear (flow) by increasing blood viscosity, arterial stiffness, or both. Both of these abnormalities are seen in association with aging, hypertension, diabetes mellitus, cigarette smoking, and obesity.<ref name="Sloop"/><ref name="Perret"/> The hemorheologic-hemodynamic theory posits that the same pathologic process, thrombosis, leads to both plaque development and its complication, superimposed thrombosis and infarction. The name reflects the fact that the interaction of hemorheologic, i.e., blood flow, and hemodynamic, i.e., blood velocity, pulsatility, and arterial geometry, factors lead to atherosclerosis. |
The '''hemorheologic–hemodynamic theory''' holds that atherosclerosis is a disease of stasis of blood, which promotes the organization of a [[thrombus]] into an [[atherosclerotic plaque]].<ref name="Sloop"/> Stasis of blood predisposes to thrombosis, as described in [[Virchow's triad]]. Risk factors for atherosclerosis create larger areas of decreased shear (flow) by increasing blood viscosity, arterial stiffness, or both. Both of these abnormalities are seen in association with aging, hypertension, diabetes mellitus, cigarette smoking, and obesity.<ref name="Sloop"/><ref name="Perret"/> The hemorheologic-hemodynamic theory posits that the same pathologic process, thrombosis, leads to both plaque development and its complication, superimposed thrombosis and infarction. The name reflects the fact that the interaction of hemorheologic, i.e., blood flow, and hemodynamic, i.e., blood velocity, pulsatility, and arterial geometry, factors lead to atherosclerosis. |
||
| ⚫ | Aggressive reduction of serum LDL-cholesterol using high dose statin therapy still leaves significant risk of adverse cardiovascular events in high-risk patients. Despite lowering serum LDL-cholesterol to less than 100 mg/dL, the estimated risk of adverse events in patients with established coronary artery disease is estimated to be 9% per year.<ref name="Hausenloy">Hausenloy DJ, Yellon DM. Targeting residual cardiovascular risk: raising high-density lipoprotein cholesterol levels. Postgraduate Medical Journal 2008;84: 590-8.</ref> The mainstream theory of atherogenesis does not explain the localization of fibrous plaques to the vicinity of changing arterial geometry, such as branches, curves, and dilatations.<ref>Kensey KR, Cho YI. The Origin of Atherosclerosis. Volume 1: An Introduction to Hemodynamics. EPP Medica, 2001, p. 84</ref> Mainstream theory provides no explanation for accelerated atherosclerosis associated with hypertension.<ref name=Perret>Perret RS, Sloop GD. Increased peak blood velocity in association with elevated blood pressure. Ultrasound in Medicine and Biology 2000;26: 1387-91.</ref> Finally, mainstream theory cannot explain the presence of fibrous plaques in synthetic arteriovenous grafts.<ref name ="Sloop3">Sloop GD, Fallon KB, Zieske AW. Atherosclerotic plaque-like lesions in synthetic arteriovenous grafts: implications for atherogenesis. Atherosclerosis 2002;1260: 133-9.</ref> |
||
===Viscosity and localized stasis=== |
===Viscosity and localized stasis=== |
||
| Line 376: | Line 376: | ||
===Insights provided by the hemorheologic–hemodynamic theory=== |
===Insights provided by the hemorheologic–hemodynamic theory=== |
||
| ⚫ | The hemorheologic-hemodynamic theory explains the existence of atherosclerotic plaques in synthetic arteriovenous grafts.<ref name="Sloop3"/> These provide an extreme hemodynamic environment, where extremely high velocity blood flows through a curved vessel. These vessels are prone to thrombosis and development of atherosclerotic plaques despite anticoagulation. These vessels lack a tunica media, which received wisdom maintains is the origin of smooth muscle cells in atherosclerotic plaques via migration. The identification of the fibrocyte provides an alternative explanation for the origin of smooth muscle cells in atherosclerotic plaques.<ref> Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proceedings of the National Academy of Science 2003;100: 4754-9.</ref> Being largely inanimate, the capacity of these vessels to respond to an injury with an inflammatory response, the inciting cause of atherosclerosis according to mainstream atherogenesis theory, would be very limited. Further, this theory explains the benefit of blood donation.<ref>Sloop GD. Possible association of a reduction in cardiovascular events with blood donation. Heart 1998;79:422.</ref> and drinking large quantities of water.<ref>Chan J, Knutsen SF, Blixx GG, Lee JW, Fraser GE. Water, other fluids, and fatal coronary heart disease: the Adventist Health Study. American Journal of Epidemiology 2002;155: 827-33.</ref> |
||
The hemorheologic-hemodynamic theory explains the significant remaining risk of adverse cardiovascular events in patients with established coronary artery disease despite aggressive lowering of LDL-cholesterol using high dose statin therapy. Despite lowering serum LDL-cholesterol to less than 100 mg/dL, the risk of major cardiovascular events in these patients is estimated to be 9% per year.<ref name="Hausenloy"/> Such therapy does not address the adverse consequences of arterial stiffening, or increased blood viscosity caused by other factors. |
|||
| ⚫ | The hemorheologic-hemodynamic theory explains the existence of atherosclerotic plaques in synthetic arteriovenous grafts.<ref name="Sloop3"/> These provide an extreme hemodynamic environment, where extremely high velocity blood flows through a curved vessel. These vessels are prone to thrombosis and development of atherosclerotic plaques despite anticoagulation. These vessels lack a tunica media, which received wisdom maintains is the origin of smooth muscle cells in atherosclerotic plaques via migration. The identification of the fibrocyte provides an alternative explanation for the origin of smooth muscle cells in atherosclerotic plaques.<ref> Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proceedings of the National Academy of Science 2003;100: 4754-9.</ref> Being largely inanimate, the capacity of these vessels to respond to an injury with an inflammatory response, the inciting cause of atherosclerosis according to mainstream atherogenesis theory, would be very limited. Further, this theory explains the benefit of blood donation.<ref>Sloop GD. Possible association of a reduction in cardiovascular events with blood donation. Heart 1998;79:422.</ref> and drinking large quantities of water.<ref>Chan J, Knutsen SF, Blixx GG, Lee JW, Fraser GE. Water, other fluids, and fatal coronary heart disease: the Adventist Health Study. American Journal of Epidemiology 2002;155: 827-33.</ref> |
||
The hemorheologic-hemodynamic eliminates reliance on the fatty streak in atherogenesis. Fatty streaks routinely resolve without sequelae.<ref>Sloop GD, Perret RS, Brahney JS, Oalmann M. A description of two morphologic patterns of aortic fatty streaks, and a hypothesis of their pathogenesis. Atherosclerosis 1998;141: 153-60.</ref><ref>Sloop GD. Insights into the relationship of fatty streaks to raised atherosclerotic lesions provided by the hemorheologic-hemodynamic theroy of atherogenesis. Medical Hypotheses 1998; 385-8.</ref> This is acknowledged in by mainstream atherogenesis theory, which is unable to predict why a particular fatty streak progresses into an atherosclerotic plaque while the majority regress.<ref name="Sloop"/> |
|||
Increased HDL particle size and increased low-shear blood viscosity caused by [[torcetrapib]] therapy could account for the increased cardiovascular mortality seen in clinical trials. |
|||
Increased peak arterial blood velocity and arterial stiffening may also play a role in sudden cardiac death associated with physical exertion. Physical exertion results in increased cardiac output and increased blood pressure, both of which could increase peak arterial velocity, although the effect of increased cardiac output on peak arterial blood velocity in this situation has not been studied. Increased [[Reynolds number]] could lead to acute coronary thrombosis as described above. |
|||
===The future=== |
|||
Validation of the hemorheologic-hemodynamic theory will require a prospective study to determine if measuring blood viscosity and peak arterial blood velocity identifies subjects at high risk for symptomatic atherosclerosis better than routine markers such as lipid analysis. |
|||
==See also== |
==See also== |
||
Revision as of 18:08, 8 March 2010
| Atherosclerosis | |
|---|---|
| Specialty | Cardiology |
Atherosclerosis (also known as arteriosclerotic vascular disease or ASVD) is the condition in which an artery wall thickens as the result of a build-up of fatty materials such as cholesterol. It is a syndrome affecting arterial blood vessels, a chronic inflammatory response in the walls of arteries, in large part due to the accumulation of macrophage white blood cells and promoted by Low-density lipoproteins (plasma proteins that carry cholesterol and triglycerides) without adequate removal of fats and cholesterol from the macrophages by functional high density lipoproteins (HDL), (see apoA-1 Milano). It is commonly referred to as a hardening or furring of the arteries. It is caused by the formation of multiple plaques within the arteries.[1]
The atheromatous plaque is divided into three distinct components:
- The atheroma ("lump of gruel", from ἀθήρα, athera, gruel in Greek,), which is the nodular accumulation of a soft, flaky, yellowish material at the center of large plaques, composed of macrophages nearest the lumen of the artery
- Underlying areas of cholesterol crystals
- Calcification at the outer base of older/more advanced lesions.
