Antioxidant: Difference between revisions
Rescuing 1 sources and tagging 0 as dead. #IABot (v2.0beta3) |
Citation bot (talk | contribs) m Alter: isbn. Add: deadurl, doi, format, authors, accessdate, url. Removed parameters. You can use this bot yourself. Report bugs here. |
||
| Line 5: | Line 5: | ||
'''Antioxidants''' are [[Chemical compound|compounds]] that inhibit the [[Redox|oxidation]]. Oxidation is a [[chemical reaction]] that can produce [[Radical (chemistry)|free radicals]], thereby leading to [[chain reaction]]s that may damage the [[cell (biology)|cells]] of organisms. Antioxidants such as [[thiol]]s or [[ascorbic acid]] (vitamin C) terminate these chain reactions. To balance the [[Oxidation state|oxidative state]], plants and animals maintain complex systems of overlapping antioxidants, such as [[glutathione]] and [[enzyme]]s (e.g., [[catalase]] and [[superoxide dismutase]]), produced internally, or the [[dietary]] antioxidants [[vitamin A]], vitamin C, and [[vitamin E]]. |
'''Antioxidants''' are [[Chemical compound|compounds]] that inhibit the [[Redox|oxidation]]. Oxidation is a [[chemical reaction]] that can produce [[Radical (chemistry)|free radicals]], thereby leading to [[chain reaction]]s that may damage the [[cell (biology)|cells]] of organisms. Antioxidants such as [[thiol]]s or [[ascorbic acid]] (vitamin C) terminate these chain reactions. To balance the [[Oxidation state|oxidative state]], plants and animals maintain complex systems of overlapping antioxidants, such as [[glutathione]] and [[enzyme]]s (e.g., [[catalase]] and [[superoxide dismutase]]), produced internally, or the [[dietary]] antioxidants [[vitamin A]], vitamin C, and [[vitamin E]]. |
||
The term "antioxidant" is mostly used for two entirely different groups of substances: [[industrial chemicals]] that are added to products to prevent oxidation, and naturally occurring compounds that are present in foods and [[Tissue (biology)|tissue]]. The former, industrial antioxidants, have diverse uses: acting as [[preservative]]s in food and cosmetics, and being [[redox|oxidation]]-inhibitors in fuels.<ref name="Ullmann">{{cite encyclopedia|doi=10.1002/14356007.a16_719.pub2|chapter=Automotive Fuels|title=Ullmann's Encyclopedia of Industrial Chemistry|year=2007|last1=Dabelstein|first1=Werner|last2=Reglitzky|first2=Arno|last3=Schütze|first3=Andrea|last4=Reders|first4=Klaus|isbn=3-527-30673- |
The term "antioxidant" is mostly used for two entirely different groups of substances: [[industrial chemicals]] that are added to products to prevent oxidation, and naturally occurring compounds that are present in foods and [[Tissue (biology)|tissue]]. The former, industrial antioxidants, have diverse uses: acting as [[preservative]]s in food and cosmetics, and being [[redox|oxidation]]-inhibitors in fuels.<ref name="Ullmann">{{cite encyclopedia|doi=10.1002/14356007.a16_719.pub2|chapter=Automotive Fuels|title=Ullmann's Encyclopedia of Industrial Chemistry|year=2007|last1=Dabelstein|first1=Werner|last2=Reglitzky|first2=Arno|last3=Schütze|first3=Andrea|last4=Reders|first4=Klaus|isbn=978-3-527-30673-2 | name-list-format = vanc |url=https://books.google.com/books?id=IY-YtwEACAAJ}}</ref> |
||
Importantly, antioxidant [[dietary supplement]]s have not yet been shown to improve health in humans, or to be effective at preventing disease.<ref>{{Cite web|url=https://nccih.nih.gov/health/antioxidants/introduction.htm|title=Antioxidants: In Depth|website=NCCIH|language=en| |
Importantly, antioxidant [[dietary supplement]]s have not yet been shown to improve health in humans, or to be effective at preventing disease.<ref>{{Cite web|url=https://nccih.nih.gov/health/antioxidants/introduction.htm|title=Antioxidants: In Depth|website=NCCIH|language=en|accessdate=20 June 2018}}</ref> Supplements of [[beta-carotene]], vitamin A, and vitamin E have no effect on [[mortality rate]]<ref>{{cite journal | authors = Bjelakovic G, Nikolova D, Gluud C | title = Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? | journal = PLoS ONE | volume = 8 | issue = 9 | pages = e74558 | year = 2013 | pmid = 24040282 | pmc = 3765487 | doi = 10.1371/journal.pone.0074558 |bibcode = 2013PLoSO...874558B }}</ref><ref>{{cite journal | authors = Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ | title = Vitamin E and all-cause mortality: a meta-analysis | journal = Current Aging Science | volume = 4 | issue = 2 | pages = 158–70 | date = July 2011 | pmid = 21235492 | pmc = 4030744 | doi = 10.2174/1874609811104020158 }}</ref> or [[cancer]] risk.<ref>{{cite journal | authors = Cortés-Jofré M, Rueda JR, Corsini-Muñoz G, Fonseca-Cortés C, Caraballoso M, Bonfill Cosp X | title = Drugs for preventing lung cancer in healthy people | journal = The Cochrane Database of Systematic Reviews | volume = 10 | issue = | pages = CD002141 | year = 2012 | pmid = 23076895 | doi = 10.1002/14651858.CD002141.pub2 }}</ref><ref>{{cite journal | authors = Jiang L, Yang KH, Tian JH, Guan QL, Yao N, Cao N, Mi DH, Wu J, Ma B, Yang SH | title = Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials | journal = Nutrition and Cancer | volume = 62 | issue = 6 | pages = 719–27 | year = 2010 | pmid = 20661819 | doi = 10.1080/01635581.2010.494335 }}</ref> Additionally, supplementation with [[selenium]] or vitamin E do not reduce the risk of [[cardiovascular disease]].<ref>{{cite journal | authors = Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S | title = Selenium supplementation for the primary prevention of cardiovascular disease | journal = The Cochrane Database of Systematic Reviews | volume = 1 | issue = 1| pages = CD009671 | year = 2013 | pmid = 23440843 | doi = 10.1002/14651858.CD009671.pub2 | pmc = 4176632 | url = http://wrap.warwick.ac.uk/53654/1/WRAP_Clarke_CD009671.pdf | format = Full text }}</ref><ref>{{cite journal | authors = Shekelle PG, Morton SC, Jungvig LK, Udani J, Spar M, Tu W, J Suttorp M, Coulter I, Newberry SJ, Hardy M | title = Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease | journal = Journal of General Internal Medicine | volume = 19 | issue = 4 | pages = 380–9 | date = April 2004 | pmid = 15061748 | pmc = 1492195 | doi = 10.1111/j.1525-1497.2004.30090.x | url = http://europepmc.org/articles/pmc1492195?pdf=render | format = Full text }}</ref> |
||
== Health effects == |
== Health effects == |
||
| Line 13: | Line 13: | ||
=== Relation to diet === |
=== Relation to diet === |
||
Although certain levels of antioxidant [[vitamins]] in the diet are required for good health, there is still considerable debate on whether antioxidant-rich foods or supplements have anti-disease activity. Moreover, if they are actually beneficial, it is unknown which antioxidants are health-promoting in the diet and in what amounts beyond typical dietary intake.<ref name="Stanner" /><ref name="Shenkin">{{cite journal | |
Although certain levels of antioxidant [[vitamins]] in the diet are required for good health, there is still considerable debate on whether antioxidant-rich foods or supplements have anti-disease activity. Moreover, if they are actually beneficial, it is unknown which antioxidants are health-promoting in the diet and in what amounts beyond typical dietary intake.<ref name="Stanner" /><ref name="Shenkin">{{cite journal | authors = Shenkin A | title = The key role of micronutrients | journal = Clinical Nutrition | volume = 25 | issue = 1 | pages = 1–13 | date = February 2006 | pmid = 16376462 | doi = 10.1016/j.clnu.2005.11.006 }}</ref><ref>{{cite journal | authors = Woodside JV, McCall D, McGartland C, Young IS | title = Micronutrients: dietary intake v. supplement use | journal = The Proceedings of the Nutrition Society | volume = 64 | issue = 4 | pages = 543–53 | date = November 2005 | pmid = 16313697 | doi = 10.1079/PNS2005464 }}</ref> Some authors dispute the hypothesis that antioxidant vitamins could prevent chronic diseases,<ref name="Stanner">{{cite journal | authors = Stanner SA, Hughes J, Kelly CN, Buttriss J | title = A review of the epidemiological evidence for the 'antioxidant hypothesis' | journal = Public Health Nutrition | volume = 7 | issue = 3 | pages = 407–22 | date = May 2004 | pmid = 15153272 | doi = 10.1079/PHN2003543 }}</ref><ref>''[http://www.dietandcancerreport.org/?p=ER Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective]''. [[World Cancer Research Fund]] (2007). {{ISBN|978-0-9722522-2-5}}.</ref> and others maintain such that hypothesis is unproven and misguided.<ref name=Hail>{{cite journal | authors = Hail N, Cortes M, Drake EN, Spallholz JE | title = Cancer chemoprevention: a radical perspective | journal = Free Radical Biology & Medicine | volume = 45 | issue = 2 | pages = 97–110 | date = July 2008 | pmid = 18454943 | doi = 10.1016/j.freeradbiomed.2008.04.004 }}</ref> |
||
[[Polyphenol]]s, which often have antioxidant properties [[in vitro]], are not necessarily antioxidants [[in vivo]] due to extensive [[metabolism]] following digestion.<ref name="lpi">{{cite web|url=http://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/flavonoids|title=Flavonoids|publisher=Linus Pauling Institute, Oregon State University, Corvallis|date=2016|accessdate=24 July 2016}}</ref> In many polyphenols the [[catechol]] group acts as an [[electron acceptor]] and is therefore responsible for the antioxidant activity.<ref>{{cite journal|pmid=26867192|year=2016|author1=Csepregi|first1=K|title=Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols|journal=Molecules|volume=21|issue=2|pages=208|last2=Neugart|first2=S|last3=Schreiner|first3=M|last4=Hideg|first4=Éva|doi=10.3390/molecules21020208}}</ref> However, this catechol group undergoes extensive metabolism upon uptake in the human body, for example by [[catechol-O-methyl transferase]], and is therefore no longer able to act as an electron acceptor. Many polyphenols may have non-antioxidant roles in minute concentrations that affect [[cell-to-cell signaling]], [[receptor (biochemistry)|receptor]] sensitivity, [[inflammation|inflammatory]] [[enzyme]] activity or [[gene regulation]].<ref name=Williams>{{cite journal | |
[[Polyphenol]]s, which often have antioxidant properties [[in vitro]], are not necessarily antioxidants [[in vivo]] due to extensive [[metabolism]] following digestion.<ref name="lpi">{{cite web|url=http://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/flavonoids|title=Flavonoids|publisher=Linus Pauling Institute, Oregon State University, Corvallis|date=2016|accessdate=24 July 2016}}</ref> In many polyphenols the [[catechol]] group acts as an [[electron acceptor]] and is therefore responsible for the antioxidant activity.<ref>{{cite journal|pmid=26867192|year=2016|author1=Csepregi|first1=K|title=Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols|journal=Molecules|volume=21|issue=2|pages=208|last2=Neugart|first2=S|last3=Schreiner|first3=M|last4=Hideg|first4=Éva|doi=10.3390/molecules21020208}}</ref> However, this catechol group undergoes extensive metabolism upon uptake in the human body, for example by [[catechol-O-methyl transferase]], and is therefore no longer able to act as an electron acceptor. Many polyphenols may have non-antioxidant roles in minute concentrations that affect [[cell-to-cell signaling]], [[receptor (biochemistry)|receptor]] sensitivity, [[inflammation|inflammatory]] [[enzyme]] activity or [[gene regulation]].<ref name=Williams>{{cite journal | authors = Williams RJ, Spencer JP, Rice-Evans C | title = Flavonoids: antioxidants or signalling molecules? | journal = Free Radical Biology & Medicine | volume = 36 | issue = 7 | pages = 838–49 | date = April 2004 | pmid = 15019969 | doi = 10.1016/j.freeradbiomed.2004.01.001 }}</ref><ref name=Virgili>{{cite journal | authors = Virgili F, Marino M | title = Regulation of cellular signals from nutritional molecules: a specific role for phytochemicals, beyond antioxidant activity | journal = Free Radical Biology & Medicine | volume = 45 | issue = 9 | pages = 1205–16 | date = November 2008 | pmid = 18762244 | doi = 10.1016/j.freeradbiomed.2008.08.001 }}</ref> |
||
Although dietary antioxidants have been investigated for potential effects on [[neurodegenerative disease]]s such as [[Alzheimers|Alzheimer's disease]], [[Parkinsons|Parkinson's disease]], and [[amyotrophic lateral sclerosis]],<ref>{{cite journal | |
