Isotopes of neptunium: Difference between revisions
XinaNicole (talk | contribs) |
XinaNicole (talk | contribs) |
||
| Line 47: | Line 47: | ||
! <br />isotopic mass (u)<br /> |
! <br />isotopic mass (u)<br /> |
||
! rowspan="2" | half-life |
! rowspan="2" | half-life |
||
! rowspan="2" | decay<br>mode(s)<ref>http://www.nucleonica.net/unc.aspx</ref><ref group="n">Abbreviations:<br>CD: [[Cluster decay]]<br>EC: [[Electron capture]]<br>IT: [[Isomeric transition]]<br>SF: [[Spontaneous fission]]</ref> |
|||
! rowspan="2" | daughter<br>isotope(s) |
|||
! rowspan="2" | nuclear<br />spin |
! rowspan="2" | nuclear<br />spin |
||
|- |
|- |
||
| Line 56: | Line 58: | ||
| 225.03391(8) |
| 225.03391(8) |
||
| 3# ms [>2 µs] |
| 3# ms [>2 µs] |
||
| [[alpha decay|α]] |
|||
| <sup>221</sup>Pa |
|||
| 9/2-# |
| 9/2-# |
||
|- |
|- |
||
| Line 63: | Line 67: | ||
| 226.03515(10)# |
| 226.03515(10)# |
||
| 35(10) ms |
| 35(10) ms |
||
| α |
|||
| <sup>222</sup>Pa |
|||
| |
| |
||
|- |
|- |
||
| {{SimpleNuclide2|Neptunium|227}} |
| rowspan=2|{{SimpleNuclide2|Neptunium|227}} |
||
| style="text-align:right" | 93 |
| rowspan=2 style="text-align:right" | 93 |
||
| style="text-align:right" | 134 |
| rowspan=2 style="text-align:right" | 134 |
||
| 227.03496(8) |
| rowspan=2|227.03496(8) |
||
| 510(60) ms |
| rowspan=2|510(60) ms |
||
| α (99.95%) |
|||
| 5/2-# |
|||
| <sup>223</sup>Pa |
|||
| rowspan=2|5/2-# |
|||
|- |
|- |
||
| β<sup>+</sup> (.05%) |
|||
| {{SimpleNuclide2|Neptunium|228}} |
|||
| <sup>227</sup>U |
|||
| style="text-align:right" | 93 |
|||
| style="text-align:right" | 135 |
|||
| 228.03618(21)# |
|||
| 61.4(14) s |
|||
| |
|||
|- |
|- |
||
| {{SimpleNuclide2|Neptunium| |
| rowspan=3|{{SimpleNuclide2|Neptunium|228}} |
||
| style="text-align:right" | 93 |
| rowspan=3 style="text-align:right" | 93 |
||
| style="text-align:right" | |
| rowspan=3 style="text-align:right" | 135 |
||
| rowspan=3|228.03618(21)# |
|||
| 229.03626(9) |
|||
| |
| rowspan=3|61.4(14) s |
||
| β<sup>+</sup> (59%) |
|||
| 5/2+# |
|||
| <sup>228</sup>U |
|||
| rowspan=3| |
|||
|- |
|- |
||
| α (41%) |
|||
| {{SimpleNuclide2|Neptunium|230}} |
|||
| <sup>224</sup>Pa |
|||
| style="text-align:right" | 93 |
|||
| style="text-align:right" | 137 |
|||
| 230.03783(6) |
|||
| 4.6(3) min |
|||
| |
|||
|- |
|- |
||
| β<sup>+</sup>, [[Spontaneous fission|SF]] (.012%) |
|||
| {{SimpleNuclide2|Neptunium|231}} |
|||
| (various) |
|||
| style="text-align:right" | 93 |
|||
| style="text-align:right" | 138 |
|||
| 231.03825(5) |
|||
| 48.8(2) min |
|||
| (5/2)(+#) |
|||
|- |
|- |
||