The following terms are similar, yet distinct, in both spelling and meaning, and can be easily confused: arteriosclerosis, arteriolosclerosis, and atherosclerosis. Arteriosclerosis is a general term describing any hardening (and loss of elasticity) of medium or large arteries (from the Greek arteria, meaning artery, and sclerosis, meaning hardening); arteriolosclerosis is any hardening (and loss of elasticity) of arterioles (small arteries); atherosclerosis is a hardening of an artery specifically due to an atheromatous plaque. The term atherogenic is used for substances or processes that cause atherosclerosis.
Atherosclerosis, though typically asymptomatic for decades, eventually produces two main problems: First, the atheromatous plaques, though long compensated for by artery enlargement (see IMT), eventually lead to plaque ruptures and clots inside the artery lumen over the ruptures. The clots heal and usually shrink but leave behind stenosis (narrowing) of the artery (both locally and in smaller downstream branches), or worse, complete closure, and, therefore, an insufficient blood supply to the tissues and organ it feeds. Second, if the compensating artery enlargement process is excessive, then a net aneurysm results.
These complications of advanced atherosclerosis are chronic, slowly progressive and cumulative. Most commonly, soft plaque suddenly ruptures (see vulnerable plaque), causing the formation of a thrombus that will rapidly slow or stop blood flow, leading to death of the tissues fed by the artery in approximately 5 minutes. This catastrophic event is called an infarction. One of the most common recognized scenarios is called coronary thrombosis of a coronary artery, causing myocardial infarction (a heart attack). Even worse is the same process in an artery to the brain, commonly called stroke. Another common scenario in very advanced disease is claudication from insufficient blood supply to the legs, typically due to a combination of both stenosis and aneurysmal segments narrowed with clots. Since atherosclerosis is a body-wide process, similar events occur also in the arteries to the brain, intestines, kidneys, legs, etc.
Yet, many infarctions involve only very small amounts of tissue and are termed clinically silent, because the person having the infarction does not notice the problem, does not seek medical help or when they do, physicians do not recognize what has happened.
Causes
Atherosclerosis develops from low-density lipoprotein molecules (LDL) becoming oxidized (ldl-ox) by free radicals, particularly reactive oxygen species (ROS). When oxidized LDL comes in contact with an artery wall, a series of reactions occur to repair the damage to the artery wall caused by oxidized LDL. The LDL molecule is globular shaped with a hollow core to carry cholesterol throughout the body. Cholesterol can move in the bloodstream only by being transported by lipoproteins.
The body's immune system responds to the damage to the artery wall caused by oxidized LDL by sending specialized white blood cells (macrophages and T-lymphocytes) to absorb the oxidized-LDL forming specialized foam cells. Unfortunately, these white blood cells are not able to process the oxidized-LDL, and ultimately grow then rupture, depositing a greater amount of oxidized cholesterol into the artery wall. This triggers more white blood cells, continuing the cycle.
Eventually, the artery becomes inflamed. The cholesterol plaque causes the muscle cells to enlarge and form a hard cover over the affected area. This hard cover is what causes a narrowing of the artery, reduces the blood flow and increases blood pressure.
Some researchers believe that atherosclerosis may be caused by an infection of the vascular smooth muscle cells. Chickens, for example, develop atherosclerosis when infected with the Marek's disease herpesvirus.[2] Herpesvirus infection of arterial smooth muscle cells has been shown to cause cholesteryl ester (CE) accumulation.[3] Cholesteryl ester accumulation is associated with atherosclerosis.
Also, cytomegalovirus (CMV) infection is associated with cardiovascular diseases[4].
Symptoms
Atherosclerosis typically begins in early adolescence, and is usually found in most major arteries, yet is asymptomatic and not detected by most diagnostic methods during life. Atheroma in arm, or more often in leg arteries, which produces decreased blood flow is called peripheral artery occlusive disease (PAOD).
According to United States data for the year 2004, for about 65% of men and 47% of women, the first symptom of atherosclerotic cardiovascular disease is heart attack or sudden cardiac death (death within one hour of onset of the symptom).
Most artery flow disrupting events occur at locations with less than 50% lumen narrowing (~20% stenosis is average). [The reader might reflect that the illustration above, like most illustrations of arterial disease, overemphasizes lumen narrowing, as opposed to compensatory external diameter enlargement (at least within smaller arteries, e.g., heart arteries) typical of the atherosclerosis process as it progresses, see Glagov[5] and the ASTEROID trial,[6] the IVUS photographs on page 8, as examples for a more accurate understanding. The relative geometry error within the illustration is common to many older illustrations, an error slowly being more commonly recognized within the last decade.]
Cardiac stress testing, traditionally the most commonly performed non-invasive testing method for blood flow limitations, in general, detects only lumen narrowing of ~75% or greater, although some physicians claim that nuclear stress methods can detect as little as 50%.
Atherogenesis
Atherogenesis is the developmental process of atheromatous plaques. It is characterized by a remodeling of arteries involving the concomitant accumulation of fatty substances called plaques. One recent theory suggests that, for unknown reasons, leukocytes, such as monocytes or basophils, begin to attack the endothelium of the artery lumen in cardiac muscle. The ensuing inflammation leads to formation of atheromatous plaques in the arterial tunica intima, a region of the vessel wall located between the endothelium and the tunica media. The bulk of these lesions is made of excess fat, collagen, and elastin. At first, as the plaques grow, only wall thickening occurs without any narrowing, stenosis of the artery opening, called the lumen; stenosis is a late event, which may never occur and is often the result of repeated plaque rupture and healing responses, not just the atherosclerosis process by itself.
A 4 minute animation of the atherosclerosis process, entitled "Pathogenesis of Acute MI", commissioned by Paul M. Ridker, MD, MPH, FACC, FAHA, at the Harvard Medical School, can be viewed at pri-med.com [1]. While the animation contains a few technical errors, partly due to the great difficulty/complexity of making a truly accurate presentation, it is better than most and correctly illustrates the principal issues.
Cellular
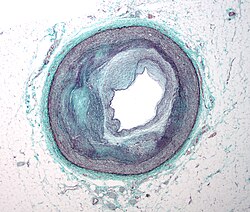
The first step of atherogenesis is the development of so called "fatty streaks", which are small sub-endothelial deposits of monocyte-derived macrophages. The primary documented driver of this process is oxidized Lipoprotein particles within the wall, beneath the endothelial cells, though upper normal or elevated concentrations of blood glucose also plays a major role and not all factors are fully understood. Fatty streaks may appear and disappear.
Low Density Lipoprotein particles in blood plasma, when they invade the endothelium and become oxidized creates a risk for cardiovascular disease. A complex set of biochemical reactions regulates the oxidation of LDL, chiefly stimulated by presence of enzymes, e.g. Lp-LpA2 and free radicals in the endothelium or blood vessel lining.
The initial damage to the blood vessel wall results in a "call for help," an inflammatory response. Monocytes (a type of white blood cell) enter the artery wall from the bloodstream, with platelets adhering to the area of insult. This may be promoted by redox signaling induction of factors such as VCAM-1, which recruit circulating monocytes. The monocytes differentiate macrophages, which ingest oxidized LDL, slowly turning into large "foam cells" – so-described because of their changed appearance resulting from the numerous internal cytoplasmic vesicles and resulting high lipid content. Under the microscope, the lesion now appears as a fatty streak. Foam cells eventually die, and further propagate the inflammatory process. There is also smooth muscle proliferation and migration from tunica media to intima responding to cytokines secreted by damaged endothelial cells. This would cause the formation of a fibrous capsule covering the fatty streak.