Although dietary antioxidants have been investigated for potential effects on [[neurodegenerative disease]]s such as [[Alzheimers|Alzheimer's disease]], [[Parkinsons|Parkinson's disease]], and [[amyotrophic lateral sclerosis]],<ref>{{cite journal | authors = Di Matteo V, Esposito E | title = Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis | journal = Current Drug Targets. CNS and Neurological Disorders | volume = 2 | issue = 2 | pages = 95–107 | date = April 2003 | pmid = 12769802 | doi = 10.2174/1568007033482959 }}</ref><ref>{{cite journal | authors = Rao AV, Balachandran B | title = Role of oxidative stress and antioxidants in neurodegenerative diseases | journal = Nutritional Neuroscience | volume = 5 | issue = 5 | pages = 291–309 | date = October 2002 | pmid = 12385592 | doi = 10.1080/1028415021000033767 }}</ref> these studies have been inconclusive.<ref>{{cite journal | authors = Crichton GE, Bryan J, Murphy KJ | title = Dietary antioxidants, cognitive function and dementia--a systematic review | journal = Plant Foods for Human Nutrition | volume = 68 | issue = 3 | pages = 279–92 | date = September 2013 | pmid = 23881465 | doi = 10.1007/s11130-013-0370-0 }}</ref><ref>{{cite journal | authors = Takeda A, Nyssen OP, Syed A, Jansen E, Bueno-de-Mesquita B, Gallo V | title = Vitamin A and carotenoids and the risk of Parkinson's disease: a systematic review and meta-analysis | journal = Neuroepidemiology | volume = 42 | issue = 1 | pages = 25–38 | year = 2014 | pmid = 24356061 | doi = 10.1159/000355849 }}</ref><ref>{{cite journal | authors = Harrison FE | title = A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer's disease | journal = Journal of Alzheimer's Disease | volume = 29 | issue = 4 | pages = 711–26 | year = 2012 | pmid = 22366772 | pmc = 3727637 | doi = 10.3233/JAD-2012-111853| url = http://europepmc.org/articles/pmc3727637?pdf=render | format = Accepted manuscript }}</ref> |
||
=== Drug candidates === |
=== Drug candidates === |
||
[[Tirilazad]] is an antioxidant steroid derivative that inhibits the lipid peroxidation that is believed to play a key role in neuronal death in stroke and head injury. It demonstrated activity in animal models of stroke,<ref>{{cite journal | |
[[Tirilazad]] is an antioxidant steroid derivative that inhibits the lipid peroxidation that is believed to play a key role in neuronal death in stroke and head injury. It demonstrated activity in animal models of stroke,<ref>{{cite journal | authors = Sena E, Wheble P, Sandercock P, Macleod M | title = Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke | journal = Stroke: A Journal of Cerebral Circulation | volume = 38 | issue = 2 | pages = 388–94 | date = February 2007 | pmid = 17204689 | doi = 10.1161/01.STR.0000254462.75851.22 }}</ref> but human trials demonstrated no effect on mortality or other outcomes in subarachnoid haemorrhage<ref>{{cite journal | authors = Zhang S, Wang L, Liu M, Wu B | title = Tirilazad for aneurysmal subarachnoid haemorrhage | journal = The Cochrane Database of Systematic Reviews | volume = | issue = 2 | pages = CD006778 | year = 2010 | pmid = 20166088 | doi = 10.1002/14651858.CD006778.pub2 }}</ref> and worsened results in ischemic stroke.<ref>{{cite journal | authors = Bath PM, Iddenden R, Bath FJ, Orgogozo JM | title = Tirilazad for acute ischaemic stroke | journal = The Cochrane Database of Systematic Reviews | volume = | issue = 4 | pages = CD002087 | year = 2001 | pmid = 11687138 | doi = 10.1002/14651858.CD002087 }}</ref> |
||
Similarly, the designed antioxidant NXY-059 exhibited efficacy in animal models, but failed to improve stroke outcomes in a clinical trial.<ref>{{cite journal | |
Similarly, the designed antioxidant NXY-059 exhibited efficacy in animal models, but failed to improve stroke outcomes in a clinical trial.<ref>{{cite journal | authors = Bath PM, Gray LJ, Bath AJ, Buchan A, Miyata T, Green AR | title = Effects of NXY-059 in experimental stroke: an individual animal meta-analysis | journal = British Journal of Pharmacology | volume = 157 | issue = 7 | pages = 1157–71 | date = August 2009 | pmid = 19422398 | pmc = 2743834 | doi = 10.1111/j.1476-5381.2009.00196.x }}</ref> As of November 2014, other antioxidants are being studied as potential neuroprotectants.<ref>{{cite journal | authors = Green AR, Ashwood T | title = Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers | journal = Current Drug Targets. CNS and Neurological Disorders | volume = 4 | issue = 2 | pages = 109–18 | date = April 2005 | pmid = 15857295 | doi = 10.2174/1568007053544156 }}</ref> |
||
Common pharmaceuticals (and supplements) with antioxidant properties may interfere with the efficacy of certain anticancer medication and radiation.<ref name="Lemmo, W Potential interactions of prescription and over-the-counter medications having antioxidant capabilities with radiation and chemotherapy 2014">{{cite journal | |
Common pharmaceuticals (and supplements) with antioxidant properties may interfere with the efficacy of certain anticancer medication and radiation.<ref name="Lemmo, W Potential interactions of prescription and over-the-counter medications having antioxidant capabilities with radiation and chemotherapy 2014">{{cite journal | authors = Lemmo W | title = Potential interactions of prescription and over-the-counter medications having antioxidant capabilities with radiation and chemotherapy | journal = International Journal of Cancer | volume = 137 | issue = 11 | pages = 2525–33 | date = September 2014 | pmid = 25220632 | doi = 10.1002/ijc.29208 }}</ref><ref>{{cite journal | authors = D'Andrea GM | title = Use of antioxidants during chemotherapy and radiotherapy should be avoided | journal = [[CA (journal)|CA: A Cancer Journal for Clinicians]] | volume = 55 | issue = 5 | pages = 319–21 | year = 2005 | pmid = 16166076 | doi = 10.3322/canjclin.55.5.319 }}</ref> |
||
A 2016 [[systematic review]] examined antioxidant medications, such as [[allopurinol]] and [[acetylcysteine]], as add on treatment for schizophrenia.<ref name=Mag2016/> Evidence was insufficient to determine benefits and there was potential for [[adverse effects]].<ref name=Mag2016>{{cite journal|last1=Magalhães| first1=P| last2=Dean|first2=O| first3=A| last3=Andreazza|title=Antioxidant treatments for schizophrenia|journal=Cochrane Database of Systematic Reviews|date=2016|volume=1|url=http://www.cochrane.org/CD008919/SCHIZ_antioxidants-add-treatment-people-schizophrenia|pages=CD008919.pub2 | |
A 2016 [[systematic review]] examined antioxidant medications, such as [[allopurinol]] and [[acetylcysteine]], as add on treatment for schizophrenia.<ref name=Mag2016/> Evidence was insufficient to determine benefits and there was potential for [[adverse effects]].<ref name=Mag2016>{{cite journal|last1=Magalhães| first1=P| last2=Dean|first2=O| first3=A| last3=Andreazza|title=Antioxidant treatments for schizophrenia|journal=Cochrane Database of Systematic Reviews|date=2016|volume=1|url=http://www.cochrane.org/CD008919/SCHIZ_antioxidants-add-treatment-people-schizophrenia|pages=CD008919.pub2 |doi=10.1002/14651858.CD008919.pub2}}</ref> |
||
=== Adverse effects === |
=== Adverse effects === |
||
| Line 33: | Line 33: | ||
[[Image:Phytate.png|thumb|right|Structure of the metal chelator [[phytic acid]].]] |
[[Image:Phytate.png|thumb|right|Structure of the metal chelator [[phytic acid]].]] |
||
Relatively strong reducing acids can have [[antinutrient]] effects by binding to [[dietary mineral]]s such as [[iron]] and [[zinc]] in the [[Human gastrointestinal tract|gastrointestinal tract]] and preventing them from being absorbed.<ref>{{cite journal | |
Relatively strong reducing acids can have [[antinutrient]] effects by binding to [[dietary mineral]]s such as [[iron]] and [[zinc]] in the [[Human gastrointestinal tract|gastrointestinal tract]] and preventing them from being absorbed.<ref>{{cite journal | authors = Hurrell RF | title = Influence of vegetable protein sources on trace element and mineral bioavailability | journal = The Journal of Nutrition | volume = 133 | issue = 9 | pages = 2973S–7S | date = September 2003 | pmid = 12949395 | url = http://jn.nutrition.org/cgi/content/full/133/9/2973S | doi = 10.1093/jn/133.9.2973S }}</ref> Notable examples are [[oxalic acid]], [[tannin]]s and [[phytic acid]], which are high in plant-based diets.<ref>{{cite journal | authors = Hunt JR | title = Bioavailability of iron, zinc, and other trace minerals from vegetarian diets | journal = The American Journal of Clinical Nutrition | volume = 78 | issue = 3 Suppl | pages = 633S–639S | date = September 2003 | pmid = 12936958 | url = http://www.ajcn.org/cgi/content/full/78/3/633S | doi = 10.1093/ajcn/78.3.633S }}</ref> [[Calcium]] and iron deficiencies are not uncommon in diets in [[developing country|developing countries]] where less meat is eaten and there is high consumption of phytic acid from beans and unleavened [[whole grain]] bread.<ref>{{cite journal | authors = Gibson RS, Perlas L, Hotz C | title = Improving the bioavailability of nutrients in plant foods at the household level | journal = The Proceedings of the Nutrition Society | volume = 65 | issue = 2 | pages = 160–8 | date = May 2006 | pmid = 16672077 | doi = 10.1079/PNS2006489 }}</ref> |
||
{| class="wikitable" style="margin-left: auto; margin-right: auto;" |
{| class="wikitable" style="margin-left: auto; margin-right: auto;" |
||
| Line 40: | Line 40: | ||
!Reducing acid present |
!Reducing acid present |
||
|- |
|- |
||
| style="text-align:center;"|[[Cocoa bean]] and chocolate, [[spinach]], [[turnip]] and [[rhubarb]].<ref name="Mosha">{{cite journal | |
| style="text-align:center;"|[[Cocoa bean]] and chocolate, [[spinach]], [[turnip]] and [[rhubarb]].<ref name="Mosha">{{cite journal | authors = Mosha TC, Gaga HE, Pace RD, Laswai HS, Mtebe K | title = Effect of blanching on the content of antinutritional factors in selected vegetables | journal = Plant Foods for Human Nutrition | volume = 47 | issue = 4 | pages = 361–7 | date = June 1995 | pmid = 8577655 | doi = 10.1007/BF01088275 }}</ref> |
||
| style="text-align:center;"|[[Oxalic acid]] |
| style="text-align:center;"|[[Oxalic acid]] |
||
|- |
|- |
||
| style="text-align:center;"|[[Whole grain]]s, maize, [[legume]]s.<ref>{{cite journal | |
| style="text-align:center;"|[[Whole grain]]s, maize, [[legume]]s.<ref>{{cite journal | authors = Sandberg AS | title = Bioavailability of minerals in legumes | journal = The British Journal of Nutrition | volume = 88 Suppl 3 | issue = Suppl 3 | pages = S281–5 | date = December 2002 | pmid = 12498628 | doi = 10.1079/BJN/2002718 }}</ref> |
||
| style="text-align:center;"|[[Phytic acid]] |
| style="text-align:center;"|[[Phytic acid]] |
||
|- |
|- |
||
| style="text-align:center;"|Tea, [[bean]]s, [[cabbage]].<ref name="Mosha" /><ref name="Beecher">{{cite journal | |
| style="text-align:center;"|Tea, [[bean]]s, [[cabbage]].<ref name="Mosha" /><ref name="Beecher">{{cite journal | authors = Beecher GR | title = Overview of dietary flavonoids: nomenclature, occurrence and intake | journal = The Journal of Nutrition | volume = 133 | issue = 10 | pages = 3248S–3254S | date = October 2003 | pmid = 14519822 | url = http://jn.nutrition.org/cgi/content/full/133/10/3248S | doi = 10.1093/jn/133.10.3248S }}</ref> |
||
| style="text-align:center;"|[[Tannin]]s |
| style="text-align:center;"|[[Tannin]]s |
||
|} |
|} |
||
[[Nonpolar]] antioxidants such as [[eugenol]]—a major component of [[oil of cloves]]—have toxicity limits that can be exceeded with the misuse of undiluted [[essential oil]]s.<ref>{{cite journal | |
[[Nonpolar]] antioxidants such as [[eugenol]]—a major component of [[oil of cloves]]—have toxicity limits that can be exceeded with the misuse of undiluted [[essential oil]]s.<ref>{{cite journal | authors = Prashar A, Locke IC, Evans CS | title = Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells | journal = Cell Proliferation | volume = 39 | issue = 4 | pages = 241–8 | date = August 2006 | pmid = 16872360 | doi = 10.1111/j.1365-2184.2006.00384.x }}</ref> Toxicity associated with high doses of water-soluble antioxidants such as ascorbic acid are less of a concern, as these compounds can be excreted rapidly in [[urine]].<ref>{{cite journal | authors = Hornig D, Vuilleumier JP, Hartmann D | title = Absorption of large, single, oral intakes of ascorbic acid | journal = International Journal for Vitamin and Nutrition Research | volume = 50 | issue = 3 | pages = 309–14 | year = 1980 | pmid = 7429760 }}</ref> More seriously, very high doses of some antioxidants may have harmful long-term effects. The beta-carotene and Retinol Efficacy Trial (CARET) study of lung cancer patients found that smokers given supplements containing beta-carotene and vitamin A had increased rates of lung cancer.<ref>{{cite journal | authors = Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Cherniack MG, Brodkin CA, Hammar S | title = Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial | journal = Journal of the National Cancer Institute | volume = 88 | issue = 21 | pages = 1550–9 | date = November 1996 | pmid = 8901853 | doi = 10.1093/jnci/88.21.1550 | url = https://academic.oup.com/jnci/article-pdf/88/21/1550/7811338/88-21-1550.pdf }}</ref> Subsequent studies confirmed these adverse effects.<ref>{{cite journal | authors = Albanes D | title = Beta-carotene and lung cancer: a case study | journal = The American Journal of Clinical Nutrition | volume = 69 | issue = 6 | pages = 1345S–50S | date = June 1999 | pmid = 10359235 | url = http://www.ajcn.org/cgi/content/full/69/6/1345S | doi = 10.1093/ajcn/69.6.1345S }}</ref> |