| {{SimpleNuclide2|Neptunium| |
| rowspan=2|{{SimpleNuclide2|Neptunium|229}} |
||
| style="text-align:right" | 93 |
| rowspan=2 style="text-align:right" | 93 |
||
| style="text-align:right" | |
| rowspan=2 style="text-align:right" | 136 |
||
| rowspan=2|229.03626(9) |
|||
| 232.04011(11)# |
|||
| |
| rowspan=2|4.0(2) min |
||
| ( |
| α (51%) |
||
| <sup>225</sup>Pa |
|||
| rowspan=2|5/2+# |
|||
|- |
|- |
||
| β<sup>+</sup> (49%) |
|||
| {{SimpleNuclide2|Neptunium|233}} |
|||
| <sup>229</sup>U |
|||
| style="text-align:right" | 93 |
|||
|- |
|||
| style="text-align:right" | 140 |
|||
| rowspan=2|{{SimpleNuclide2|Neptunium|230}} |
|||
| 233.04074(5) |
|||
| rowspan=2 style="text-align:right" | 93 |
|||
| 36.2(1) min |
|||
| rowspan=2 style="text-align:right" | 137 |
|||
| (5/2+) |
|||
| rowspan=2|230.03783(6) |
|||
| rowspan=2|4.6(3) min |
|||
| β<sup>+</sup> (97%) |
|||
| <sup>230</sup>U |
|||
| rowspan=2| |
|||
|- |
|||
| α (3%) |
|||
| <sup>226</sup>Pa |
|||
|- |
|||
| rowspan=2|{{SimpleNuclide2|Neptunium|231}} |
|||
| rowspan=2 style="text-align:right" | 93 |
|||
| rowspan=2 style="text-align:right" | 138 |
|||
| rowspan=2|231.03825(5) |
|||
| rowspan=2|48.8(2) min |
|||
| β<sup>+</sup> (98%) |
|||
| <sup>231</sup>U |
|||
| rowspan=2|(5/2)(+#) |
|||
|- |
|||
| α (2%) |
|||
| <sup>227</sup>Pa |
|||
|- |
|||
| rowspan=2|{{SimpleNuclide2|Neptunium|232}} |
|||
| rowspan=2 style="text-align:right" | 93 |
|||
| rowspan=2 style="text-align:right" | 139 |
|||
| rowspan=2|232.04011(11)# |
|||
| rowspan=2|14.7(3) min |
|||
| β<sup>+</sup> (99.99%) |
|||
| <sup>232</sup>U |
|||
| rowspan=2|(4+) |
|||
|- |
|||
| α (.003%) |
|||
| <sup>228</sup>Pa |
|||
|- |
|||
| rowspan=2|{{SimpleNuclide2|Neptunium|233}} |
|||
| rowspan=2 style="text-align:right" | 93 |
|||
| rowspan=2 style="text-align:right" | 140 |
|||
| rowspan=2|233.04074(5) |
|||
| rowspan=2|36.2(1) min |
|||
| β<sup>+</sup> (99.99%) |
|||
| <sup>233</sup>U |
|||
| rowspan=2|(5/2+) |
|||
|- |
|||
| α (.001%) |
|||
| <sup>229</sup>Pa |
|||
|- |
|- |
||
| {{SimpleNuclide2|Neptunium|234}} |
| {{SimpleNuclide2|Neptunium|234}} |
||
| Line 119: | Line 163: | ||
| 234.042895(9) |
| 234.042895(9) |
||
| 4.4(1) d |
| 4.4(1) d |
||
| β<sup>+</sup> |
|||
| <sup>234</sup>U |
|||
| (0+) |
| (0+) |
||
|- |
|- |
||
| {{SimpleNuclide2|Neptunium|235}} |
| rowspan=2|{{SimpleNuclide2|Neptunium|235}} |
||
| style="text-align:right" | 93 |
| rowspan=2 style="text-align:right" | 93 |
||
| style="text-align:right" | 142 |
| rowspan=2 style="text-align:right" | 142 |
||
| 235.0440633(21) |
| rowspan=2|235.0440633(21) |