Calcification and lipids
Intracellular microcalcifications form within vascular smooth muscle cells of the surrounding muscular layer, specifically in the muscle cells adjacent to the atheromas. In time, as cells die, this leads to extracellular calcium deposits between the muscular wall and outer portion of the atheromatous plaques. A similar form of an intramural calcification, presenting the picture of an early phase of arteriosclerosis, appears to be induced by a number of drugs that have an antiproliferative mechanism of action (Rainer Liedtke 2008).
Cholesterol is delivered into the vessel wall by cholesterol-containing low-density lipoprotein (LDL) particles. To attract and stimulate macrophages, the cholesterol must be released from the LDL particles and oxidized, a key step in the ongoing inflammatory process. The process is worsened if there is insufficient high-density lipoprotein (HDL), the lipoprotein particle that removes cholesterol from tissues and carries it back to the liver.
The foam cells and platelets encourage the migration and proliferation of smooth muscle cells, which in turn ingest lipids, become replaced by collagen and transform into foam cells themselves. A protective fibrous cap normally forms between the fatty deposits and the artery lining (the intima).
These capped fatty deposits (now called 'atheromas') produce enzymes that cause the artery to enlarge over time. As long as the artery enlarges sufficiently to compensate for the extra thickness of the atheroma, then no narrowing ("stenosis") of the opening ("lumen") occurs. The artery becomes expanded with an egg-shaped cross-section, still with a circular opening. If the enlargement is beyond proportion to the atheroma thickness, then an aneurysm is created.[5]
Visible features

Although arteries are not typically studied microscopically, two plaque types can be distinguished[2]:
- The fibro-lipid (fibro-fatty) plaque is characterized by an accumulation of lipid-laden cells underneath the intima of the arteries, typically without narrowing the lumen due to compensatory expansion of the bounding muscular layer of the artery wall. Beneath the endothelium there is a "fibrous cap" covering the atheromatous "core" of the plaque. The core consists of lipid-laden cells (macrophages and smooth muscle cells) with elevated tissue cholesterol and cholesterol ester content, fibrin, proteoglycans, collagen, elastin, and cellular debris. In advanced plaques, the central core of the plaque usually contains extracellular cholesterol deposits (released from dead cells), which form areas of cholesterol crystals with empty, needle-like clefts. At the periphery of the plaque are younger "foamy" cells and capillaries. These plaques usually produce the most damage to the individual when they rupture.
- The fibrous plaque is also localized under the intima, within the wall of the artery resulting in thickening and expansion of the wall and, sometimes, spotty localized narrowing of the lumen with some atrophy of the muscular layer. The fibrous plaque contains collagen fibers (eosinophilic), precipitates of calcium (hematoxylinophilic) and, rarely, lipid-laden cells.
In effect, the muscular portion of the artery wall forms small aneurysms just large enough to hold the atheroma that are present. The muscular portion of artery walls usually remain strong, even after they have remodeled to compensate for the atheromatous plaques.
However, atheromas within the vessel wall are soft and fragile with little elasticity. Arteries constantly expand and contract with each heartbeat, i.e., the pulse. In addition, the calcification deposits between the outer portion of the atheroma and the muscular wall, as they progress, lead to a loss of elasticity and stiffening of the artery as a whole.
The calcification deposits, after they have become sufficiently advanced, are partially visible on coronary artery computed tomography or electron beam tomography (EBT) as rings of increased radiographic density, forming halos around the outer edges of the atheromatous plaques, within the artery wall. On CT, >130 units on the Hounsfield scale (some argue for 90 units) has been the radiographic density usually accepted as clearly representing tissue calcification within arteries. These deposits demonstrate unequivocal evidence of the disease, relatively advanced, even though the lumen of the artery is often still normal by angiographic or intravascular ultrasound.
Rupture and stenosis
Although the disease process tends to be slowly progressive over decades, it usually remains asymptomatic until an atheroma ulcerates which leads to immediate blood clotting at the site of atheroma ulcer. This triggers a cascade of events that leads to clot enlargement which may quickly obstruct the lumen (opening) of the artery itself. A complete blockage leads to ischemia of the myocardial (heart) muscle and damage. This process is the myocardial infarction or "heart attack."
If the heart attack is not fatal, fibrous organization of the clot within the lumen ensues, covering the rupture but also producing stenosis or closure of the lumen, or over time and after repeated ruptures, resulting in a persistent, usually localized stenosis or blockage of the artery lumen. Stenoses can be slowly progressive, whereas plaque ulceration is a sudden event that occurs specifically in atheromas with thinner/weaker fibrous caps that have become "unstable."
Repeated plaque ruptures, ones not resulting in total lumen closure, combined with the clot patch over the rupture and healing response to stabilize the clot, is the process that produces most stenoses over time. The stenotic areas tend to become more stable, despite increased flow velocities at these narrowings. Most major blood-flow-stopping events occur at large plaques, which, prior to their rupture, produced very little if any stenosis.
From clinical trials, 20% is the average stenosis at plaques that subsequently rupture with resulting complete artery closure. Most severe clinical events do not occur at plaques that produce high-grade stenosis. From clinical trials, only 14% of heart attacks occur from artery closure at plaques producing a 75% or greater stenosis prior to the vessel closing.
If the fibrous cap separating a soft atheroma from the bloodstream within the artery ruptures, tissue fragments are exposed and released, and blood enters the atheroma within the wall and sometimes results in a sudden expansion of the atheroma size. Tissue fragments are very clot-promoting, containing collagen and tissue factor; they activate platelets and activate the system of coagulation. The result is the formation of a thrombus (blood clot) overlying the atheroma, which obstructs blood flow acutely. With the obstruction of blood flow, downstream tissues are starved of oxygen and nutrients. If this is the myocardium (heart muscle), angina (cardiac chest pain) or myocardial infarction (heart attack) develops.
Diagnosis of plaque-related disease

Areas of severe narrowing, stenosis, detectable by angiography, and to a lesser extent "stress testing" have long been the focus of human diagnostic techniques for cardiovascular disease, in general. However, these methods focus on detecting only severe narrowing, not the underlying atherosclerosis disease. As demonstrated by human clinical studies, most severe events occur in locations with heavy plaque, yet little or no lumen narrowing present before debilitating events suddenly occur. Plaque rupture can lead to artery lumen occlusion within seconds to minutes, and potential permanent debility and sometimes sudden death.
Plaques that have ruptured are called complicated plaques. The lipid matrix breaks through the thinning collagen gap and when the lipids come in contact with the blood, clotting occurs. After rupture the platelet adhesion causes the clotting cascade to contact with the lipid pool causing a thrombus to form. This thrombus will eventually grow and travel throughout the body. The thrombus will travel through different arteries and veins and eventually become lodged in an area that narrows. Once the area is blocked, blood and oxygen will not be able to supply the vessels and will cause death of cells and lead to necrosis and poisoning. Serious complicated plaques can cause death of organ tissues, causing serious complications to that organ system.
Greater than 75% lumen stenosis used to be considered by cardiologists as the hallmark of clinically significant disease because it is typically only at this severity of narrowing of the larger heart arteries that recurring episodes of angina and detectable abnormalities by stress testing methods are seen. However, clinical trials have shown that only about 14% of clinically-debilitating events occur at locations with this, or greater severity of narrowing. The majority of events occur due to atheroma plaque rupture at areas without narrowing sufficient enough to produce any angina or stress test abnormalities. Thus, since the later-1990s, greater attention is being focused on the "vulnerable plaque."[7]
Though any artery in the body can be involved, usually only severe narrowing or obstruction of some arteries, those that supply more critically-important organs are recognized. Obstruction of arteries supplying the heart muscle result in a heart attack. Obstruction of arteries supplying the brain result in a stroke. These events are life-changing, and often result in irreversible loss of function because lost heart muscle and brain cells do not grow back to any significant extent, typically less than 2%.
Over the last couple of decades, methods other than angiography and stress-testing have been increasingly developed as ways to better detect atherosclerotic disease before it becomes symptomatic. These have included both (a) anatomic detection methods and (b) physiologic measurement methods.
Examples of anatomic methods include: (1) coronary calcium scoring by CT, (2) carotid IMT (intimal media thickness) measurement by ultrasound, and (3) IVUS.