||
These harmful effects may also be seen in non-smokers, as one [[meta-analysis]] including data from approximately 230,000 patients showed that β-carotene, vitamin A or vitamin E supplementation is associated with increased mortality, but saw no significant effect from vitamin C.<ref name=Bjelakovic>{{cite journal | |
These harmful effects may also be seen in non-smokers, as one [[meta-analysis]] including data from approximately 230,000 patients showed that β-carotene, vitamin A or vitamin E supplementation is associated with increased mortality, but saw no significant effect from vitamin C.<ref name=Bjelakovic>{{cite journal | authors = Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C | title = Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis | journal = JAMA | volume = 297 | issue = 8 | pages = 842–57 | date = February 2007 | pmid = 17327526 | doi = 10.1001/jama.297.8.842 | url = http://jama.ama-assn.org/cgi/content/abstract/297/8/842 }}</ref> No health risk was seen when all the randomized controlled studies were examined together, but an increase in mortality was detected when only high-quality and low-bias risk trials were examined separately.<ref name=Bj2012/> As the majority of these low-bias trials dealt with either [[Old age|elderly people]], or people with disease, these results may not apply to the general population.<ref>[https://www.sciencedaily.com/releases/2007/02/070228172604.htm Study Citing Antioxidant Vitamin Risks Based On Flawed Methodology, Experts Argue] News release from [[Oregon State University]] published on ScienceDaily. Retrieved 19 April 2007</ref> This meta-analysis was later repeated and extended by the same authors, with the new analysis published by the [[Cochrane Collaboration]]; this analysis confirmed the previous results.<ref name=Bj2012>{{cite journal | authors = Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C | title = Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases | journal = The Cochrane Database of Systematic Reviews | volume = 3 | issue = 3 | pages = CD007176 | date = 14 March 2012 | pmid = 22419320 | doi = 10.1002/14651858.CD007176.pub2 | url = http://www.scielo.br/pdf/spmj/v133n2/1516-3180-spmj-133-02-00164.pdf | format = Submitted manuscript }}</ref> These two publications are consistent with some previous meta-analyzes that also suggested that Vitamin E supplementation increased mortality,<ref>{{cite journal | authors = Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E | title = Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality | journal = Annals of Internal Medicine | volume = 142 | issue = 1 | pages = 37–46 | date = January 2005 | pmid = 15537682 | doi = 10.7326/0003-4819-142-1-200501040-00110 }}</ref> and that antioxidant supplements increased the risk of [[Colorectal cancer|colon cancer]].<ref name="Bjelakovic G 2036">{{cite journal | authors = Bjelakovic G, Nagorni A, Nikolova D, Simonetti RG, Bjelakovic M, Gluud C | title = Meta-analysis: antioxidant supplements for primary and secondary prevention of colorectal adenoma | journal = Alimentary Pharmacology & Therapeutics | volume = 24 | issue = 2 | pages = 281–91 | date = July 2006 | pmid = 16842454 | doi = 10.1111/j.1365-2036.2006.02970.x }}</ref> [[Beta-carotene]] may also increase lung cancer.<ref name="Bjelakovic G 2036"/><ref>{{cite journal | authors = Cortés-Jofré M, Rueda JR, Corsini-Muñoz G, Fonseca-Cortés C, Caraballoso M, Bonfill Cosp X | title = Drugs for preventing lung cancer in healthy people | journal = The Cochrane Database of Systematic Reviews | volume = 10 | pages = CD002141 | date = 17 October 2012 | pmid = 23076895 | doi = 10.1002/14651858.CD002141.pub2 }}</ref> Overall, the large number of clinical trials carried out on antioxidant supplements suggest that either these products have no effect on health, or that they cause a small increase in mortality in elderly or vulnerable populations.<ref name="Stanner" /><ref name="Shenkin" /><ref name="Bjelakovic" /> |
||
While antioxidant supplementation is widely used in attempts to prevent the development of cancer, antioxidants may interfere with cancer treatments,<ref>{{cite journal | |
While antioxidant supplementation is widely used in attempts to prevent the development of cancer, antioxidants may interfere with cancer treatments,<ref>{{cite journal | authors = Schumacker PT | title = Reactive oxygen species in cancer cells: live by the sword, die by the sword | journal = Cancer Cell | volume = 10 | issue = 3 | pages = 175–6 | date = September 2006 | pmid = 16959608 | doi = 10.1016/j.ccr.2006.08.015 }}</ref> since the environment of cancer cells causes high levels of oxidative stress, making these cells more susceptible to the further oxidative stress induced by treatments. As a result, by reducing the redox stress in cancer cells, antioxidant supplements (and pharmaceuticals) could decrease the effectiveness of [[radiotherapy]] and [[chemotherapy]].<ref name="Lemmo, W Potential interactions of prescription and over-the-counter medications having antioxidant capabilities with radiation and chemotherapy 2014"/><ref>{{cite journal | authors = Seifried HE, McDonald SS, Anderson DE, Greenwald P, Milner JA | title = The antioxidant conundrum in cancer | journal = Cancer Research | volume = 63 | issue = 15 | pages = 4295–8 | date = August 2003 | pmid = 12907593 | url = http://cancerres.aacrjournals.org/cgi/content/full/63/15/4295 }}</ref><ref>{{cite journal | authors = Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB | title = Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? | journal = Journal of the National Cancer Institute | volume = 100 | issue = 11 | pages = 773–83 | date = June 2008 | pmid = 18505970 | doi = 10.1093/jnci/djn148 }}</ref> On the other hand, other reviews have suggested that antioxidants could reduce [[adverse effect|side effects]] or increase survival times.<ref>{{cite journal | authors = Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C | title = Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials | journal = International Journal of Cancer | volume = 123 | issue = 6 | pages = 1227–39 | date = September 2008 | pmid = 18623084 | doi = 10.1002/ijc.23754 }}</ref><ref>{{cite journal | authors = Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C | title = Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials | journal = Cancer Treatment Reviews | volume = 33 | issue = 5 | pages = 407–18 | date = August 2007 | pmid = 17367938 | doi = 10.1016/j.ctrv.2007.01.005 }}</ref> |
||
== Oxidative challenge in biology == |
== Oxidative challenge in biology == |
||
| Line 61: | Line 61: | ||
[[Image:L-ascorbic-acid-3D-balls.png|thumb|right|The structure of the antioxidant [[vitamin]] [[ascorbic acid]] (vitamin C).]] |
[[Image:L-ascorbic-acid-3D-balls.png|thumb|right|The structure of the antioxidant [[vitamin]] [[ascorbic acid]] (vitamin C).]] |
||
A [[paradox]] in [[metabolism]] is that, while the vast majority of complex [[life|life on Earth]] requires [[oxygen]] for its existence, oxygen is a highly reactive molecule that damages living organisms by producing [[reactive oxygen species]].<ref name="Davies">{{cite journal | |
A [[paradox]] in [[metabolism]] is that, while the vast majority of complex [[life|life on Earth]] requires [[oxygen]] for its existence, oxygen is a highly reactive molecule that damages living organisms by producing [[reactive oxygen species]].<ref name="Davies">{{cite journal | authors = Davies KJ | title = Oxidative stress: the paradox of aerobic life | journal = Biochemical Society Symposium | volume = 61 | pages = 1–31 | year = 1995 | pmid = 8660387 | doi=10.1042/bss0610001}}</ref> Consequently, organisms contain a complex network of antioxidant [[metabolite]]s and [[enzyme]]s that work together to prevent oxidative damage to cellular components such as [[DNA]], [[protein]]s and [[lipid]]s.<ref name="Sies">{{cite journal | authors = Sies H | title = Oxidative stress: oxidants and antioxidants | journal = Experimental Physiology | volume = 82 | issue = 2 | pages = 291–5 | date = March 1997 | pmid = 9129943 | doi = 10.1113/expphysiol.1997.sp004024 | url = http://ep.physoc.org/content/82/2/291.long }}</ref><ref name="Vertuani">{{cite journal | authors = Vertuani S, Angusti A, Manfredini S | title = The antioxidants and pro-antioxidants network: an overview | journal = Current Pharmaceutical Design | volume = 10 | issue = 14 | pages = 1677–94 | year = 2004 | pmid = 15134565 | doi = 10.2174/1381612043384655 }}</ref> In general, antioxidant systems either prevent these reactive species from being formed, or remove them before they can damage vital components of the cell.<ref name="Davies" /><ref name="Sies" /> However, reactive oxygen species also have useful cellular functions, such as [[redox signaling]]. Thus, the function of antioxidant systems is not to remove oxidants entirely, but instead to keep them at an optimum level.<ref>{{cite journal | authors = Rhee SG | title = Cell signaling. H2O2, a necessary evil for cell signaling | journal = Science | volume = 312 | issue = 5782 | pages = 1882–3 | date = June 2006 | pmid = 16809515 | doi = 10.1126/science.1130481 }}</ref> |
||
The reactive oxygen species produced in cells include [[hydrogen peroxide]] (H<sub>2</sub>O<sub>2</sub>), [[hypochlorous acid]] (HClO), and [[free radical]]s such as the [[hydroxyl radical]] (·OH) and the [[superoxide|superoxide anion]] (O<sub>2</sub><sup>−</sup>).<ref name="emfafb">{{cite journal | |
The reactive oxygen species produced in cells include [[hydrogen peroxide]] (H<sub>2</sub>O<sub>2</sub>), [[hypochlorous acid]] (HClO), and [[free radical]]s such as the [[hydroxyl radical]] (·OH) and the [[superoxide|superoxide anion]] (O<sub>2</sub><sup>−</sup>).<ref name="emfafb">{{cite journal | authors = Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J | title = Free radicals and antioxidants in normal physiological functions and human disease | journal = The International Journal of Biochemistry & Cell Biology | volume = 39 | issue = 1 | pages = 44–84 | year = 2007 | pmid = 16978905 | doi = 10.1016/j.biocel.2006.07.001 }}</ref> The hydroxyl radical is particularly unstable and will react rapidly and non-specifically with most biological molecules. This species is produced from hydrogen peroxide in [[catalysis|metal-catalyzed]] redox reactions such as the [[Fenton reaction]].<ref name="ReferenceA">{{cite journal | authors = Stohs SJ, Bagchi D | title = Oxidative mechanisms in the toxicity of metal ions | journal = Free Radical Biology & Medicine | volume = 18 | issue = 2 | pages = 321–36 | date = February 1995 | pmid = 7744317 | doi = 10.1016/0891-5849(94)00159-H | url = http://www8.umoncton.ca/umcm-gauthier_didier/bc6423/2SO/Strohs95.pdf | format = Submitted manuscript }}</ref> These oxidants can damage cells by starting chemical chain reactions such as [[lipid peroxidation]], or by oxidizing DNA or proteins.<ref name="Sies" /> Damage to DNA can cause [[mutation]]s and possibly cancer, if not reversed by [[DNA repair]] mechanisms,<ref>{{cite journal | authors = Nakabeppu Y, Sakumi K, Sakamoto K, Tsuchimoto D, Tsuzuki T, Nakatsu Y | title = Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids | journal = Biological Chemistry | volume = 387 | issue = 4 | pages = 373–9 | date = April 2006 | pmid = 16606334 | doi = 10.1515/BC.2006.050 }}</ref><ref>{{cite journal | authors = Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J | title = Role of oxygen radicals in DNA damage and cancer incidence | journal = Molecular and Cellular Biochemistry | volume = 266 | issue = 1–2 | pages = 37–56 | date = November 2004 | pmid = 15646026 | doi = 10.1023/B:MCBI.0000049134.69131.89 }}</ref> while damage to [[protein]]s causes enzyme inhibition, [[denaturation (biochemistry)|denaturation]] and [[proteasome|protein degradation]].<ref>{{cite journal | authors = Stadtman ER | title = Protein oxidation and aging | journal = Science | volume = 257 | issue = 5074 | pages = 1220–4 | date = August 1992 | pmid = 1355616 | doi = 10.1126/science.1355616 | bibcode = 1992Sci...257.1220S | url = https://zenodo.org/record/1230934/files/article.pdf | format = Submitted manuscript }}</ref> |