||
| 396.1(12) d |
| rowspan=2|396.1(12) d |
||
| [[Electron capture|EC]] |
|||
| 5/2+ |
|||
| <sup>235</sup>U |
|||
| rowspan=2|5/2+ |
|||
|- |
|- |
||
| α (.0026%) |
|||
| {{SimpleNuclide2|Neptunium|236}} |
|||
| <sup>231</sup>Pa |
|||
| style="text-align:right" | 93 |
|||
| style="text-align:right" | 143 |
|||
| 236.04657(5) |
|||
| 1.54(6)E+5 a |
|||
| (6-) |
|||
|- |
|- |
||
| |
| rowspan=3|{{SimpleNuclide2|Neptunium|236}} |
||
| |
| rowspan=3 style="text-align:right" | 93 |
||
| rowspan=3 style="text-align:right" | 143 |
|||
| 22.5(4) h |
|||
| rowspan=3|236.04657(5) |
|||
| 1 |
|||
| rowspan=3|1.54(6)E+5 a |
|||
| EC (87.3%) |
|||
| <sup>236</sup>U |
|||
| rowspan=3|(6-) |
|||
|- |
|- |
||
| β<sup>-</sup> (12.5%) |
|||
| {{SimpleNuclide2|Neptunium|237}} |
|||
| <sup>236</sup>Pu |
|||
| style="text-align:right" | 93 |
|||
|- |
|||
| style="text-align:right" | 144 |
|||
| |
| α (.16%) |
||
| <sup>232</sup>Pa |
|||
| 2.144(7)E+6 a |
|||
|- |
|||
| 5/2+ |
|||
| rowspan=2 style="text-indent:1em" | {{SimpleNuclide2|Neptunium|236m}} |
|||
| rowspan=2 colspan="3" style="text-indent:2em" | 60(50) keV |
|||
| rowspan=2|22.5(4) h |
|||
| EC (52%) |
|||
| <sup>236</sup>U |
|||
| rowspan=2|1 |
|||
|- |
|||
| β<sup>-</sup> (48%) |
|||
| <sup>236</sup>Pu |
|||
|- |
|||
| rowspan=3|{{SimpleNuclide2|Neptunium|237}} |
|||
| rowspan=3 style="text-align:right" | 93 |
|||
| rowspan=3 style="text-align:right" | 144 |
|||
| rowspan=3|237.0481734(20) |
|||
| rowspan=3|2.144(7)E+6 a |
|||
| α |
|||
| <sup>233</sup>Pa |
|||
| rowspan=3|5/2+ |
|||
|- |
|||
| [[Spontaneous fission|SF]] (2E-10%) |
|||
| (various) |
|||
|- |
|||
| [[Cluster decay|CD]] (4E-12%) |
|||
| <sup>207</sup>Tl, <sup>30</sup>Mg |
|||
|- |
|- |
||
| {{SimpleNuclide2|Neptunium|238}} |
| {{SimpleNuclide2|Neptunium|238}} |
||
| Line 152: | Line 224: | ||
| 238.0509464(20) |
| 238.0509464(20) |
||
| 2.117(2) d |
| 2.117(2) d |
||
| β<sup>-</sup> |
|||
| <sup>238</sup>Pu |
|||
| 2+ |
| 2+ |
||
|- |
|- |
||
| Line 157: | Line 231: | ||
| colspan="3" style="text-indent:2em" | 2300(200)# keV |
| colspan="3" style="text-indent:2em" | 2300(200)# keV |
||
| 112(39) ns |
| 112(39) ns |
||
| |
|||
| |
|||
| |
| |
||
|- |
|- |
||
| Line 164: | Line 240: | ||
| 239.0529390(22) |
| 239.0529390(22) |
||
| 2.356(3) d |
| 2.356(3) d |
||
| β<sup>-</sup> |
|||
| <sup>239</sup>Pu |
|||
| 5/2+ |
| 5/2+ |
||
|- |
|- |
||
| Line 171: | Line 249: | ||
| 240.056162(16) |
| 240.056162(16) |
||
| 61.9(2) min |
| 61.9(2) min |
||
| β<sup>-</sup> |
|||
| <sup>240</sup>Pu |
|||
| (5+) |
| (5+) |
||