Examples of physiologic methods include: (1) lipoprotein subclass analysis, (2) HbA1c, (3) hs-CRP, and (4) homocysteine.
The example of the metabolic syndrome combines both anatomic (abdominal girth) and physiologic (blood pressure, elevated blood glucose) methods.
Advantages of these two approaches: The anatomic methods directly measure some aspect of the actual atherosclerotic disease process itself, thus offer potential for earlier detection, including before symptoms start, disease staging and tracking of disease progression. The physiologic methods are often less expensive and safer and changing them for the better may slow disease progression, in some cases with marked improvement.
Disadvantages of these two approaches: The anatomic methods are generally more expensive and several are invasive, such as IVUS. The physiologic methods do not quantify the current state of the disease or directly track progression. For both, clinicians and third party payers have been slow to accept the usefulness of these newer approaches.
Physiologic factors that increase risk
Various anatomic, physiological & behavioral risk factors for atherosclerosis are known.[8] These can be divided into various categories: congenital vs acquired, modifiable or not, classical or non-classical. The points labelled '+' in the following list form the core components of "metabolic syndrome".
Factors add to each other multiplicatively, with two factors increasing the risk of atherosclerosis fourfold.[9] Hyperlipidemia, hypertension and cigarette smoking together increases the risk seven times.[9]
Modifiable
- Having diabetes[9] or Impaired glucose tolerance (IGT) +
- Dyslipoproteinemia[9] (unhealthy patterns of serum proteins carrying fats & cholesterol): +
- High serum concentration of low-density lipoprotein (LDL, "bad if elevated concentrations and small"), and / or very low density lipoprotein (VLDL) particles, i.e., "lipoprotein subclass analysis"
- Low serum concentration of functioning high density lipoprotein (HDL "protective if large and high enough" particles), i.e., "lipoprotein subclass analysis"
- An LDL:HDL ratio greater than 3:1
- Tobacco smoking, increases risk by 200% after several pack years [9]
- Having high blood pressure +, on its own increasing risk by 60%[9]
- Elevated serum C-reactive protein concentrations[9][10]
Nonmodifiable
- Advanced age [9]
- Male sex[9]
- Having close relatives who have had some complication of atherosclerosis (eg. coronary heart disease or stroke)[9]
- Genetic abnormalities,[9] e.g. familial hypercholesterolemia
Lesser or uncertain
The following factors are of relatively lesser importance, are uncertain or nonquantitated:
- Being obese[9] (in particular central obesity, also referred to as abdominal or male-type obesity) +
- A sedentary lifestyle[9]
- Postmenopausal estrogen deficiency[9]
- High carbohydrate intake[9]
- Intake of trans fat[9]
- Elevated serum levels of triglycerides +
- Elevated serum levels of homocysteine
- Elevated serum levels of uric acid (also responsible for gout)
- Elevated serum fibrinogen concentrations
- Elevated serum lipoprotein(a) concentrations [9]
- Chronic systemic inflammation as reflected by upper normal WBC concentrations, elevated hs-CRP and many other blood chemistry markers, most only research level at present, not clinically done.[11]
- Stress[9] or symptoms of clinical depression
- Hyperthyroidism (an over-active thyroid)
- Elevated serum insulin levels + [12]
- Short sleep duration [13]
- Chlamydia pneumoniae infection[9]
Dietary risk factors
The relation between dietary fat and atherosclerosis is a contentious field. The USDA, in its food pyramid, promotes a low-fat diet, based largely on its view that fat in the diet is atherogenic. The American Heart Association, the American Diabetes Association and the National Cholesterol Education Program make similar recommendations. In contrast, Prof Walter Willett (Harvard School of Public Health, PI of the second Nurses' Health Study) recommends much higher levels, especially of monounsaturated and polyunsaturated fat.[14] Writing in Science, Gary Taubes detailed that political considerations played into the recommendations of government bodies.[15] These differing views reach a consensus, though, against consumption of trans fats.
The role of dietary oxidized fats / lipid peroxidation (rancid fats) in humans is not clear. Laboratory animals fed rancid fats develop atherosclerosis. Rats fed DHA-containing oils experienced marked disruptions to their antioxidant systems, as well as accumulated significant amounts of peroxide in their blood, livers and kidneys.[16] In another study, rabbits fed atherogenic diets containing various oils were found to undergo the greatest amount of oxidative susceptibility of LDL via polyunsaturated oils.[17] In a study involving rabbits fed heated soybean oil, "grossly induced atherosclerosis and marked liver damage were histologically and clinically demonstrated".[18]
Rancid fats and oils taste very bad even in small amounts; people avoid eating them.[19] It is very difficult to measure or estimate the actual human consumption of these substances.[20] In addition, the majority of oils consumed in the United States are refined, bleached, deodorized and degummed by manufacturers. The resultant oils are colorless, odorless, tasteless and have a longer shelf life than their unrefined counterparts.[21] This extensive processing serves to make peroxidated, rancid oils much more elusive to detection via the various human senses than the unprocessed alternatives.
The French paradox is the observation that despite having a diet similar to those United States in terms of fat intake, rates of heart disease are lower in France. There is evidence to suggest the French paradox is due to underestimation of the rates of heart disease in France.[22]
Prognosis
Lipoprotein imbalances, upper normal and especially elevated blood sugar, i.e., diabetes and high blood pressure are risk factors for atherosclerosis; homocysteine, stopping smoking, taking anticoagulants (anti-clotting agents), which target clotting factors, taking omega-3 oils from fatty fish or plant oils such as flax or canola oils, exercising and losing weight are the usual focus of treatments that have proven to be helpful in clinical trials. The target serum cholesterol level is ideally equal or less than 4 mmol/L (160 mg/dL), and triglycerides equal or less than 2 mmol/L (180 mg/dL).
Evidence has increased that people with diabetes, despite their not having clinically-detectable atherosclotic disease, have more severe debility from atherosclerotic events over time than even non-diabetics that have already suffered atherosclerotic events. Thus diabetes has been upgraded to be viewed as an advanced atherosclerotic disease equivalent.
Treatment
If atherosclerosis leads to symptoms, some symptoms such as angina pectoris can be treated. Non-pharmaceutical means are usually the first method of treatment, such as cessation of smoking and practicing regular exercise. If these methods do not work, medicines are usually the next step in treating cardiovascular diseases, and, with improvements, have increasingly become the most effective method over the long term. However, medicines are criticized for their expense, patented control and occasional undesired effects.
Statins
In general, the group of medications referred to as statins has been the most popular and are widely prescribed for treating atherosclerosis. They have relatively few short-term or longer-term undesirable side-effects, and multiple comparative treatment/placebo trials have fairly consistently shown strong effects in reducing atherosclerotic disease 'events' and generally ~25% comparative mortality reduction in clinical trials, although one study design, ALLHAT,[23] was less strongly favorable.
The newest statin, rosuvastatin, has been the first to demonstrate regression of atherosclerotic plaque within the coronary arteries by IVUS (intravascular ultrasound evaluation).[6] The study was set up to demonstrate effect primarily on atherosclerosis volume within a 2 year time-frame in people with active/symptomatic disease (angina frequency also declined markedly) but not global clinical outcomes, which was expected to require longer trial time periods; these longer trials remain in progress.
However, for most people, changing their physiologic behaviors, from the usual high risk to greatly reduced risk, requires a combination of several compounds, taken on a daily basis and indefinitely. More and more human treatment trials have been done and are ongoing that demonstrate improved outcome for those people using more-complex and effective treatment regimens that change physiologic behaviour patterns to more closely resemble those that humans exhibit in childhood at a time before fatty streaks begin forming.
The statins, and some other medications, have been shown to have antioxidant effects, possibly part of their basis for some of their therapeutic success in reducing cardiac 'events'.