||
The use of oxygen as part of the process for generating metabolic energy produces reactive oxygen species.<ref name="Raha">{{cite journal | |
The use of oxygen as part of the process for generating metabolic energy produces reactive oxygen species.<ref name="Raha">{{cite journal | authors = Raha S, Robinson BH | title = Mitochondria, oxygen free radicals, disease and ageing | journal = Trends in Biochemical Sciences | volume = 25 | issue = 10 | pages = 502–8 | date = October 2000 | pmid = 11050436 | doi = 10.1016/S0968-0004(00)01674-1 }}</ref> In this process, the superoxide anion is produced as a [[by-product]] of several steps in the [[electron transport chain]].<ref>{{cite journal | authors = Lenaz G | title = The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology | journal = IUBMB Life | volume = 52 | issue = 3–5 | pages = 159–64 | year = 2001 | pmid = 11798028 | doi = 10.1080/15216540152845957 }}</ref> Particularly important is the reduction of [[coenzyme Q]] in [[complex III]], since a highly reactive free radical is formed as an intermediate (Q'''·'''<sup>−</sup>). This unstable intermediate can lead to electron "leakage", when electrons jump directly to oxygen and form the superoxide anion, instead of moving through the normal series of well-controlled reactions of the electron transport chain.<ref>{{cite journal | authors = Finkel T, Holbrook NJ | title = Oxidants, oxidative stress and the biology of ageing | journal = Nature | volume = 408 | issue = 6809 | pages = 239–47 | date = November 2000 | pmid = 11089981 | doi = 10.1038/35041687 | bibcode = 2000Natur.408..239F }}</ref> Peroxide is also produced from the oxidation of reduced [[flavoprotein]]s, such as [[complex I]].<ref>{{cite journal | authors = Hirst J, King MS, Pryde KR | title = The production of reactive oxygen species by complex I | journal = Biochemical Society Transactions | volume = 36 | issue = Pt 5 | pages = 976–80 | date = October 2008 | pmid = 18793173 | doi = 10.1042/BST0360976 }}</ref> However, although these enzymes can produce oxidants, the relative importance of the electron transfer chain to other processes that generate peroxide is unclear.<ref>{{cite journal | authors = Seaver LC, Imlay JA | title = Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? | journal = The Journal of Biological Chemistry | volume = 279 | issue = 47 | pages = 48742–50 | date = November 2004 | pmid = 15361522 | doi = 10.1074/jbc.M408754200 }}</ref><ref name="Pathways Ofoxidativedamage">{{cite journal | authors = Imlay JA | title = Pathways of oxidative damage | journal = Annual Review of Microbiology | volume = 57 | pages = 395–418 | year = 2003 | pmid = 14527285 | doi = 10.1146/annurev.micro.57.030502.090938 }}</ref> In plants, [[algae]], and [[cyanobacteria]], reactive oxygen species are also produced during [[photosynthesis]],<ref>{{cite journal | authors = Demmig-Adams B, Adams WW | title = Antioxidants in photosynthesis and human nutrition | journal = Science | volume = 298 | issue = 5601 | pages = 2149–53 | date = December 2002 | pmid = 12481128 | doi = 10.1126/science.1078002 | bibcode = 2002Sci...298.2149D }}</ref> particularly under conditions of high [[irradiance|light intensity]].<ref>{{cite journal | authors = Krieger-Liszkay A | title = Singlet oxygen production in photosynthesis | journal = Journal of Experimental Botany | volume = 56 | issue = 411 | pages = 337–46 | date = January 2005 | pmid = 15310815 | doi = 10.1093/jxb/erh237 | citeseerx = 10.1.1.327.9651 }}</ref> This effect is partly offset by the involvement of [[carotenoid]]s in [[photoinhibition]], and in algae and cyanobacteria, by large amount of [[iodide]] and [[selenium]],<ref>{{cite journal|last1=Kupper|first1=F. C.|last2=Carpenter|first2=L. J.|authorlink2=Lucy Carpenter|last3=McFiggans|first3=G. B.|last4=Palmer|first4=C. J.|last5=Waite|first5=T. J.|last6=Boneberg|first6=E.-M.|last7=Woitsch|first7=S.|last8=Weiller|first8=M.|last9=Abela|first9=R.|last10=Grolimund|first10=D.|last11=Potin|first11=P.|last12=Butler|first12=A.|last13=Luther|first13=G. W.|last14=Kroneck|first14=P. M. H.|last15=Meyer-Klaucke|first15=W.|last16=Feiters|first16=M. C.|title=Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry|journal=[[Proceedings of the National Academy of Sciences]]|volume=105|issue=19|year=2008|pages=6954–6958|issn=0027-8424| pmid = 18458346 | pmc = 2383960 | doi = 10.1073/pnas.0709959105 | bibcode = 2008PNAS..105.6954K }}</ref> which involves these antioxidants reacting with over-reduced forms of the [[photosynthetic reaction centre]]s to prevent the production of reactive oxygen species.<ref>{{cite journal | authors = Szabó I, Bergantino E, Giacometti GM | title = Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation | journal = EMBO Reports | volume = 6 | issue = 7 | pages = 629–34 | date = July 2005 | pmid = 15995679 | pmc = 1369118 | doi = 10.1038/sj.embor.7400460 }}</ref><ref>{{cite journal | authors = Kerfeld CA | title = Water-soluble carotenoid proteins of cyanobacteria | journal = Archives of Biochemistry and Biophysics | volume = 430 | issue = 1 | pages = 2–9 | date = October 2004 | pmid = 15325905 | doi = 10.1016/j.abb.2004.03.018 | url = https://cloudfront.escholarship.org/dist/prd/content/qt3dm533x9/qt3dm533x9.pdf | format = Submitted manuscript }}</ref> |
||
== Examples of bioactive antioxidant compounds == |
== Examples of bioactive antioxidant compounds == |
||
Antioxidants are classified into two broad divisions, depending on whether they are soluble in water ([[hydrophile|hydrophilic]]) or in lipids ([[Lipophilicity|lipophilic]]). In general, water-soluble antioxidants react with oxidants in the cell [[cytosol]] and the [[blood plasma]], while lipid-soluble antioxidants protect [[cell membrane]]s from lipid peroxidation.<ref name="Sies" /> These compounds may be synthesized in the body or obtained from the diet.<ref name="Vertuani" /> The different antioxidants are present at a wide range of concentrations in [[Bodily fluid|body fluids]] and tissues, with some such as glutathione or [[ubiquinone]] mostly present within cells, while others such as [[uric acid]] are more evenly distributed (see table below). Some antioxidants are only found in a few organisms and these compounds can be important in [[pathogen]]s and can be [[virulence factor]]s.<ref>{{cite journal | |
Antioxidants are classified into two broad divisions, depending on whether they are soluble in water ([[hydrophile|hydrophilic]]) or in lipids ([[Lipophilicity|lipophilic]]). In general, water-soluble antioxidants react with oxidants in the cell [[cytosol]] and the [[blood plasma]], while lipid-soluble antioxidants protect [[cell membrane]]s from lipid peroxidation.<ref name="Sies" /> These compounds may be synthesized in the body or obtained from the diet.<ref name="Vertuani" /> The different antioxidants are present at a wide range of concentrations in [[Bodily fluid|body fluids]] and tissues, with some such as glutathione or [[ubiquinone]] mostly present within cells, while others such as [[uric acid]] are more evenly distributed (see table below). Some antioxidants are only found in a few organisms and these compounds can be important in [[pathogen]]s and can be [[virulence factor]]s.<ref>{{cite journal | authors = Miller RA, Britigan BE | title = Role of oxidants in microbial pathophysiology | journal = Clinical Microbiology Reviews | volume = 10 | issue = 1 | pages = 1–18 | date = January 1997 | pmid = 8993856 | pmc = 172912 }}</ref> |
||
The relative importance and interactions between these different antioxidants is a very complex question, with the various antioxidant compounds and antioxidant enzyme systems having [[synergy|synergistic]] and interdependent effects on one another.<ref>{{cite journal | |
The relative importance and interactions between these different antioxidants is a very complex question, with the various antioxidant compounds and antioxidant enzyme systems having [[synergy|synergistic]] and interdependent effects on one another.<ref>{{cite journal | authors = Chaudière J, Ferrari-Iliou R | title = Intracellular antioxidants: from chemical to biochemical mechanisms | journal = Food and Chemical Toxicology | volume = 37 | issue = 9–10 | pages = 949–62 | year = 1999 | pmid = 10541450 | doi = 10.1016/S0278-6915(99)00090-3 }}</ref><ref>{{cite journal | authors = Sies H | title = Strategies of antioxidant defense | journal = European Journal of Biochemistry / FEBS | volume = 215 | issue = 2 | pages = 213–9 | date = July 1993 | pmid = 7688300 | doi = 10.1111/j.1432-1033.1993.tb18025.x }}</ref> The action of one antioxidant may therefore depend on the proper function of other members of the antioxidant system.<ref name="Vertuani" /> The amount of protection provided by any one antioxidant will also depend on its concentration, its reactivity towards the particular reactive oxygen species being considered, and the status of the antioxidants with which it interacts.<ref name="Vertuani" /> |
||
Some compounds contribute to antioxidant defense by [[chelation|chelating]] [[transition metal]]s and preventing them from catalyzing the production of free radicals in the cell. Particularly important is the ability to sequester iron, which is the function of [[iron-binding proteins]] such as [[transferrin]] and [[ferritin]].<ref name="Pathways Ofoxidativedamage"/> [[Selenium]] and zinc are commonly referred to as ''antioxidant nutrients'', but these [[chemical element]]s have no antioxidant action themselves and are instead required for the activity of some antioxidant enzymes, as is discussed below. |
Some compounds contribute to antioxidant defense by [[chelation|chelating]] [[transition metal]]s and preventing them from catalyzing the production of free radicals in the cell. Particularly important is the ability to sequester iron, which is the function of [[iron-binding proteins]] such as [[transferrin]] and [[ferritin]].<ref name="Pathways Ofoxidativedamage"/> [[Selenium]] and zinc are commonly referred to as ''antioxidant nutrients'', but these [[chemical element]]s have no antioxidant action themselves and are instead required for the activity of some antioxidant enzymes, as is discussed below. |
||
| Line 83: | Line 83: | ||
| [[Ascorbic acid]] ([[vitamin C]]) |
| [[Ascorbic acid]] ([[vitamin C]]) |
||
| Water |
| Water |
||
| 50–60<ref>{{cite journal | |
| 50–60<ref>{{cite journal | authors = Khaw KT, Woodhouse P | title = Interrelation of vitamin C, infection, haemostatic factors, and cardiovascular disease | journal = BMJ | volume = 310 | issue = 6994 | pages = 1559–63 | date = June 1995 | pmid = 7787643 | pmc = 2549940 | doi = 10.1136/bmj.310.6994.1559 | url = http://bmj.com/cgi/pmidlookup?view=long&pmid=7787643 }}</ref> |
||
| 260 (human)<ref name="Evelson">{{cite journal | |
| 260 (human)<ref name="Evelson">{{cite journal | authors = Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi EA | title = Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols | journal = Archives of Biochemistry and Biophysics | volume = 388 | issue = 2 | pages = 261–6 | date = April 2001 | pmid = 11368163 | doi = 10.1006/abbi.2001.2292 }}</ref> |
||
|- |
|- |
||
| [[Glutathione]] |
| [[Glutathione]] |
||
| Water |
| Water |
||
| 4<ref>{{cite journal | |
| 4<ref>{{cite journal | authors = Morrison JA, Jacobsen DW, Sprecher DL, Robinson K, Khoury P, Daniels SR | title = Serum glutathione in adolescent males predicts parental coronary heart disease | journal = Circulation | volume = 100 | issue = 22 | pages = 2244–7 | date = November 1999 | pmid = 10577998 | doi = 10.1161/01.CIR.100.22.2244 | url = http://circ.ahajournals.org/content/100/22/2244.full.pdf }}</ref> |
||
| 6,400 (human)<ref name="Evelson" /> |
| 6,400 (human)<ref name="Evelson" /> |
||
|- |
|- |
||
| [[Lipoic acid]] |
| [[Lipoic acid]] |
||
| Water |
| Water |
||
| 0.1–0.7<ref>{{cite journal | |