|- |
|- |
||
| style="text-indent:1em" | {{SimpleNuclide2|Neptunium|240m}} |
| rowspan=2 style="text-indent:1em" | {{SimpleNuclide2|Neptunium|240m}} |
||
| colspan="3" style="text-indent:2em" | 20(15) keV |
| rowspan=2 colspan="3" style="text-indent:2em" | 20(15) keV |
||
| 7.22(2) min |
| rowspan=2|7.22(2) min |
||
| 1(+) |
| rowspan=2|1(+) |
||
| β<sup>-</sup> (99.89%) |
|||
| <sup>240</sup>Pu |
|||
|- |
|||
| [[Isomeric transition|IT]] (.11%) |
|||
| <sup>240</sup>Np |
|||
|- |
|- |
||
| {{SimpleNuclide2|Neptunium|241}} |
| {{SimpleNuclide2|Neptunium|241}} |
||
| Line 183: | Line 268: | ||
| 241.05825(8) |
| 241.05825(8) |
||
| 13.9(2) min |
| 13.9(2) min |
||
| β<sup>-</sup> |
|||
| <sup>241</sup>Pu |
|||
| (5/2+) |
| (5/2+) |
||
|- |
|- |
||
| Line 190: | Line 277: | ||
| 242.06164(21) |
| 242.06164(21) |
||
| 2.2(2) min |
| 2.2(2) min |
||
| β<sup>-</sup> |
|||
| <sup>242</sup>Pu |
|||
| (1+) |
| (1+) |
||
|- |
|- |
||
| Line 195: | Line 284: | ||
| colspan="3" style="text-indent:2em" | 0(50)# keV |
| colspan="3" style="text-indent:2em" | 0(50)# keV |
||
| 5.5(1) min |
| 5.5(1) min |
||
| |
|||
| |
|||
| 6+# |
| 6+# |
||
|- |
|- |
||
| Line 202: | Line 293: | ||
| 243.06428(3)# |
| 243.06428(3)# |
||
| 1.85(15) min |
| 1.85(15) min |
||
| β<sup>-</sup> |
|||
| <sup>243</sup>Pu |
|||
| (5/2-) |
| (5/2-) |
||
|- |
|- |
||
| Line 209: | Line 302: | ||
| 244.06785(32)# |
| 244.06785(32)# |
||
| 2.29(16) min |
| 2.29(16) min |
||
| β<sup>-</sup> |
|||
| <sup>244</sup>Pu |
|||
| (7-) |
| (7-) |
||
|} |
|} |
||
Revision as of 12:24, 18 May 2011
Neptunium (Np) has no stable isotopes. A standard atomic mass cannot be given.
19 neptunium radioisotopes have been characterized, with the most stable being 237
Np
with a half-life of 2.14 million years, 236
Np
with a half-life of 154,000 years, and 235
Np
with a half-life of 396.1 days. All of the remaining radioactive isotopes have half-lives that are less than 4.5 days, and the majority of these have half lifes that are less than 50 minutes. This element also has 4 meta states, with the most stable being 236m
Np
(t½ 22.5 hours).
The isotopes of neptunium range in atomic weight from 225.0339 u (225
Np
) to 244.068 u (244
Np
). The primary decay mode before the most stable isotope, 237
Np
, is electron capture (with a good deal of alpha emission), and the primary mode after is beta emission. The primary decay products before 237
Np
are element 92 (uranium) isotopes (alpha emission produces element 91, protactinium, however) and the primary products after are element 94 (plutonium) isotopes.