The success of statin drugs in clinical trials is based on some reductions in mortality rates, however by trial design biased toward men and middle-age, the data is as, as yet, less strongly clear for women and people over the age of 70[24]. For example, in the Scandinavian Simvastatin Survival Study (4S), the first large placebo controlled, randomized clinical trial of a statin in people with advanced disease who had already suffered a heart attack, the overall mortality rate reduction for those taking the statin, vs. placebo, was 30%. For the subgroup of people in the trial that had Diabetes Mellitus, the mortality rate reduction between statin and placebo was 54%. 4S was a 5.4-year trial that started in 1989 and was published in 1995 after completion. There were 3 more dead women at trial's end on statin than in the group on placebo drug whether chance or some relation to the statin remains unclear. The ASTEROID trial has been the first to show actual disease volume regression[6] (see page 8 of the paper, which shows cross-sectional areas of the total heart artery wall at start and 2 years of rosuvastatin 40 mg/day treatment); however, its design was not able to "prove" the mortality reduction issue since it did not include a placebo group, the individuals offered treatment within the trial had advanced disease and promoting a comparison placebo arm was judged to be unethical.
Primary and secondary prevention
Combinations of statins, niacin, intestinal cholesterol absorption-inhibiting supplements (ezetimibe and others, and to a much lesser extent fibrates) have been the most successful in changing common but sub-optimal lipoprotein patterns and group outcomes. In the many secondary prevention and several primary prevention trials, several classes of lipoprotein expression (less correctly termed "cholesterol-lowering") altering agents have consistently reduced not only heart attack, stroke and hospitalization but also all-cause mortality rates. The first of the large secondary prevention comparative statin/placebo treatment trials was the Scandinavian Simvastatin Survival Study. (4S) [25] with over 15 more extending through the more recent ASTEROID [26] trial published in 2006. The first primary prevention comparative treatment trial was AFCAPS/TexCAPS [27] with multiple later comparative statin/placebo treatment trials including EXCEL.[28], ASCOT[29] and SPARCL.[30] [31] While the statin trials have all been clearly favorable for improved human outcomes, only ASTEROID showed evidence of atherosclerotic regression (slight). For both human and animal trials, those which have shown evidence of disease regression had all utilized more aggressive combination agent treatment strategies, nearly always including niacin.[8]
Diet and dietary supplements
Vitamin B3, AKA niacin, in pharmacologic doses, (generally 1,000 to 3,000 mg/day), sold in many OTC and prescription formulations, tends to improve (a) HDL levels, size and function, (b) shift LDL particle distribution to larger particle size and (c) lower lipoprotein(a), an atherosclerosis promoting genetic variant of LDL. Additionally, individual responses to daily niacin, while mostly evident after a month at effective doses, tends to continue to slowly improve further over time. (However, careful patient understanding of how to achieve this without nuisance symptoms is needed, though not often achieved.) Research work on increasing HDL particle concentration and function, beyond the usual niacin effect/response, even more important, is slowly advancing.
Dietary changes to achieve benefit have been more controversial, generally far less effective and less widely adhered to with success. One key reason for this is that most cholesterol, typically 80-90%, within the body is created and controlled by internal production by all cells in the body (true of all animals), with typically slightly greater relative production by hepatic/liver cells. (Cell structure relies on fat membranes to separate and organize intracellular water, proteins and nucleic acids and cholesterol is one of the components of all animal cell membranes.)
Caldwell B Esselstyn Jr. MD has had an article published in Preventive Cardiology 2001;4: 171-177 in which he has published angiograms showing regression of atherosclerosis brought about by a very low fat vegan diet in some cases with cholesterol lowering medications.[32]
While the absolute production quantities vary with the individual, group averages for total human body content of cholesterol within the U.S. population commonly run about ~35,000 mg (assuming lean build; varies with body weight and build) and ~1,000 mg/day ongoing production. Dietary intake plays a smaller role, 200–300 mg/day being common values; for pure vegetarians, essentially 0 mg/day, but this typically does not change the situation very much because internal production increases to largely compensate for the reduced intake. For many, especially those with greater than optimal body mass and increased glucose levels, reducing carbohydrate (especially simple forms) intake, not fats or cholesterol, is often more effective for improving lipoprotein expression patterns, weight and blood glucose values. For this reason, medical authorities much less frequently promote the low dietary fat concepts than was commonly the case prior to about year 2005. However, evidence has increased that processed, particularly industrial non-enzymatic hydrogenation produced trans fats, as opposed to the natural cis-configured fats, which living cells primarily produce, is a significant health hazard.
Dietary supplements of Omega-3 oils, especially those from the muscle of some deep salt water living fish species, also have clinical evidence of significant protective effects as confirmed by 6 double blind placebo controlled human clinical trials[citation needed].
There is also a variety of evidence, though less robust, that homocysteine and uric acid levels, including within the normal range promote atherosclerosis and that lowering these levels is helpful, up to a point.
In animals Vitamin C deficiency has been confirmed as an important role in development of hypercholesterolemia and atherosclerosis, but due to ethical reasons placebo-controlled human studies are impossible to do.[33] Vitamin C acts as an antioxidant in vessels and inhibits inflammatory process.[34] It has therapeutic properties on high blood pressure and its fluctuation,[35][36] and arterial stiffness in diabetes.[37] Vitamin C is also a natural regulator of cholesterol[38] and higher doses (over 150 mg/kg daily) may confer significant protection against atherosclerosis even in the situation of elevated cholesterol levels.[39][40]
The scale of vitamin C benefits on cardiovascular system led several authors to the theory, that vitamin C deficiency is the primary cause of cardiovascular diseases.[41] The theory was unified by twice Nobel prize winner Linus Pauling and Matthias Rath. They suggest, that clinical manifestations of cardiovascular diseases are merely overshoot of body defense mechanisms, that are involved in stabilisation of vascular wall, after it is weakened by the vitamin C deficiency and the subsequent collagen degradation. They discuss several metabolic and genetic predispositions and their pathomechanism.[42]
Trials on Vitamin E have been done, but they have failed to find a beneficial effect, for various reasons, but for some patients at high risk for atherosclerosis there may be some benefits.[43]
Menaquinone (Vitamin K2), but not phylloquinone (Vitamin K1), intake is associated with reduced risk of CHD mortality, all-cause mortality and severe aortic calcification.[44][45][46]
It has been suggested that excess iron may be involved in development of atherosclerosis[47][48], but one study found reducing body iron stores in patients with symptomatic peripheral artery disease through phlebotomy did not significantly decrease all-cause mortality or death plus nonfatal myocardial infarction and stroke.[49] Further studies may be warranted.
Surgical intervention
Other physical treatments, helpful in the short term, include minimally invasive angioplasty procedures that may include stents to physically expand narrowed arteries[50] and major invasive surgery, such as bypass surgery, to create additional blood supply connections that go around the more severely narrowed areas.
Prophylaxis
Patients at risk for atherosclerosis-related diseases are increasingly being treated prophylactically with low-dose aspirin and a statin. The high incidence of cardiovascular disease led Wald and Law[51] to propose a Polypill, a once-daily pill containing these two types of drugs in addition to an ACE inhibitor, diuretic, beta blocker, and folic acid. They maintain that high uptake by the general population by such a Polypill would reduce cardiovascular mortality by 80%. It must be emphasized however that this is purely theoretical, as the Polypill has never been tested in a clinical trial.
Medical treatments often focus predominantly on the symptoms. However, over time, the treatments which focus on decreasing the underlying atherosclerosis processes, as opposed to simply treating the symptoms resulting from the atherosclerosis, have been shown by clinical trials to be more effective.
In summary, the key to the more effective approaches has been better understanding of the widespread and insidious nature of the disease and to combine multiple different treatment strategies, not rely on just one or a few approaches. In addition, for those approaches, such as lipoprotein transport behaviors, which have been shown to produce the most success, adopting more aggressive combination treatment strategies has generally produced better results, both before and especially after people are symptomatic.
Because many blood thinners, particularly salicylates such as warfarin and aspirin thin the blood by interfering with Vitamin K, there is recent evidence that blood thinners which work by this mechanism, can actually worsen arterial calcification in the long term even though they thin the blood in the short term.[52][53] [3][4]
Recent research
An indication of the role of HDL on atherosclerosis has been with the rare Apo-A1 Milano human genetic variant of this HDL protein. A small short-term trial using bacterial synthetized human Apo-A1 Milano HDL in people with unstable angina produced fairly dramatic reduction in measured coronary plaque volume in only 6 weeks vs. the usual increase in plaque volume in those randomized to placebo. The trial was published in JAMA in early 2006. Ongoing work starting in the 1990s may lead to human clinical trials—probably by about 2008. These may use synthesized Apo-A1 Milano HDL directly. Or they may use gene-transfer methods to pass the ability to synthesize the Apo-A1 Milano HDLipoprotein.