| 0.1–0.7<ref>{{cite journal | authors = Teichert J, Preiss R | title = HPLC-methods for determination of lipoic acid and its reduced form in human plasma | journal = International Journal of Clinical Pharmacology, Therapy, and Toxicology | volume = 30 | issue = 11 | pages = 511–2 | date = November 1992 | pmid = 1490813 }}</ref> |
||
| 4–5 (rat)<ref>{{cite journal | |
| 4–5 (rat)<ref>{{cite journal | authors = Akiba S, Matsugo S, Packer L, Konishi T | title = Assay of protein-bound lipoic acid in tissues by a new enzymatic method | journal = Analytical Biochemistry | volume = 258 | issue = 2 | pages = 299–304 | date = May 1998 | pmid = 9570844 | doi = 10.1006/abio.1998.2615 }}</ref> |
||
|- |
|- |
||
| [[Uric acid]] |
| [[Uric acid]] |
||
| Water |
| Water |
||
| 200–400<ref name=Glantzounis>{{cite journal | |
| 200–400<ref name=Glantzounis>{{cite journal | authors = Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA | title = Uric acid and oxidative stress | journal = Current Pharmaceutical Design | volume = 11 | issue = 32 | pages = 4145–51 | year = 2005 | pmid = 16375736 | doi = 10.2174/138161205774913255 }}</ref> |
||
| 1,600 (human)<ref name="Evelson" /> |
| 1,600 (human)<ref name="Evelson" /> |
||
|- |
|- |
||
| [[Carotene]]s |
| [[Carotene]]s |
||
| Lipid |
| Lipid |
||
| [[carotene|β-carotene]]: 0.5–1<ref>{{cite journal | |
| [[carotene|β-carotene]]: 0.5–1<ref>{{cite journal | authors = El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H | title = Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake | journal = The American Journal of Clinical Nutrition | volume = 76 | issue = 1 | pages = 172–9 | date = July 2002 | pmid = 12081831 | url = http://www.ajcn.org/content/76/1/172.long | doi = 10.1093/ajcn/76.1.172 }}</ref> |
||
[[retinol]] (vitamin A): 1–3<ref name="Sowell">{{cite journal | |
[[retinol]] (vitamin A): 1–3<ref name="Sowell">{{cite journal | authors = Sowell AL, Huff DL, Yeager PR, Caudill SP, Gunter EW | title = Retinol, alpha-tocopherol, lutein/zeaxanthin, beta-cryptoxanthin, lycopene, alpha-carotene, trans-beta-carotene, and four retinyl esters in serum determined simultaneously by reversed-phase HPLC with multiwavelength detection | journal = Clinical Chemistry | volume = 40 | issue = 3 | pages = 411–6 | date = March 1994 | pmid = 8131277 | url = http://www.clinchem.org/cgi/pmidlookup?view=long&pmid=8131277 }}</ref> |
||
| 5 (human, total carotenoids)<ref>{{cite journal | |
| 5 (human, total carotenoids)<ref>{{cite journal | authors = Stahl W, Schwarz W, Sundquist AR, Sies H | title = cis-trans isomers of lycopene and beta-carotene in human serum and tissues | journal = Archives of Biochemistry and Biophysics | volume = 294 | issue = 1 | pages = 173–7 | date = April 1992 | pmid = 1550343 | doi = 10.1016/0003-9861(92)90153-N }}</ref> |
||
|- |
|- |
||
| [[tocopherol|α-Tocopherol]] (vitamin E) |
| [[tocopherol|α-Tocopherol]] (vitamin E) |
||
| Line 114: | Line 114: | ||
| [[Coenzyme Q|Ubiquinol]] (coenzyme Q) |
| [[Coenzyme Q|Ubiquinol]] (coenzyme Q) |
||
| Lipid |
| Lipid |
||
| 5<ref>{{cite journal | |
| 5<ref>{{cite journal | authors = Zita C, Overvad K, Mortensen SA, Sindberg CD, Moesgaard S, Hunter DA | title = Serum coenzyme Q10 concentrations in healthy men supplemented with 30 mg or 100 mg coenzyme Q10 for two months in a randomised controlled study | journal = BioFactors | volume = 18 | issue = 1–4 | pages = 185–93 | year = 2003 | pmid = 14695934 | doi = 10.1002/biof.5520180221 }}</ref> |
||
| 200 (human)<ref name="Turunen">{{cite journal | |
| 200 (human)<ref name="Turunen">{{cite journal | authors = Turunen M, Olsson J, Dallner G | title = Metabolism and function of coenzyme Q | journal = Biochimica et Biophysica Acta | volume = 1660 | issue = 1–2 | pages = 171–99 | date = January 2004 | pmid = 14757233 | doi = 10.1016/j.bbamem.2003.11.012 }}</ref> |
||
|} |
|} |
||
=== Uric acid === |
=== Uric acid === |
||
Uric acid is by far the highest concentration antioxidant in human blood. Uric acid (UA) is an antioxidant oxypurine produced from [[xanthine]] by the enzyme [[xanthine oxidase]], and is an intermediate product of [[purine]] metabolism.<ref name = Enomoto2005>{{cite journal | |
Uric acid is by far the highest concentration antioxidant in human blood. Uric acid (UA) is an antioxidant oxypurine produced from [[xanthine]] by the enzyme [[xanthine oxidase]], and is an intermediate product of [[purine]] metabolism.<ref name = Enomoto2005>{{cite journal | authors = Enomoto A, Endou H | title = Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease | journal = Clinical and Experimental Nephrology | volume = 9 | issue = 3 | pages = 195–205 | date = September 2005 | pmid = 16189627 | doi = 10.1007/s10157-005-0368-5 }}</ref> In almost all land animals, [[urate oxidase]] further catalyzes the oxidation of uric acid to [[allantoin]],<ref name=Wu1989>{{cite journal | authors = Wu XW, Lee CC, Muzny DM, Caskey CT | title = Urate oxidase: primary structure and evolutionary implications | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 86 | issue = 23 | pages = 9412–6 | date = December 1989 | pmid = 2594778 | pmc = 298506 | doi = 10.1073/pnas.86.23.9412 | bibcode = 1989PNAS...86.9412W }}</ref> but in humans and most higher primates, the urate oxidase gene is nonfunctional, so that UA is not further broken down.<ref name=Wu1989/><ref name=Wu1992>{{cite journal | authors = Wu XW, Muzny DM, Lee CC, Caskey CT | title = Two independent mutational events in the loss of urate oxidase during hominoid evolution | journal = Journal of Molecular Evolution | volume = 34 | issue = 1 | pages = 78–84 | date = January 1992 | pmid = 1556746 | doi = 10.1007/BF00163854 | bibcode = 1992JMolE..34...78W }}</ref> The evolutionary reasons for this loss of urate conversion to allantoin remain the topic of active speculation.<ref name=Alvarez2010>{{cite journal | authors = Álvarez-Lario B, Macarrón-Vicente J | title = Uric acid and evolution | journal = Rheumatology | volume = 49 | issue = 11 | pages = 2010–5 | date = November 2010 | pmid = 20627967 | doi = 10.1093/rheumatology/keq204 }}</ref><ref name=Watanabe2002>{{cite journal | authors = Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, Mazzali M, Johnson RJ | title = Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity | journal = Hypertension | volume = 40 | issue = 3 | pages = 355–60 | date = September 2002 | pmid = 12215479 | doi = 10.1161/01.HYP.0000028589.66335.AA }}</ref> The antioxidant effects of uric acid have led researchers to suggest this mutation was beneficial to early primates and humans.<ref name=Watanabe2002/><ref name=Johnson2010>{{cite journal | authors = Johnson RJ, Andrews P, Benner SA, Oliver W | title = Theodore E. Woodward award. The evolution of obesity: insights from the mid-Miocene | journal = Transactions of the American Clinical and Climatological Association | volume = 121 | pages = 295–305; discussion 305–8 | year = 2010 | pmid = 20697570 | pmc = 2917125 }}</ref> Studies of high altitude acclimatization support the hypothesis that urate acts as an antioxidant by mitigating the oxidative stress caused by high-altitude hypoxia.<ref name=Baillie2007>{{cite journal | authors = Baillie JK, Bates MG, Thompson AA, Waring WS, Partridge RW, Schnopp MF, Simpson A, Gulliver-Sloan F, Maxwell SR, Webb DJ | title = Endogenous urate production augments plasma antioxidant capacity in healthy lowland subjects exposed to high altitude | journal = Chest | volume = 131 | issue = 5 | pages = 1473–8 | date = May 2007 | pmid = 17494796 | doi = 10.1378/chest.06-2235 }}</ref> |
||
Uric acid has the highest concentration of any blood antioxidant<ref name=Glantzounis/> and provides over half of the total antioxidant capacity of human serum.<ref>{{cite journal | |
Uric acid has the highest concentration of any blood antioxidant<ref name=Glantzounis/> and provides over half of the total antioxidant capacity of human serum.<ref>{{cite journal | authors = Becker BF | title = Towards the physiological function of uric acid | journal = Free Radical Biology & Medicine | volume = 14 | issue = 6 | pages = 615–31 | date = June 1993 | pmid = 8325534 | doi = 10.1016/0891-5849(93)90143-I }}</ref> Uric acid's antioxidant activities are also complex, given that it does not react with some oxidants, such as [[superoxide]], but does act against [[peroxynitrite]],<ref name = Sautin2008>{{cite journal | authors = Sautin YY, Johnson RJ | title = Uric acid: the oxidant-antioxidant paradox | journal = Nucleosides, Nucleotides & Nucleic Acids | volume = 27 | issue = 6 | pages = 608–19 | date = June 2008 | pmid = 18600514 | pmc = 2895915 | doi = 10.1080/15257770802138558 | url = http://europepmc.org/articles/pmc2895915?pdf=render | format = Accepted manuscript }}</ref> [[peroxide]]s, and [[hypochlorous acid]].<ref name=Enomoto2005/> Concerns over elevated UA's contribution to [[gout]] must be considered as one of many risk factors.<ref name=Eggebeen2007>{{cite journal | authors = Eggebeen AT | title = Gout: an update | journal = American Family Physician | volume = 76 | issue = 6 | pages = 801–8 | date = September 2007 | pmid = 17910294 | url = http://www.aafp.org/link_out?pmid=17910294 }}</ref> By itself, UA-related risk of gout at high levels (415–530 μmol/L) is only 0.5% per year with an increase to 4.5% per year at UA [[supersaturation|supersaturation levels]] (535+ μmol/L).<ref name=Campion1987>{{cite journal | authors = Campion EW, Glynn RJ, DeLabry LO | title = Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study | journal = The American Journal of Medicine | volume = 82 | issue = 3 | pages = 421–6 | date = March 1987 | pmid = 3826098 | doi = 10.1016/0002-9343(87)90441-4 }}</ref> Many of these aforementioned studies determined UA's antioxidant actions within normal physiological levels,<ref name=Baillie2007/><ref name=Sautin2008/> and some found antioxidant activity at levels as high as 285 μmol/L.<ref name=Nazarewicz2007>{{cite journal | authors = Nazarewicz RR, Ziolkowski W, Vaccaro PS, Ghafourifar P | title = Effect of short-term ketogenic diet on redox status of human blood | journal = Rejuvenation Research | volume = 10 | issue = 4 | pages = 435–40 | date = December 2007 | pmid = 17663642 | doi = 10.1089/rej.2007.0540 }}</ref> |
||
=== Vitamin C === |
=== Vitamin C === |
||
[[Ascorbic acid]] or "[[vitamin C]]" is a [[monosaccharide]] oxidation-reduction ([[redox]]) [[catalyst]] found in both animals and plants. As one of the enzymes needed to make ascorbic acid has been lost by [[mutation]] during [[primate]] [[evolution]], humans must obtain it from the diet; it is therefore a vitamin.<ref>{{cite journal | |
[[Ascorbic acid]] or "[[vitamin C]]" is a [[monosaccharide]] oxidation-reduction ([[redox]]) [[catalyst]] found in both animals and plants. As one of the enzymes needed to make ascorbic acid has been lost by [[mutation]] during [[primate]] [[evolution]], humans must obtain it from the diet; it is therefore a vitamin.<ref>{{cite journal | authors = Smirnoff N | title = L-ascorbic acid biosynthesis | journal = Vitamins and Hormones | volume = 61 | pages = 241–66 | year = 2001 | pmid = 11153268 | doi = 10.1016/S0083-6729(01)61008-2 | isbn = 978-0-12-709861-6 | series = Vitamins & Hormones | url = https://books.google.com/books?id=wVNFlAEACAAJ }}</ref> Most other animals are able to produce this compound in their bodies and do not require it in their diets.<ref>{{cite journal | authors = Linster CL, Van Schaftingen E | title = Vitamin C. Biosynthesis, recycling and degradation in mammals | journal = The FEBS Journal | volume = 274 | issue = 1 | pages = 1–22 | date = January 2007 | pmid = 17222174 | doi = 10.1111/j.1742-4658.2006.05607.x }}</ref> Ascorbic acid is required for the conversion of the [[collagen#Collagen I formation|procollagen]] to [[collagen]] by oxidizing [[proline]] residues to [[hydroxyproline]]. In other cells, it is maintained in its reduced form by reaction with glutathione, which can be catalysed by [[protein disulfide isomerase]] and [[glutaredoxin]]s.<ref name="MeisterA">{{cite journal | authors = Meister A | title = Glutathione-ascorbic acid antioxidant system in animals | journal = The Journal of Biological Chemistry | volume = 269 | issue = 13 | pages = 9397–400 | date = April 1994 | pmid = 8144521 | url = http://www.jbc.org/cgi/pmidlookup?view=long&pmid=8144521 }}</ref><ref>{{cite journal | authors = Wells WW, Xu DP, Yang YF, Rocque PA | title = Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity | journal = The Journal of Biological Chemistry | volume = 265 | issue = 26 | pages = 15361–4 | date = September 1990 | pmid = 2394726 | url = http://www.jbc.org/cgi/pmidlookup?view=long&pmid=2394726 }}</ref> Ascorbic acid is a redox catalyst which can reduce, and thereby neutralize, reactive oxygen species such as hydrogen peroxide.<ref>{{cite journal | authors = Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M | title = Vitamin C as an antioxidant: evaluation of its role in disease prevention | journal = Journal of the American College of Nutrition | volume = 22 | issue = 1 | pages = 18–35 | date = February 2003 | pmid = 12569111 | doi = 10.1080/07315724.2003.10719272 | url = http://www.jacn.org/cgi/pmidlookup?view=long&pmid=12569111 }}</ref> In addition to its direct antioxidant effects, ascorbic acid is also a [[substrate (biochemistry)|substrate]] for the redox enzyme [[ascorbate peroxidase]], a function that is particularly important in stress resistance in plants.<ref>{{cite journal | authors = Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K | title = Regulation and function of ascorbate peroxidase isoenzymes | journal = Journal of Experimental Botany | volume = 53 | issue = 372 | pages = 1305–19 | date = May 2002 | pmid = 11997377 | doi = 10.1093/jexbot/53.372.1305 }}</ref> Ascorbic acid is present at high levels in all parts of plants and can reach concentrations of 20 [[millimolar]] in [[chloroplast]]s.<ref>{{cite journal | authors = Smirnoff N, Wheeler GL | title = Ascorbic acid in plants: biosynthesis and function | journal = Critical Reviews in Biochemistry and Molecular Biology | volume = 35 | issue = 4 | pages = 291–314 | year = 2000 | pmid = 11005203 | doi = 10.1080/10409230008984166 }}</ref> |