Some notable isotopes
Neptunium-235
Neptunium-235 is a radioactive isotope of neptunium with 93 electrons and protons and 142 neutrons. It has a half-life of 400 days. This isotope of Neptunium either decays by:
- Emitting an alpha particle - Here, the decay energy is 5.2 MeV and the decay product is Protactinium-231.
- Electron capture - Here, the decay energy is 0.125 MeV and the decay product is Uranium-235
This particular isotope of neptunium has a weight of 235.0440633 grams/mole. It is not abundant in nature because it is unstable.
Neptunium-236
Neptunium-236 is a radioactive isotope of neptunium with 93 electrons and protons and 143 neutrons. It has a half-life of 154,000 years. It can decay by the following methods -
- Electron capture - here, the decay energy is 0.95 MeV and the decay product is Uranium-236.
- Beta emission - Here, the decay energy is 0.94 MeV and the decay product is Plutonium-236.
- Alpha emission - Here, the decay energy is 5.024 MeV and the decay product is Protactinium-232
This particular isotope of neptunium has a mass of 236.04657 grams/mole. It is a fissile material with a critical mass of 7 kg. It is not found on earth because it is unstable.
possible parent nuclides: alpha from Am-240
Neptunium-237
| Actinides[1] by decay chain | Half-life range (a) |
Fission products of 235U by yield[2] | ||||||
|---|---|---|---|---|---|---|---|---|
| 4n | 4n + 1 | 4n + 2 | 4n + 3 | 4.5–7% | 0.04–1.25% | <0.001% | ||
| 228Ra№ | 4–6 a | 155Euþ | ||||||
| 244Cmƒ | 241Puƒ | 250Cf | 227Ac№ | 10–29 a | 90Sr | 85Kr | 113mCdþ | |
| 232Uƒ | 238Puƒ | 243Cmƒ | 29–97 a | 137Cs | 151Smþ | 121mSn | ||
| 248Bk[3] | 249Cfƒ | 242mAmƒ | 141–351 a |
No fission products have a half-life | ||||
| 241Amƒ | 251Cfƒ[4] | 430–900 a | ||||||
| 226Ra№ | 247Bk | 1.3–1.6 ka | ||||||
| 240Pu | 229Th | 246Cmƒ | 243Amƒ | 4.7–7.4 ka | ||||
| 245Cmƒ | 250Cm | 8.3–8.5 ka | ||||||
| 239Puƒ | 24.1 ka | |||||||
| 230Th№ | 231Pa№ | 32–76 ka | ||||||
| 236Npƒ | 233Uƒ | 234U№ | 150–250 ka | 99Tc₡ | 126Sn | |||
| 248Cm | 242Pu | 327–375 ka | 79Se₡ | |||||
| 1.53 Ma | 93Zr | |||||||
| 237Npƒ | 2.1–6.5 Ma | 135Cs₡ | 107Pd | |||||
| 236U | 247Cmƒ | 15–24 Ma | 129I₡ | |||||
| 244Pu | 80 Ma |
... nor beyond 15.7 Ma[5] | ||||||
| 232Th№ | 238U№ | 235Uƒ№ | 0.7–14.1 Ga | |||||
| ||||||||
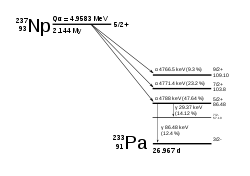
237
Np
decays via the neptunium series to thallium, unlike most other actinides which decay to isotopes of lead.
237
Np
was recently shown to be capable of sustaining a chain reaction with fast neutrons, as in a nuclear weapon.[6] However, it has a low probability of fission on bombardment with thermal neutrons, which makes it unsuitable as a fuel for nuclear power plants.