Methods to increase high-density lipoprotein (HDL) particle concentrations, which in some animal studies largely reverses and remove atheromas, are being developed and researched.
Niacin has HDL raising effects (by 10 - 30%) and showed clinical trial benefit in the Coronary Drug Project and is commonly used in combination with other lipoprotein agents to improve efficacy of changing lipoprotein for the better. However most individuals have nuisance symptoms with short term flushing reactions, especially initially, and so working with a physician with a history of successful experience with niacin implementation, careful selection of brand, dosing strategy, etc. are usually critical to success.
However, increasing HDL by any means is not necessarily helpful. For example, the drug torcetrapib is the most effective agent currently known for raising HDL (by up to 60%). However, in clinical trials it also raised deaths by 60%. All studies regarding this drug were halted in December 2006.[54]
The ERASE trial is a newer trial of an HDL booster which has shown promise.[55]
The ASTEROID trial used a high-dose of rosuvastatin—the statin with typically the most potent dose/response correlation track record (both for LDLipoproteins and HDLipoproteins.) It found plaque (intima + media volume) reduction.[6] Several additional rosuvastatin treatment/placebo trials for evaluating other clinical outcomes are in progress.
The actions of macrophages drive atherosclerotic plaque progression. Immunomodulation of atherosclerosis is the term for techniques which modulate immune system function in order to suppress this macrophage action.[56] Immunomodulation has been pursued with considerable success in both mice and rabbits since about 2002. Plans for human trials, hoped for by about 2008, are in progress.
Research on genetic expression and control mechanisms is progressing. Topics include
- PPAR, known to be important in blood sugar and variants of lipoprotein production and function;
- The multiple variants of the proteins that form the lipoprotein transport particles.
Some controversial research has suggested a link between atherosclerosis and the presence of several different nanobacteria in the arteries, e.g., Chlamydophila pneumoniae, though trials of current antibiotic treatments known to be usually effective in suppressing growth or killing these bacteria have not been successful in improving outcomes.[57]
The immunomodulation approaches mentioned above, because they deal with innate responses of the host to promote atherosclerosis, have far greater prospects for success.
The Hemorheologic-hemodynamic theory
The hemorheologic–hemodynamic theory holds that atherosclerosis is a disease of stasis of blood, which promotes the organization of a thrombus into an atherosclerotic plaque.[58] Stasis of blood predisposes to thrombosis, as described in Virchow's triad. Risk factors for atherosclerosis create larger areas of decreased shear (flow) by increasing blood viscosity, arterial stiffness, or both. Both of these abnormalities are seen in association with aging, hypertension, diabetes mellitus, cigarette smoking, and obesity.[58][59] The hemorheologic-hemodynamic theory posits that the same pathologic process, thrombosis, leads to both plaque development and its complication, superimposed thrombosis and infarction. The name reflects the fact that the interaction of hemorheologic, i.e., blood flow, and hemodynamic, i.e., blood velocity, pulsatility, and arterial geometry, factors lead to atherosclerosis.
Aggressive reduction of serum LDL-cholesterol using high dose statin therapy still leaves significant risk of adverse cardiovascular events in high-risk patients. Despite lowering serum LDL-cholesterol to less than 100 mg/dL, the estimated risk of adverse events in patients with established coronary artery disease is estimated to be 9% per year.[60] The mainstream theory of atherogenesis does not explain the localization of fibrous plaques to the vicinity of changing arterial geometry, such as branches, curves, and dilatations.[61] Mainstream theory provides no explanation for accelerated atherosclerosis associated with hypertension.[59] Finally, mainstream theory cannot explain the presence of fibrous plaques in synthetic arteriovenous grafts.[62]
Viscosity and localized stasis
In arteries during systole, there is a gradient of blood velocity with the highest velocity in the center of the vessel and the lowest against the arterial wall.[63] In areas of vascular branching, curving and dilatation, focal blood pooling occurs in the low shear environment against the arterial wall if blood velocity exceeds a critical value of Reynolds number (see below). This phenomenon is seen in nature when rapidly flowing water encounters an obstruction, forming eddies and pools. Blood is a non-Newtonian fluid, and its viscosity progressively increases with decreasing shear. In areas of pooling, a vicious cycle can develop in which increased viscosity leads to decreased flow, further increasing viscosity and decreasing flow, leading ultimately to stasis and thrombosis in the absence of adequate fibrinolytic activity. Decreased blood flow promotes thrombosis by decreasing influx of fibrinolytic molecules and decreasing efflux of activated clotting factors. Platelets activated by high shear[64] in the central column of blood can be directed to the vicinity of the arterial wall by eddy currents. In these areas, decreased blood flow decreases endothelial production of molecules with antithrombotic activity such as nitric oxide and prostacyclin, further promoting thrombosis. This is akin to endothelial dysfunction which in mainstream atherogensis theory is thought to be caused by putative cytopathic effects of oxidized low-density lipoprotein.
Arterial stiffening
Increased arterial stiffness accelerates atherosclerosis by increasing peak arterial blood velocity, thereby increasing Reynold's number, which indicates the propensity for a flowing fluid to develop pools and eddy currents in association with changing arterial geometry. In the normally compliant aorta, a portion of each stroke volume is stored in systole and propelled with lower velocity in diastole,[65] creating blood flow throughout most of the cardiac cycle. An area of pooling created by high velocity would disappear during slow diastolic flow, and any accumulated microthrombus would be dispersed. In a perfectly stiff aorta, the entire stroke volume would be expelled in systole. Given constant stroke volume, conservation of mass requires increasing peak arterial blood velocity with increasing arterial stiffness.[59] In addition, no low velocity diastolic flow will occur, so that a pool formed during high velocity systolic flow will persist throughout the cardiac cycle. This will allow the time necessary for thrombus growth and subsequent organization (see below). Additionally, increased peak arterial velocity will augment shear-mediated platelet activiation.
Organization of mural thrombi
J.B. Duguid promoted the idea that atherosclerotic plaques develop from organization of mural thrombi in the 1940s and 50's.[66] Arterial thrombi tend to remain localized to the low shear environment of the arterial wall because of high blood velocity in the central portion of the artery. These thrombi are know as "mural" or "parietal."[67] In veins, the velocity gradient between the center of the vessel and the vessel wall is small. Thus, thrombi in veins are more likely to become occlusive, as in deep venous thrombosis. This is why atherosclerosis is limited to arteries. If thrombi persist, whether in arteries or veins, they undergo organization, in which circulating fibrocytes[68] colonize the thrombus and differentiate into cells capable of producing collagen and markers of smooth muscle differentiation, such as smooth muscle actin.
Role of lipoproteins in atherogenesis
The hemorheologic-hemodynamic theory predicts that low-density lipoprotein (LDL) should increase blood viscosity and high-density liporotein (HDL) should decrease blood viscosity, which has been demonstrated experimentally.[69] Erythrocytes are separated by a minimum intercellular distance of approximately 15 nanometers caused by electrostatic repulsion due to sialic acid on the cell membrane surface. LDL has a particle diameter of 18 to 40 nanometers, large enough to simultaneously bind to two erythrocytes and form erythrocyte aggregates. Erythrocyte aggregates increase blood viscosity at low shear by increasing the inertia of the suspended particles. HDL, with a particle diameter of 8 to 12 nanometers, is too small to promote erythrocyte aggregation. Instead, by competing with LDL for binding to erythrocytes, it antagonizes erythrocyte aggregation and decreases blood viscosity. Erythrocyte aggregates are weak, and progressively distrupted with increasing shear. Given the relationship of LDL to blood viscosity, it is not surprising that hypercholesterolemia is a risk factor for both atherosclerosis and deep venous thrombosis.[70]
Insights provided by the hemorheologic–hemodynamic theory
The hemorheologic-hemodynamic theory explains the existence of atherosclerotic plaques in synthetic arteriovenous grafts.[62] These provide an extreme hemodynamic environment, where extremely high velocity blood flows through a curved vessel. These vessels are prone to thrombosis and development of atherosclerotic plaques despite anticoagulation. These vessels lack a tunica media, which received wisdom maintains is the origin of smooth muscle cells in atherosclerotic plaques via migration. The identification of the fibrocyte provides an alternative explanation for the origin of smooth muscle cells in atherosclerotic plaques.[71] Being largely inanimate, the capacity of these vessels to respond to an injury with an inflammatory response, the inciting cause of atherosclerosis according to mainstream atherogenesis theory, would be very limited. Further, this theory explains the benefit of blood donation.[72] and drinking large quantities of water.[73]
See also
- Angiogram
- Arterial stiffness
- Atheroma
- Chelation therapy
- Coronary circulation
- Coronary catheterization
- Fatty streaks
- Monckeberg's arteriosclerosis
- Intravascular ultrasound
Footnotes
- ^ Maton, Anthea (1993). Human Biology and Health. Englewood Cliffs, New Jersey, USA: Prentice Hall. ISBN 0-13-981176-1. OCLC 32308337.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fabricant CG, Fabricant J (1999). "Atherosclerosis induced by infection with Marek's disease herpesvirus in chickens". Am Heart J. 138 (5 Pt 2): S465–8. doi:10.1016/S0002-8703(99)70276-0. PMID 10539849.