||
=== Glutathione === |
=== Glutathione === |
||
[[Image:Lipid peroxidation.svg|thumb|right|The [[Radical (chemistry)|free radical]] mechanism of lipid peroxidation.]] |
[[Image:Lipid peroxidation.svg|thumb|right|The [[Radical (chemistry)|free radical]] mechanism of lipid peroxidation.]] |
||
[[Glutathione]] is a [[cysteine]]-containing [[peptide]] found in most forms of aerobic life.<ref name="MeisterB">{{cite journal | |
[[Glutathione]] is a [[cysteine]]-containing [[peptide]] found in most forms of aerobic life.<ref name="MeisterB">{{cite journal | authors = Meister A, Anderson ME | title = Glutathione | journal = Annual Review of Biochemistry | volume = 52 | pages = 711–60 | year = 1983 | pmid = 6137189 | doi = 10.1146/annurev.bi.52.070183.003431 }}</ref> It is not required in the diet and is instead synthesized in cells from its constituent [[amino acid]]s.<ref>{{cite journal | authors = Meister A | title = Glutathione metabolism and its selective modification | journal = The Journal of Biological Chemistry | volume = 263 | issue = 33 | pages = 17205–8 | date = November 1988 | pmid = 3053703 | url = http://www.jbc.org/cgi/pmidlookup?view=long&pmid=3053703 }}</ref> Glutathione has antioxidant properties since the [[thiol]] group in its [[cysteine]] [[wikt:moiety|moiety]] is a reducing agent and can be reversibly oxidized and reduced. In cells, glutathione is maintained in the reduced form by the enzyme [[glutathione reductase]] and in turn reduces other metabolites and enzyme systems, such as ascorbate in the [[glutathione-ascorbate cycle]], [[glutathione peroxidase]]s and [[glutaredoxin]]s, as well as reacting directly with oxidants.<ref name="MeisterA" /> Due to its high concentration and its central role in maintaining the cell's redox state, glutathione is one of the most important cellular antioxidants.<ref name="MeisterB" /> In some organisms glutathione is replaced by other thiols, such as by [[mycothiol]] in the [[Actinomycete]]s, [[bacillithiol]] in some [[Gram-positive bacteria]],<ref name="pmid20308541">{{cite journal | authors = Gaballa A, Newton GL, Antelmann H, Parsonage D, Upton H, Rawat M, Claiborne A, Fahey RC, Helmann JD | title = Biosynthesis and functions of bacillithiol, a major low-molecular-weight thiol in Bacilli | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 107 | issue = 14 | pages = 6482–6 | date = April 2010 | pmid = 20308541 | pmc = 2851989 | doi = 10.1073/pnas.1000928107 |bibcode = 2010PNAS..107.6482G }}</ref><ref name=Newton>{{cite journal | authors = Newton GL, Rawat M, La Clair JJ, Jothivasan VK, Budiarto T, Hamilton CJ, Claiborne A, Helmann JD, Fahey RC | title = Bacillithiol is an antioxidant thiol produced in Bacilli | journal = Nature Chemical Biology | volume = 5 | issue = 9 | pages = 625–627 | date = September 2009 | pmid = 19578333 | pmc = 3510479 | doi = 10.1038/nchembio.189 | url = http://www.nature.com/nchembio/journal/v5/n9/full/nchembio.189.html }}</ref> or by [[trypanothione]] in the [[Kinetoplastida|Kinetoplastids]].<ref>{{cite journal | authors = Fahey RC | title = Novel thiols of prokaryotes | journal = Annual Review of Microbiology | volume = 55 | pages = 333–56 | year = 2001 | pmid = 11544359 | doi = 10.1146/annurev.micro.55.1.333 }}</ref><ref>{{cite journal | authors = Fairlamb AH, Cerami A | title = Metabolism and functions of trypanothione in the Kinetoplastida | journal = Annual Review of Microbiology | volume = 46 | pages = 695–729 | year = 1992 | pmid = 1444271 | doi = 10.1146/annurev.mi.46.100192.003403 }}</ref> |
||
=== Vitamin E === |
=== Vitamin E === |
||
[[Vitamin E]] is the collective name for a set of eight related [[tocopherol]]s and [[tocotrienol]]s, which are [[fat-soluble]] vitamins with antioxidant properties.<ref name="Herrera">{{cite journal | |
[[Vitamin E]] is the collective name for a set of eight related [[tocopherol]]s and [[tocotrienol]]s, which are [[fat-soluble]] vitamins with antioxidant properties.<ref name="Herrera">{{cite journal | authors = Herrera E, Barbas C | title = Vitamin E: action, metabolism and perspectives | journal = Journal of Physiology and Biochemistry | volume = 57 | issue = 2 | pages = 43–56 | date = March 2001 | pmid = 11579997 | doi = 10.1007/BF03179812 }}</ref><ref>{{cite journal | authors = Packer L, Weber SU, Rimbach G | title = Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling | journal = The Journal of Nutrition | volume = 131 | issue = 2 | pages = 369S–73S | date = February 2001 | pmid = 11160563 | url = http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=11160563 | doi = 10.1093/jn/131.2.369S }}</ref> Of these, α-tocopherol has been most studied as it has the highest [[bioavailability]], with the body preferentially absorbing and metabolising this form.<ref name="Brigelius">{{cite journal | authors = Brigelius-Flohé R, Traber MG | title = Vitamin E: function and metabolism | journal = FASEB Journal | volume = 13 | issue = 10 | pages = 1145–55 | date = July 1999 | pmid = 10385606 | url = http://www.fasebj.org/cgi/pmidlookup?view=long&pmid=10385606 | citeseerx = 10.1.1.337.5276 | doi=10.1096/fasebj.13.10.1145}}</ref> |
||
It has been claimed that the α-tocopherol form is the most important lipid-soluble antioxidant, and that it protects membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction.<ref name="Herrera" /><ref>{{cite journal | |
It has been claimed that the α-tocopherol form is the most important lipid-soluble antioxidant, and that it protects membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction.<ref name="Herrera" /><ref>{{cite journal | authors = Traber MG, Atkinson J | title = Vitamin E, antioxidant and nothing more | journal = Free Radical Biology & Medicine | volume = 43 | issue = 1 | pages = 4–15 | date = July 2007 | pmid = 17561088 | pmc = 2040110 | doi = 10.1016/j.freeradbiomed.2007.03.024 | url = http://europepmc.org/articles/pmc2040110?pdf=render | format = Accepted manuscript }}</ref> This removes the free radical intermediates and prevents the propagation reaction from continuing. This reaction produces oxidised α-tocopheroxyl radicals that can be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol or ubiquinol.<ref>{{cite journal | authors = Wang X, Quinn PJ | title = Vitamin E and its function in membranes | journal = Progress in Lipid Research | volume = 38 | issue = 4 | pages = 309–36 | date = July 1999 | pmid = 10793887 | doi = 10.1016/S0163-7827(99)00008-9 }}</ref> This is in line with findings showing that α-tocopherol, but not water-soluble antioxidants, efficiently protects glutathione peroxidase 4 ([[GPX4]])-deficient cells from cell death.<ref>{{cite journal | authors = Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Rådmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M | title = Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death | journal = Cell Metabolism | volume = 8 | issue = 3 | pages = 237–48 | date = September 2008 | pmid = 18762024 | doi = 10.1016/j.cmet.2008.07.005 }}</ref> GPx4 is the only known enzyme that efficiently reduces lipid-hydroperoxides within biological membranes. |
||
However, the roles and importance of the various forms of vitamin E are presently unclear,<ref>{{cite journal | |
However, the roles and importance of the various forms of vitamin E are presently unclear,<ref>{{cite journal | authors = Brigelius-Flohé R, Davies KJ | title = Is vitamin E an antioxidant, a regulator of signal transduction and gene expression, or a 'junk' food? Comments on the two accompanying papers: "Molecular mechanism of alpha-tocopherol action" by A. Azzi and "Vitamin E, antioxidant and nothing more" by M. Traber and J. Atkinson | journal = Free Radical Biology & Medicine | volume = 43 | issue = 1 | pages = 2–3 | date = July 2007 | pmid = 17561087 | doi = 10.1016/j.freeradbiomed.2007.05.016 }}</ref><ref>{{cite journal | authors = Atkinson J, Epand RF, Epand RM | title = Tocopherols and tocotrienols in membranes: a critical review | journal = Free Radical Biology & Medicine | volume = 44 | issue = 5 | pages = 739–64 | date = March 2008 | pmid = 18160049 | doi = 10.1016/j.freeradbiomed.2007.11.010 }}</ref> and it has even been suggested that the most important function of α-tocopherol is as a [[cell signaling|signaling molecule]], with this molecule having no significant role in antioxidant metabolism.<ref name="Azzi">{{cite journal | authors = Azzi A | title = Molecular mechanism of alpha-tocopherol action | journal = Free Radical Biology & Medicine | volume = 43 | issue = 1 | pages = 16–21 | date = July 2007 | pmid = 17561089 | doi = 10.1016/j.freeradbiomed.2007.03.013 }}</ref><ref>{{cite journal|authors=Zingg JM, Azzi A |title=Non-antioxidant activities of vitamin E |journal=Current Medicinal Chemistry |volume=11 |issue=9 |pages=1113–33 |date=May 2004 |pmid=15134510 |doi=10.2174/0929867043365332 |url=http://www.benthamdirect.org/pages/content.php?CMC/2004/00000011/00000009/0005C.SGM |deadurl=yes |archiveurl=https://web.archive.org/web/20111006103310/http://www.benthamdirect.org/pages/content.php?CMC%2F2004%2F00000011%2F00000009%2F0005C.SGM |archivedate=6 October 2011 |df= }}</ref> The functions of the other forms of vitamin E are even less well understood, although γ-tocopherol is a [[nucleophile]] that may react with [[electrophile|electrophilic]] mutagens,<ref name="Brigelius" /> and tocotrienols may be important in protecting [[neuron]]s from damage.<ref>{{cite journal | authors = Sen CK, Khanna S, Roy S | title = Tocotrienols: Vitamin E beyond tocopherols | journal = Life Sciences | volume = 78 | issue = 18 | pages = 2088–98 | date = March 2006 | pmid = 16458936 | pmc = 1790869 | doi = 10.1016/j.lfs.2005.12.001 | url = http://europepmc.org/articles/pmc1790869?pdf=render | format = Accepted manuscript }}</ref> |
||
== Pro-oxidant activities == |
== Pro-oxidant activities == |
||
{{further|Pro-oxidant}} |
{{further|Pro-oxidant}} |
||
Antioxidants that are reducing agents can also act as pro-oxidants. For example, vitamin C has antioxidant activity when it reduces oxidizing substances such as hydrogen peroxide,<ref>{{cite journal | |
Antioxidants that are reducing agents can also act as pro-oxidants. For example, vitamin C has antioxidant activity when it reduces oxidizing substances such as hydrogen peroxide,<ref>{{cite journal | authors = Duarte TL, Lunec J | title = Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C | journal = Free Radical Research | volume = 39 | issue = 7 | pages = 671–86 | date = July 2005 | pmid = 16036346 | doi = 10.1080/10715760500104025 }}</ref> however, it will also reduce metal ions that generate free radicals through the [[Fenton's reagent|Fenton reaction]].<ref name="ReferenceA"/><ref name="Carr">{{cite journal | authors = Carr A, Frei B | title = Does vitamin C act as a pro-oxidant under physiological conditions? | journal = FASEB Journal | volume = 13 | issue = 9 | pages = 1007–24 | date = June 1999 | pmid = 10336883 | url = http://www.fasebj.org/cgi/content/full/13/9/1007 }}</ref> |