237
Np
is the only neptunium isotope produced in significant quantity in the nuclear fuel cycle, both by successive neutron capture on uranium-235 (which fissions most but not all of the time) and uranium-236, or (n,2n) reactions where a fast neutron occasionally knocks a neutron loose from uranium-238 or isotopes of plutonium. Over the long term, 237
Np
also forms in spent nuclear fuel as the decay product of americium-241.
237
Np
is projected to be one of the most mobile nuclides at the Yucca Mountain nuclear waste repository.
Table
| nuclide symbol |
Z(p) | N(n) | isotopic mass (u) |
half-life | decay mode(s)[7][n 1] |
daughter isotope(s) |
nuclear spin |
|---|---|---|---|---|---|---|---|
| excitation energy | |||||||
| 225 Np |
93 | 132 | 225.03391(8) | 3# ms [>2 µs] | α | 221Pa | 9/2-# |
| 226 Np |
93 | 133 | 226.03515(10)# | 35(10) ms | α | 222Pa | |
| 227 Np |
93 | 134 | 227.03496(8) | 510(60) ms | α (99.95%) | 223Pa | 5/2-# |
| β+ (.05%) | 227U | ||||||
| 228 Np |
93 | 135 | 228.03618(21)# | 61.4(14) s | β+ (59%) | 228U | |
| α (41%) | 224Pa | ||||||
| β+, SF (.012%) | (various) | ||||||
| 229 Np |
93 | 136 | 229.03626(9) | 4.0(2) min | α (51%) | 225Pa | 5/2+# |
| β+ (49%) | 229U | ||||||
| 230 Np |
93 | 137 | 230.03783(6) | 4.6(3) min | β+ (97%) | 230U | |
| α (3%) | 226Pa | ||||||
| 231 Np |
93 | 138 | 231.03825(5) | 48.8(2) min | β+ (98%) | 231U | (5/2)(+#) |
| α (2%) | 227Pa | ||||||
| 232 Np |
93 | 139 | 232.04011(11)# | 14.7(3) min | β+ (99.99%) | 232U | (4+) |
| α (.003%) | 228Pa | ||||||
| 233 Np |
93 | 140 | 233.04074(5) | 36.2(1) min | β+ (99.99%) | 233U | (5/2+) |
| α (.001%) | 229Pa | ||||||
| 234 Np |
93 | 141 | 234.042895(9) | 4.4(1) d | β+ | 234U | (0+) |
| 235 Np |
93 | 142 | 235.0440633(21) | 396.1(12) d | EC | 235U | 5/2+ |
| α (.0026%) | 231Pa | ||||||
| 236 Np |
93 | 143 | 236.04657(5) | 1.54(6)E+5 a | EC (87.3%) | 236U | (6-) |
| β- (12.5%) | 236Pu | ||||||
| α (.16%) | 232Pa | ||||||
| 236m Np |
60(50) keV | 22.5(4) h | EC (52%) | 236U | 1 | ||
| β- (48%) | 236Pu | ||||||
| 237 Np |
93 | 144 | 237.0481734(20) | 2.144(7)E+6 a | α | 233Pa | 5/2+ |
| SF (2E-10%) | (various) | ||||||
| CD (4E-12%) | 207Tl, 30Mg | ||||||
| 238 Np |
93 | 145 | 238.0509464(20) | 2.117(2) d | β- | 238Pu | 2+ |
| 238m Np |
2300(200)# keV | 112(39) ns | |||||
| 239 Np |
93 | 146 | 239.0529390(22) | 2.356(3) d | β- | 239Pu | 5/2+ |
| 240 Np |
93 | 147 | 240.056162(16) | 61.9(2) min | β- | 240Pu | (5+) |
| 240m Np |
20(15) keV | 7.22(2) min | 1(+) | β- (99.89%) | 240Pu | ||
| IT (.11%) | 240Np | ||||||
| 241 Np |
93 | 148 | 241.05825(8) | 13.9(2) min | β- | 241Pu | (5/2+) |
| 242 Np |
93 | 149 | 242.06164(21) | 2.2(2) min | β- | 242Pu | (1+) |
| 242m Np |
0(50)# keV | 5.5(1) min | 6+# | ||||
| 243 Np |
93 | 150 | 243.06428(3)# | 1.85(15) min | β- | 243Pu | (5/2-) |
| 244 Np |
93 | 151 | 244.06785(32)# | 2.29(16) min | β- | 244Pu | (7-) |
- ^ Abbreviations:
CD: Cluster decay
EC: Electron capture
IT: Isomeric transition
SF: Spontaneous fission
Notes
- Values marked # are not purely derived from experimental data, but at least partly from systematic trends. Spins with weak assignment arguments are enclosed in parentheses.