- ^ Hsu HY, Nicholson AC, Pomerantz KB, Kaner RJ, Hajjar DP (1995). "Altered cholesterol trafficking in herpesvirus-infected arterial cells. Evidence for viral protein kinase-mediated cholesterol accumulation". J Biol Chem. 270 (33): 19630–7. doi:10.1074/jbc.270.33.19630. PMID 7642651.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Cheng J, Ke Q, Jin Z, Wang H, Kocher O, Morgan JP, Zhang J, Crumpacker CS (2009). "Cytomegalovirus Infection Causes an Increase of Arterial Blood Pressure". PLoS Pathog. 5 (5): e1000427. doi:10.1371/journal.ppat.1000427. PMC 2673691. PMID 19436702.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ (1987). "Compensatory enlargement of human atherosclerotic coronary arteries". N. Engl. J. Med. 316 (22): 1371–5. PMID 3574413.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Nissen. ""Effect of Very High-Intensity Statin Therapy on Regression of Coronary Atherosclerosis–The ASTEROID Trial"" (PDF). JAMA.
- ^ Maseri A, Fuster V (2003). "Is there a vulnerable plaque?". Circulation. 107 (16): 2068–71. doi:10.1161/01.CIR.0000070585.48035.D1. PMID 12719286.
- ^ a b Blankenhorn DH, Hodis HN (1993). "Atherosclerosis--reversal with therapy". The Western journal of medicine. 159 (2): 172–9. PMC 1022223. PMID 8212682.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e f g h i j k l m n o p q r s Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). Robbins Basic Pathology: With STUDENT CONSULT Online Access. Philadelphia: Saunders. p. 345. ISBN 1-4160-2973-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) 8th edition. - ^ Narain VS, Gupta N, Sethi R, Puri A, Dwivedi SK, Saran RK, Puri VK. Clinical correlation of multiple biomarkers for risk assessment in patients with acute coronary syndrome. Indian Heart J. 2008 Nov-Dec;60(6):536-42.
- ^ Bhatt DL, Topol EJ (2002). "Need to test the arterial inflammation hypothesis". Circulation. 106 (1): 136–40. doi:10.1161/01.CIR.0000021112.29409.A2. PMID 12093783.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Griffin M, Frazer A, Johnson A, Collins P, Owens D, Tomkin GH (1998). "Cellular cholesterol synthesis--the relationship to post-prandial glucose and insulin following weight loss". Atherosclerosis. 138 (2): 313–8. doi:10.1016/S0021-9150(98)00036-7. PMID 9690914.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ King, Cr; Knutson, Kl; Rathouz, Pj; Sidney, S; Liu, K; Lauderdale, Ds (2008). "Short sleep duration and incident coronary artery calcification". JAMA : the journal of the American Medical Association. 300 (24): 2859–66. doi:10.1001/jama.2008.867. PMC 2661105. PMID 19109114.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Food Pyramids: Nutrition Source, Harvard School of Public Health". Retrieved 2007-11-25.
- ^ Taubes G (2001). "Nutrition. The soft science of dietary fat". Science (New York, N.Y.). 291 (5513): 2536–45. doi:10.1126/science.291.5513.2536. PMID 11286266.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Song JH, Fujimoto K, Miyazawa T (2000). "Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid-containing oils". J. Nutr. 130 (12): 3028–33. PMID 11110863.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yap SC, Choo YM, Hew NF; et al. (1995). "Oxidative susceptibility of low density lipoprotein from rabbits fed atherogenic diets containing coconut, palm, or soybean oils". Lipids. 30 (12): 1145–50. doi:10.1007/BF02536616. PMID 8614305.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Greco AV, Mingrone G (1990). "Serum and biliary lipid pattern in rabbits feeding a diet enriched with unsaturated fatty acids". Exp Pathol. 40 (1): 19–33. PMID 2279534.
- ^ Mattes RD (2005). "Fat taste and lipid metabolism in humans". Physiol. Behav. 86 (5): 691–7. doi:10.1016/j.physbeh.2005.08.058. PMID 16249011.
The rancid odor of an oxidized fat is readily detectable
- ^ Dobarganes C, Márquez-Ruiz G (2003). "Oxidized fats in foods". Curr Opin Clin Nutr Metab Care. 6 (2): 157–63. doi:10.1097/01.mco.0000058585.27240.ee. PMID 12589185.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ How Bad Are Cooking Oils? by Udo Erasmus, PhD
- ^ "Drink Like the French, Die Like the French"
- ^ The Allhat Officers And Coordinators For The Allhat Collaborative Research Group, (2002). "Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT)". JAMA. 288 (23): 2998–3007. doi:10.1001/jama.288.23.2998. PMID 12479764.
{{cite journal}}:|access-date=requires|url=(help)CS1 maint: extra punctuation (link) - ^ Vos E, Rose CP (2005). "Questioning the benefits of statins". CMAJ. 173 (10): 1207, author reply 1210. doi:10.1503/cmaj.1050120. PMC 1277053. PMID 16275976.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ T. E. Strandberg, S. Lehto, K. Pyörälä, A. Kesäniemi, H. Oksa (1997-01-11). "Cholesterol lowering after participation in the Scandinavian Simvastatin Survival Study (4S) in Finland". European Heart Journal. 18 (18(11)): 1725–1727, . PMID 9402446. Retrieved 2007-11-18.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Nissen SE, Nicholls SJ, Sipahi I; et al. (2006). "Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial" (PDF). JAMA. 295 (13): 1556–65. doi:10.1001/jama.295.13.jpc60002. PMID 16533939.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Downs JR, Clearfield M, Weis S; et al. (1998). "Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study". JAMA : the journal of the American Medical Association. 279 (20): 1615–22. doi:10.1001/jama.279.20.1615. PMID 9613910.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bradford RH, Shear CL, Chremos AN; et al. (1991). "Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia". Arch. Intern. Med. 151 (1): 43–9. doi:10.1001/archinte.151.1.43. PMID 1985608.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Sever PS, Poulter NR, Dahlöf B; et al. (2005). "Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial--lipid-lowering arm (ASCOT-LLA)". Diabetes Care. 28 (5): 1151–7. doi:10.2337/diacare.28.5.1151. PMID 15855581.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Linda Brookes, MSc. "SPARCL: Stroke Prevention by Aggressive Reduction in Cholesterol Levels". Medscape. Retrieved 2007-11-19.
- ^ Amarenco P, Bogousslavsky J, Callahan AS; et al. (2003). "Design and baseline characteristics of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) study". Cerebrovascular diseases. 16 (4): 389–95. doi:10.1159/000072562. PMID 14584489.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Resolving the Coronary Artery Disease Epidemic through Plant-Based Nutrition Caldwell B. Esselstyn, Jr., MD
- ^ Ginter E (2007). "Chronic vitamin C deficiency increases the risk of cardiovascular diseases". Bratisl Lek Listy. 108 (9): 417–21. PMID 18225482.