||
: 2 Fe<sup>3+</sup> + Ascorbate → 2 Fe<sup>2+</sup> + Dehydroascorbate |
: 2 Fe<sup>3+</sup> + Ascorbate → 2 Fe<sup>2+</sup> + Dehydroascorbate |
||
:: 2 Fe<sup>2+</sup> + 2 H<sub>2</sub>O<sub>2</sub> → 2 Fe<sup>3+</sup> + 2 OH'''·''' + 2 OH<sup>−</sup> |
:: 2 Fe<sup>2+</sup> + 2 H<sub>2</sub>O<sub>2</sub> → 2 Fe<sup>3+</sup> + 2 OH'''·''' + 2 OH<sup>−</sup> |
||
The relative importance of the antioxidant and pro-oxidant activities of antioxidants is an area of current research, but vitamin C, which exerts its effects as a vitamin by oxidizing polypeptides, appears to have a mostly antioxidant action in the human body.<ref name="Carr" /> However, less data is available for other dietary antioxidants, such as vitamin E,<ref>{{cite journal | |
The relative importance of the antioxidant and pro-oxidant activities of antioxidants is an area of current research, but vitamin C, which exerts its effects as a vitamin by oxidizing polypeptides, appears to have a mostly antioxidant action in the human body.<ref name="Carr" /> However, less data is available for other dietary antioxidants, such as vitamin E,<ref>{{cite journal | authors = Schneider C | title = Chemistry and biology of vitamin E | journal = Molecular Nutrition & Food Research | volume = 49 | issue = 1 | pages = 7–30 | date = January 2005 | pmid = 15580660 | doi = 10.1002/mnfr.200400049 }}</ref> or the [[polyphenol antioxidant|polyphenols]].<ref>{{cite journal | authors = Halliwell B | title = Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? | journal = Archives of Biochemistry and Biophysics | volume = 476 | issue = 2 | pages = 107–112 | date = August 2008 | pmid = 18284912 | doi = 10.1016/j.abb.2008.01.028 }}</ref><ref name="Ristow_2010">{{cite journal | authors = Ristow M, Zarse K | title = How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis) | journal = Experimental Gerontology | volume = 45 | issue = 6 | pages = 410–418 | date = June 2010 | pmid = 20350594 | doi = 10.1016/j.exger.2010.03.014 }}</ref> Likewise, the pathogenesis of diseases involving hyperuricemia likely involve uric acid's direct and indirect pro-oxidant properties. |
||
That is, paradoxically, agents which are normally considered antioxidants can act as conditional [[pro-oxidant]]s and actually increase oxidative stress. Besides ascorbate, medically important conditional pro-oxidants include uric acid and sulfhydryl amino acids such as [[homocysteine]]. Typically, this involves some transition-series metal such as copper or iron as catalyst. The potential role of the pro-oxidant role of uric acid in (e.g.) atherosclerosis and ischemic stroke is considered above. Another example is the postulated role of [[homocysteine]] in atherosclerosis. |
That is, paradoxically, agents which are normally considered antioxidants can act as conditional [[pro-oxidant]]s and actually increase oxidative stress. Besides ascorbate, medically important conditional pro-oxidants include uric acid and sulfhydryl amino acids such as [[homocysteine]]. Typically, this involves some transition-series metal such as copper or iron as catalyst. The potential role of the pro-oxidant role of uric acid in (e.g.) atherosclerosis and ischemic stroke is considered above. Another example is the postulated role of [[homocysteine]] in atherosclerosis. |
||
| Line 155: | Line 155: | ||
\underset{Oxygen}{O2} -> \underset{Superoxide}{*O2^-} ->[{{} \atop \ce{Superoxide \atop dismutase}}] \underset{Hydrogen \atop peroxide}{H2O2} ->[{{} \atop \ce{Peroxidases \atop catalase}}] \underset{Water}{H2O} |
\underset{Oxygen}{O2} -> \underset{Superoxide}{*O2^-} ->[{{} \atop \ce{Superoxide \atop dismutase}}] \underset{Hydrogen \atop peroxide}{H2O2} ->[{{} \atop \ce{Peroxidases \atop catalase}}] \underset{Water}{H2O} |
||
</chem>}} |
</chem>}} |
||
As with the chemical antioxidants, cells are protected against oxidative stress by an interacting network of antioxidant enzymes.<ref name="Davies" /><ref name="Sies" /> Here, the superoxide released by processes such as [[oxidative phosphorylation]] is first converted to hydrogen peroxide and then further reduced to give water. This detoxification pathway is the result of multiple enzymes, with superoxide dismutases catalysing the first step and then catalases and various peroxidases removing hydrogen peroxide. As with antioxidant metabolites, the contributions of these enzymes to antioxidant defenses can be hard to separate from one another, but the generation of [[Genetically modified organism|transgenic mice]] lacking just one antioxidant enzyme can be informative.<ref name=Magnenat>{{cite journal | |
As with the chemical antioxidants, cells are protected against oxidative stress by an interacting network of antioxidant enzymes.<ref name="Davies" /><ref name="Sies" /> Here, the superoxide released by processes such as [[oxidative phosphorylation]] is first converted to hydrogen peroxide and then further reduced to give water. This detoxification pathway is the result of multiple enzymes, with superoxide dismutases catalysing the first step and then catalases and various peroxidases removing hydrogen peroxide. As with antioxidant metabolites, the contributions of these enzymes to antioxidant defenses can be hard to separate from one another, but the generation of [[Genetically modified organism|transgenic mice]] lacking just one antioxidant enzyme can be informative.<ref name=Magnenat>{{cite journal | authors = Ho YS, Magnenat JL, Gargano M, Cao J | title = The nature of antioxidant defense mechanisms: a lesson from transgenic studies | journal = Environmental Health Perspectives | volume = 106 Suppl 5 | issue = Suppl 5 | pages = 1219–28 | date = October 1998 | pmid = 9788901 | pmc = 1533365 | doi = 10.2307/3433989 | jstor = 3433989 | url = http://europepmc.org/articles/pmc1533365?pdf=render | format = Full text }}</ref> |
||
=== Superoxide dismutase, catalase, and peroxiredoxins === |
=== Superoxide dismutase, catalase, and peroxiredoxins === |
||
[[Superoxide dismutase]]s (SODs) are a class of closely related enzymes that catalyze the breakdown of the superoxide anion into oxygen and hydrogen peroxide.<ref>{{cite journal | |
[[Superoxide dismutase]]s (SODs) are a class of closely related enzymes that catalyze the breakdown of the superoxide anion into oxygen and hydrogen peroxide.<ref>{{cite journal | authors = Zelko IN, Mariani TJ, Folz RJ | title = Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression | journal = Free Radical Biology & Medicine | volume = 33 | issue = 3 | pages = 337–49 | date = August 2002 | pmid = 12126755 | doi = 10.1016/S0891-5849(02)00905-X }}</ref><ref name="Bannister">{{cite journal | authors = Bannister JV, Bannister WH, Rotilio G | title = Aspects of the structure, function, and applications of superoxide dismutase | journal = CRC Critical Reviews in Biochemistry | volume = 22 | issue = 2 | pages = 111–80 | year = 1987 | pmid = 3315461 | doi = 10.3109/10409238709083738 }}</ref> SOD enzymes are present in almost all aerobic cells and in extracellular fluids.<ref>{{cite journal | authors = Johnson F, Giulivi C | title = Superoxide dismutases and their impact upon human health | journal = Molecular Aspects of Medicine | volume = 26 | issue = 4–5 | pages = 340–52 | year = 2005 | pmid = 16099495 | doi = 10.1016/j.mam.2005.07.006 }}</ref> Superoxide dismutase enzymes contain metal ion cofactors that, depending on the isozyme, can be copper, zinc, [[manganese]] or iron. In humans, the copper/zinc SOD is present in the [[cytosol]], while manganese SOD is present in the [[mitochondrion]].<ref name="Bannister" /> There also exists a third form of SOD in [[extracellular fluid]]s, which contains copper and zinc in its active sites.<ref>{{cite journal | authors = Nozik-Grayck E, Suliman HB, Piantadosi CA | title = Extracellular superoxide dismutase | journal = The International Journal of Biochemistry & Cell Biology | volume = 37 | issue = 12 | pages = 2466–71 | date = December 2005 | pmid = 16087389 | doi = 10.1016/j.biocel.2005.06.012 }}</ref> The mitochondrial isozyme seems to be the most biologically important of these three, since mice lacking this enzyme die soon after birth.<ref>{{cite journal | authors = Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC | title = A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase | journal = Nature Genetics | volume = 18 | issue = 2 | pages = 159–63 | date = February 1998 | pmid = 9462746 | doi = 10.1038/ng0298-159 }}</ref> In contrast, the mice lacking copper/zinc SOD (Sod1) are viable but have numerous pathologies and a reduced lifespan (see article on [[superoxide]]), while mice without the extracellular SOD have minimal defects (sensitive to [[hyperoxia]]).<ref name="Magnenat" /><ref>{{cite journal | authors = Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Scott RW, Snider WD | title = Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury | journal = Nature Genetics | volume = 13 | issue = 1 | pages = 43–7 | date = May 1996 | pmid = 8673102 | doi = 10.1038/ng0596-43 }}</ref> In plants, SOD isozymes are present in the cytosol and mitochondria, with an iron SOD found in [[chloroplast]]s that is absent from [[vertebrate]]s and [[yeast]].<ref>{{cite journal | authors = Van Camp W, Inzé D, Van Montagu M | title = The regulation and function of tobacco superoxide dismutases | journal = Free Radical Biology & Medicine | volume = 23 | issue = 3 | pages = 515–20 | year = 1997 | pmid = 9214590 | doi = 10.1016/S0891-5849(97)00112-3 }}</ref> |
||
[[Catalase]]s are enzymes that catalyse the conversion of hydrogen peroxide to water and oxygen, using either an iron or manganese cofactor.<ref>{{cite journal | |
[[Catalase]]s are enzymes that catalyse the conversion of hydrogen peroxide to water and oxygen, using either an iron or manganese cofactor.<ref>{{cite journal | authors = Chelikani P, Fita I, Loewen PC | title = Diversity of structures and properties among catalases | journal = Cellular and Molecular Life Sciences | volume = 61 | issue = 2 | pages = 192–208 | date = January 2004 | pmid = 14745498 | doi = 10.1007/s00018-003-3206-5 | url = https://digital.csic.es/bitstream/10261/111097/1/accesoRestringido.pdf | format = Submitted manuscript }}</ref><ref>{{cite journal | authors = Zámocký M, Koller F | title = Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis | journal = Progress in Biophysics and Molecular Biology | volume = 72 | issue = 1 | pages = 19–66 | year = 1999 | pmid = 10446501 | doi = 10.1016/S0079-6107(98)00058-3 }}</ref> This protein is localized to [[peroxisome]]s in most [[eukaryote|eukaryotic]] cells.<ref>{{cite journal | authors = del Río LA, Sandalio LM, Palma JM, Bueno P, Corpas FJ | title = Metabolism of oxygen radicals in peroxisomes and cellular implications | journal = Free Radical Biology & Medicine | volume = 13 | issue = 5 | pages = 557–80 | date = November 1992 | pmid = 1334030 | doi = 10.1016/0891-5849(92)90150-F }}</ref> Catalase is an unusual enzyme since, although hydrogen peroxide is its only substrate, it follows a [[enzyme kinetics|ping-pong mechanism]]. Here, its cofactor is oxidised by one molecule of hydrogen peroxide and then regenerated by transferring the bound oxygen to a second molecule of substrate.<ref>{{cite journal | authors = Hiner AN, Raven EL, Thorneley RN, García-Cánovas F, Rodríguez-López JN | title = Mechanisms of compound I formation in heme peroxidases | journal = Journal of Inorganic Biochemistry | volume = 91 | issue = 1 | pages = 27–34 | date = July 2002 | pmid = 12121759 | doi = 10.1016/S0162-0134(02)00390-2 }}</ref> Despite its apparent importance in hydrogen peroxide removal, humans with genetic deficiency of catalase — "[[acatalasemia]]" — or mice [[Genetic engineering|genetically engineered]] to lack catalase completely, suffer few ill effects.<ref>{{cite journal | authors = Mueller S, Riedel HD, Stremmel W | title = Direct evidence for catalase as the predominant H2O2 -removing enzyme in human erythrocytes | journal = Blood | volume = 90 | issue = 12 | pages = 4973–8 | date = December 1997 | pmid = 9389716 | url = http://www.bloodjournal.org/cgi/content/full/90/12/4973 }}</ref><ref>{{cite journal | authors = Ogata M | title = Acatalasemia | journal = Human Genetics | volume = 86 | issue = 4 | pages = 331–40 | date = February 1991 | pmid = 1999334 | doi = 10.1007/BF00201829 }}</ref> |