- Uncertainties are given in concise form in parentheses after the corresponding last digits. Uncertainty values denote one standard deviation, except isotopic composition and standard atomic mass from IUPAC which use expanded uncertainties.
References
- ^ Plus radium (element 88). While actually a sub-actinide, it immediately precedes actinium (89) and follows a three-element gap of instability after polonium (84) where no nuclides have half-lives of at least four years (the longest-lived nuclide in the gap is radon-222 with a half life of less than four days). Radium's longest lived isotope, at 1,600 years, thus merits the element's inclusion here.
- ^ Specifically from thermal neutron fission of uranium-235, e.g. in a typical nuclear reactor.
- ^ Milsted, J.; Friedman, A. M.; Stevens, C. M. (1965). "The alpha half-life of berkelium-247; a new long-lived isomer of berkelium-248". Nuclear Physics. 71 (2): 299. Bibcode:1965NucPh..71..299M. doi:10.1016/0029-5582(65)90719-4.
"The isotopic analyses disclosed a species of mass 248 in constant abundance in three samples analysed over a period of about 10 months. This was ascribed to an isomer of Bk248 with a half-life greater than 9 [years]. No growth of Cf248 was detected, and a lower limit for the β− half-life can be set at about 104 [years]. No alpha activity attributable to the new isomer has been detected; the alpha half-life is probably greater than 300 [years]." - ^ This is the heaviest nuclide with a half-life of at least four years before the "sea of instability".
- ^ Excluding those "classically stable" nuclides with half-lives significantly in excess of 232Th; e.g., while 113mCd has a half-life of only fourteen years, that of 113Cd is eight quadrillion years.
- ^ P. Weiss (26 October 2002). "Little-studied metal goes critical - Neptunium Nukes?". Science News. Retrieved 2006-09-29.
- ^ http://www.nucleonica.net/unc.aspx
- Isotope masses from:
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- Isotopic compositions and standard atomic masses from:
- J. R. de Laeter, J. K. Böhlke, P. De Bièvre, H. Hidaka, H. S. Peiser, K. J. R. Rosman and P. D. P. Taylor (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - M. E. Wieser (2006). "Atomic weights of the elements 2005 (IUPAC Technical Report)". Pure and Applied Chemistry. 78 (11): 2051–2066. doi:10.1351/pac200678112051.
{{cite journal}}: Unknown parameter|laysummary=ignored (help)
- J. R. de Laeter, J. K. Böhlke, P. De Bièvre, H. Hidaka, H. S. Peiser, K. J. R. Rosman and P. D. P. Taylor (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
- Half-life, spin, and isomer data selected from the following sources. See editing notes on this article's talk page.
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - National Nuclear Data Center. "NuDat 2.1 database". Brookhaven National Laboratory. Retrieved September 2005.
{{cite web}}: Check date values in:|accessdate=(help) - N. E. Holden (2004). "Table of the Isotopes". In D. R. Lide (ed.). CRC Handbook of Chemistry and Physics (85th ed.). CRC Press. Section 11. ISBN 978-0849304859.
{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help)
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