- ^ Böhm F, Settergren M, Pernow J (2007). "Vitamin C blocks vascular dysfunction and release of interleukin-6 induced by endothelin-1 in humans in vivo". Atherosclerosis. 190 (2): 408–15. doi:10.1016/j.atherosclerosis.2006.02.018. PMID 16527283.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sato K, Dohi Y, Kojima M, Miyagawa K, Takase H, Katada E, Suzuki S. (2006). "Effects of ascorbic acid on ambulatory blood pressure in elderly patients with refractory hypertension". Arzneimittelforschung. 56 (7): 535–40. PMID 16927536.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gladys Block, Christopher D Jensen, Edward P Norkus, Mark Hudes and Patricia B Crawford (2008). "Vitamin C in plasma is inversely related to blood pressure and change in blood pressure during the previous year in young Black and White women". Nutrition Journal. 7 (35): 535–40. doi:10.1186/1475-2891-7-35.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Brian A. Mullan; Ian S. Young; Howard Fee; David R. McCance (2002). "Ascorbic Acid Reduces Blood Pressure and Arterial Stiffness in Type 2 Diabetes". Hypertension. 40 (6): 804. doi:10.1161/01.HYP.0000039961.13718.00. PMID 12468561.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ HJ Harwood Jr, YJ Greene and PW Stacpoole (June 5, 1986). "Inhibition of human leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase activity by ascorbic acid. An effect mediated by the free radical monodehydroascorbate". J. Biol. Chem. 261 (16): 7127–7135. PMID 3711081.
- ^ Das S, Ray R, Snehlata, Das N, Srivastava LM (2006). "Effect of ascorbic acid on prevention of hypercholesterolemia induced atherosclerosis". Mol Cell Biochem. 285(1-2) (1–2): 143–7. doi:10.1007/s11010-005-9070-x. PMID 16479321.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Klenner F.R. (1974). "Significance of high daily intake of ascorbic acid in preventive medicine". J. Int. Acad. Prev. Med. 1: 45–49.
- ^ Stone, I. (1972). The Healing Factor: Vitamin C Against Disease. Grosset and Dunlap, New York/Perigee Books, published by The Putnam Publishing Group. ISBN 0-399-50764-7.
- ^ Rath M, Pauling L (1992). "A Unified Theory of Human Cardiovascular Disease Leading the Way to the Abolition of This Disease as a Cause for Human Mortality" (PDF). Journal of Orthomolecular Medicine. 7 (1): 5–15.
- ^ Robinson I, de Serna DG, Gutierrez A, Schade DS (2006). "Vitamin E in humans: an explanation of clinical trial failure". Endocr Pract. 12 (5): 576–82. PMID 17002935.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Geleijnse JM, Vermeer C, Grobbee DE; et al. (2004). "Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study". J. Nutr. 134 (11): 3100–5. PMID 15514282.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Erkkilä AT, Booth SL (2008). "Vitamin K intake and atherosclerosis". Curr. Opin. Lipidol. 19 (1): 39–42. doi:10.1097/MOL.0b013e3282f1c57f. PMID 18196985.
- ^ Wallin R, Schurgers L, Wajih N (2008). "Effects of the blood coagulation vitamin K as an inhibitor of arterial calcification". Thromb. Res. 122 (3): 411. doi:10.1016/j.thromres.2007.12.005. PMC 2529147. PMID 18234293.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brewer GJ (2007). "Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer's disease". Exp. Biol. Med. (Maywood). 232 (2): 323–35. PMID 17259340.
- ^ Sullivan JL, Mascitelli L (2007). "[Current status of the iron hypothesis of cardiovascular diseases]". Recenti Prog Med (in Italian). 98 (7–8): 373–7. PMID 17685184.
- ^ Zacharski LR, Chow BK, Howes PS; et al. (2007). "Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial". JAMA. 297 (6): 603–10. doi:10.1001/jama.297.6.603. PMID 17299195.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Heart disease and stents". Cypher Stent. Retrieved 2008-04-01.
- ^ Wald NJ, Law MR (2003). "A strategy to reduce cardiovascular disease by more than 80%". BMJ (Clinical research ed.). 326 (7404): 1419. doi:10.1136/bmj.326.7404.1419. PMC 162259. PMID 12829553.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Price PA, Faus SA, Williamson MK (2000). "Warfarin-induced artery calcification is accelerated by growth and vitamin D". Arteriosclerosis, thrombosis, and vascular biology. 20 (2): 317–27. PMID 10669626.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Geleijnse JM, Vermeer C, Grobbee DE; et al. (2004). "Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study". J Nutr. 134 (11): 3100–5. PMID 15514282.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Barter PJ, Caulfield M, Eriksson M; et al. (2007). "Effects of torcetrapib in patients at high risk for coronary events". N Engl J Med. 357 (21): 2109–22. doi:10.1056/NEJMoa0706628. PMID 17984165.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sue Hughes (March 26, 2007). "ERASE: New HDL mimetic shows promise". HeartWire.
- ^ Jan Nilsson; Göran K. Hansson; Prediman K. Shah (2005). "Immunomodulation of Atherosclerosis – Implications for Vaccine Development—ATVB In Focus". Arteriosclerosis, Thrombosis, and Vascular Biology. 5 (1): 18–28. doi:10.1161/01.ATV.0000149142.42590.a2. PMID 15514204.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ M Stitzinger (2007). "Lipids, inflammation and atherosclerosis" (pdf). The digital repository of Leiden University. Retrieved 2007-11-02.
Results of clinical trials investigating anti-chlamydial antibiotics as an addition to standard therapy in patients with coronary artery disease have been inconsistent. Therefore, Andraws et al. conducted a meta- analysis of these clinical trials and found that evidence available to date does not demonstrate an overall benefit of antibiotic therapy in reducing mortality or cardiovascular events in patients with coronary artery disease.
{{cite web}}: line feed character in|quote=at position 75 (help) - ^ a b Cite error: The named reference
Sloopwas invoked but never defined (see the help page). - ^ a b c Perret RS, Sloop GD. Increased peak blood velocity in association with elevated blood pressure. Ultrasound in Medicine and Biology 2000;26: 1387-91.
- ^ Hausenloy DJ, Yellon DM. Targeting residual cardiovascular risk: raising high-density lipoprotein cholesterol levels. Postgraduate Medical Journal 2008;84: 590-8.
- ^ Kensey KR, Cho YI. The Origin of Atherosclerosis. Volume 1: An Introduction to Hemodynamics. EPP Medica, 2001, p. 84
- ^ a b Sloop GD, Fallon KB, Zieske AW. Atherosclerotic plaque-like lesions in synthetic arteriovenous grafts: implications for atherogenesis. Atherosclerosis 2002;1260: 133-9.
- ^ Kensey KR, Cho YI. The Origin of Atherosclerosis. Volume 1: An Introduction to Hemodynamics. EPP Medica, 2001, p.22.
- ^ Brown CH 3rd, Leverett LB, Lewis CW, Alfrey CP Jr, Hellums JD. Morphological, biochemical, and functional changes in human platelets subjected to shear stress. Journal of Laboratory and Clinical Medicine 1975;86:462-71.
- ^ Kensey and Cho, pp. 68-73.
- ^ Duguid JB. The thrombogenic hypothesis and its implications. Postgraduate Medical Journal 1960;36: 226-9.
- ^ Majno G, Joris I. Cells, Tissues, and Disease. Blackwell Science, 1996, pp. 634, 648.
- ^ Bellini A, Mattoli S,. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparitive fibrosis. Laboratory Investigation 2007;87: 858-70.
- ^ Sloop GD, Garber DW. The effects of low-density lipoprotein and high-density lipoprotein on blood viscosity correlate with their association with risk of atherosclerosis in humans. Clinical Science 1997;92:473-9.
- ^ Kawasaki T, Kambayashi J, Ariyoshi H, Sakon M, Suehisa E, Monden M. Hypercholesterolemia as a risk factor for deep-vein thrombosis. Thrombosis Research 1997;88:67-73.
- ^ Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proceedings of the National Academy of Science 2003;100: 4754-9.
- ^ Sloop GD. Possible association of a reduction in cardiovascular events with blood donation. Heart 1998;79:422.
- ^ Chan J, Knutsen SF, Blixx GG, Lee JW, Fraser GE. Water, other fluids, and fatal coronary heart disease: the Adventist Health Study. American Journal of Epidemiology 2002;155: 827-33.