||
[[Image:Peroxiredoxin.png|thumb|[[Quaternary structure|Decameric]] structure of AhpC, a [[bacterial]] 2-cysteine [[peroxiredoxin]] from ''[[Salmonella enterica|Salmonella typhimurium]]''.<ref>{{cite journal | |
[[Image:Peroxiredoxin.png|thumb|[[Quaternary structure|Decameric]] structure of AhpC, a [[bacterial]] 2-cysteine [[peroxiredoxin]] from ''[[Salmonella enterica|Salmonella typhimurium]]''.<ref>{{cite journal |authors=Parsonage D, Youngblood D, Sarma G, Wood Z, Karplus P, Poole L |title=Analysis of the link between enzymatic activity and oligomeric state in AhpC, a bacterial peroxiredoxin |journal=Biochemistry |volume=44 |issue=31 |pages=10583–92 |year=2005 |pmid=16060667 |doi=10.1021/bi050448i |pmc=3832347|url=http://europepmc.org/articles/pmc3832347?pdf=render |format=Accepted manuscript }} [http://www.rcsb.org/pdb/explore.do?structureId=1YEX PDB 1YEX]</ref>]] |
||
[[Peroxiredoxin]]s are peroxidases that catalyze the reduction of hydrogen peroxide, [[organic peroxide|organic hydroperoxides]], as well as [[peroxynitrite]].<ref>{{cite journal | |
[[Peroxiredoxin]]s are peroxidases that catalyze the reduction of hydrogen peroxide, [[organic peroxide|organic hydroperoxides]], as well as [[peroxynitrite]].<ref>{{cite journal | authors = Rhee SG, Chae HZ, Kim K | title = Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling | journal = Free Radical Biology & Medicine | volume = 38 | issue = 12 | pages = 1543–52 | date = June 2005 | pmid = 15917183 | doi = 10.1016/j.freeradbiomed.2005.02.026 }}</ref> They are divided into three classes: typical 2-cysteine peroxiredoxins; atypical 2-cysteine peroxiredoxins; and 1-cysteine peroxiredoxins.<ref>{{cite journal | authors = Wood ZA, Schröder E, Robin Harris J, Poole LB | title = Structure, mechanism and regulation of peroxiredoxins | journal = Trends in Biochemical Sciences | volume = 28 | issue = 1 | pages = 32–40 | date = January 2003 | pmid = 12517450 | doi = 10.1016/S0968-0004(02)00003-8 }}</ref> These enzymes share the same basic catalytic mechanism, in which a redox-active cysteine (the peroxidatic cysteine) in the [[active site]] is oxidized to a [[sulfenic acid]] by the peroxide substrate.<ref>{{cite journal | authors = Claiborne A, Yeh JI, Mallett TC, Luba J, Crane EJ, Charrier V, Parsonage D | title = Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation | journal = Biochemistry | volume = 38 | issue = 47 | pages = 15407–16 | date = November 1999 | pmid = 10569923 | doi = 10.1021/bi992025k }}</ref> Over-oxidation of this cysteine residue in peroxiredoxins inactivates these enzymes, but this can be reversed by the action of [[sulfiredoxin]].<ref>{{cite journal | authors = Jönsson TJ, Lowther WT | title = The peroxiredoxin repair proteins | journal = Sub-Cellular Biochemistry | volume = 44 | pages = 115–41 | year = 2007 | pmid = 18084892 | pmc = 2391273 | doi = 10.1007/978-1-4020-6051-9_6 | isbn = 978-1-4020-6050-2 | series = Subcellular Biochemistry | url = https://books.google.com/books?id=gHwEOH7vDmUC }}</ref> Peroxiredoxins seem to be important in antioxidant metabolism, as mice lacking peroxiredoxin 1 or 2 have shortened lifespan and suffer from [[hemolytic anaemia]], while plants use peroxiredoxins to remove hydrogen peroxide generated in chloroplasts.<ref>{{cite journal | authors = Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, Van Etten RA | title = Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression | journal = Nature | volume = 424 | issue = 6948 | pages = 561–5 | date = July 2003 | pmid = 12891360 | doi = 10.1038/nature01819 |bibcode = 2003Natur.424..561N | url = https://cloudfront.escholarship.org/dist/prd/content/qt8m75q3ct/qt8m75q3ct.pdf?t=nhvrjt | format = Full text }}</ref><ref>{{cite journal | authors = Lee TH, Kim SU, Yu SL, Kim SH, Park DS, Moon HB, Dho SH, Kwon KS, Kwon HJ, Han YH, Jeong S, Kang SW, Shin HS, Lee KK, Rhee SG, Yu DY | title = Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice | journal = Blood | volume = 101 | issue = 12 | pages = 5033–8 | date = June 2003 | pmid = 12586629 | doi = 10.1182/blood-2002-08-2548 | url = http://www.bloodjournal.org/cgi/content/full/101/12/5033 }}</ref><ref>{{cite journal | authors = Dietz KJ, Jacob S, Oelze ML, Laxa M, Tognetti V, de Miranda SM, Baier M, Finkemeier I | title = The function of peroxiredoxins in plant organelle redox metabolism | journal = Journal of Experimental Botany | volume = 57 | issue = 8 | pages = 1697–709 | year = 2006 | pmid = 16606633 | doi = 10.1093/jxb/erj160 }}</ref> |
||
=== Thioredoxin and glutathione systems === |
=== Thioredoxin and glutathione systems === |
||
The [[thioredoxin]] system contains the 12-k[[atomic mass unit|Da]] protein thioredoxin and its companion [[thioredoxin reductase]].<ref>{{cite journal | |
The [[thioredoxin]] system contains the 12-k[[atomic mass unit|Da]] protein thioredoxin and its companion [[thioredoxin reductase]].<ref>{{cite journal | authors = Nordberg J, Arnér ES | title = Reactive oxygen species, antioxidants, and the mammalian thioredoxin system | journal = Free Radical Biology & Medicine | volume = 31 | issue = 11 | pages = 1287–312 | date = December 2001 | pmid = 11728801 | doi = 10.1016/S0891-5849(01)00724-9 }}</ref> Proteins related to thioredoxin are present in all sequenced organisms. Plants, such as ''[[Arabidopsis thaliana]],'' have a particularly great diversity of isoforms.<ref>{{cite journal | authors = Vieira Dos Santos C, Rey P | title = Plant thioredoxins are key actors in the oxidative stress response | journal = Trends in Plant Science | volume = 11 | issue = 7 | pages = 329–34 | date = July 2006 | pmid = 16782394 | doi = 10.1016/j.tplants.2006.05.005 }}</ref> The active site of thioredoxin consists of two [[vicinal (chemistry)|neighboring]] cysteines, as part of a highly conserved CXXC [[sequence motif|motif]], that can cycle between an active dithiol form (reduced) and an oxidized [[disulfide]] form. In its active state, thioredoxin acts as an efficient reducing agent, scavenging reactive oxygen species and maintaining other proteins in their reduced state.<ref>{{cite journal | authors = Arnér ES, Holmgren A | title = Physiological functions of thioredoxin and thioredoxin reductase | journal = European Journal of Biochemistry / FEBS | volume = 267 | issue = 20 | pages = 6102–9 | date = October 2000 | pmid = 11012661 | doi = 10.1046/j.1432-1327.2000.01701.x | url = http://www.blackwell-synergy.com/doi/full/10.1046/j.1432-1327.2000.01701.x }}</ref> After being oxidized, the active thioredoxin is regenerated by the action of thioredoxin reductase, using [[NADPH]] as an [[electron donor]].<ref>{{cite journal | authors = Mustacich D, Powis G | title = Thioredoxin reductase | journal = The Biochemical Journal | volume = 346 | issue = 1 | pages = 1–8 | date = February 2000 | pmid = 10657232 | pmc = 1220815 | doi = 10.1042/0264-6021:3460001 }}</ref> |
||
The [[glutathione]] system includes glutathione, [[glutathione reductase]], [[glutathione peroxidase]]s, and [[glutathione S-transferase|glutathione ''S''-transferases]].<ref name="MeisterB" /> This system is found in animals, plants and microorganisms.<ref name="MeisterB" /><ref>{{cite journal | |
The [[glutathione]] system includes glutathione, [[glutathione reductase]], [[glutathione peroxidase]]s, and [[glutathione S-transferase|glutathione ''S''-transferases]].<ref name="MeisterB" /> This system is found in animals, plants and microorganisms.<ref name="MeisterB" /><ref>{{cite journal | authors = Creissen G, Broadbent P, Stevens R, Wellburn AR, Mullineaux P | title = Manipulation of glutathione metabolism in transgenic plants | journal = Biochemical Society Transactions | volume = 24 | issue = 2 | pages = 465–9 | date = May 1996 | pmid = 8736785 | doi = 10.1042/bst0240465 }}</ref> Glutathione peroxidase is an enzyme containing four [[selenium]]-[[cofactor (biochemistry)|cofactors]] that catalyzes the breakdown of hydrogen peroxide and organic hydroperoxides. There are at least four different glutathione peroxidase [[isozyme]]s in animals.<ref>{{cite journal | authors = Brigelius-Flohé R | title = Tissue-specific functions of individual glutathione peroxidases | journal = Free Radical Biology & Medicine | volume = 27 | issue = 9–10 | pages = 951–65 | date = November 1999 | pmid = 10569628 | doi = 10.1016/S0891-5849(99)00173-2 }}</ref> Glutathione peroxidase 1 is the most abundant and is a very efficient scavenger of hydrogen peroxide, while glutathione peroxidase 4 is most active with lipid hydroperoxides. Surprisingly, glutathione peroxidase 1 is dispensable, as mice lacking this enzyme have normal lifespans,<ref>{{cite journal | authors = Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD | title = Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia | journal = The Journal of Biological Chemistry | volume = 272 | issue = 26 | pages = 16644–51 | date = June 1997 | pmid = 9195979 | doi = 10.1074/jbc.272.26.16644 }}</ref> but they are hypersensitive to induced oxidative stress.<ref>{{cite journal | authors = de Haan JB, Bladier C, Griffiths P, Kelner M, O'Shea RD, Cheung NS, Bronson RT, Silvestro MJ, Wild S, Zheng SS, Beart PM, Hertzog PJ, Kola I | title = Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide | journal = The Journal of Biological Chemistry | volume = 273 | issue = 35 | pages = 22528–36 | date = August 1998 | pmid = 9712879 | doi = 10.1074/jbc.273.35.22528 }}</ref> In addition, the glutathione ''S''-transferases show high activity with lipid peroxides.<ref>{{cite journal | authors = Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC | title = Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis | journal = Antioxidants & Redox Signaling | volume = 6 | issue = 2 | pages = 289–300 | date = April 2004 | pmid = 15025930 | doi = 10.1089/152308604322899350 }}</ref> These enzymes are at particularly high levels in the liver and also serve in [[detoxification]] metabolism.<ref>{{cite journal | authors = Hayes JD, Flanagan JU, Jowsey IR | title = Glutathione transferases | journal = Annual Review of Pharmacology and Toxicology | volume = 45 | pages = 51–88 | year = 2005 | pmid = 15822171 | doi = 10.1146/annurev.pharmtox.45.120403.095857 }}</ref> |
||
== Oxidative stress in disease == |
== Oxidative stress in disease == |
||
{{further|Pathology|Free-radical theory|Oxidative stress}} |
{{further|Pathology|Free-radical theory|Oxidative stress}} |
||
Oxidative stress is thought to contribute to the development of a wide range of diseases including [[Alzheimer's disease]],<ref>{{cite journal | |
Oxidative stress is thought to contribute to the development of a wide range of diseases including [[Alzheimer's disease]],<ref>{{cite journal | authors = Christen Y | title = Oxidative stress and Alzheimer disease | journal = The American Journal of Clinical Nutrition | volume = 71 | issue = 2 | pages = 621S–629S | date = February 2000 | pmid = 10681270 | url = http://www.ajcn.org/cgi/content/full/71/2/621s | doi = 10.1093/ajcn/71.2.621s }}</ref><ref>{{cite journal | authors = Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA | title = Involvement of oxidative stress in Alzheimer disease | journal = Journal of Neuropathology and Experimental Neurology | volume = 65 | issue = 7 | pages = 631–41 | date = July 2006 | pmid = 16825950 | doi = 10.1097/01.jnen.0000228136.58062.bf }}</ref> [[Parkinson's disease]],<ref>{{cite journal | authors = Wood-Kaczmar A, Gandhi S, Wood NW | title = Understanding the molecular causes of Parkinson's disease | journal = Trends in Molecular Medicine | volume = 12 | issue = 11 | pages = 521–8 | date = November 2006 | pmid = 17027339 | doi = 10.1016/j.molmed.2006.09.007 }}</ref> the pathologies caused by [[diabetes]],<ref>{{cite journal | authors = Davì G, Falco A, Patrono C | title = Lipid peroxidation in diabetes mellitus | journal = Antioxidants & Redox Signaling | volume = 7 | issue = 1–2 | pages = 256–68 | year = 2005 | pmid = 15650413 | doi = 10.1089/ars.2005.7.256 }}</ref><ref>{{cite journal | authors = Giugliano D, Ceriello A, Paolisso G | title = Oxidative stress and diabetic vascular complications | journal = Diabetes Care | volume = 19 | issue = 3 | pages = 257–67 | date = March 1996 | pmid = 8742574 | doi = 10.2337/diacare.19.3.257 }}</ref> [[rheumatoid arthritis]],<ref>{{cite journal | authors = Hitchon CA, El-Gabalawy HS | title = Oxidation in rheumatoid arthritis | journal = [[Arthritis Research & Therapy]] | volume = 6 | issue = 6 | pages = 265–78 | year = 2004 | pmid = 15535839 | pmc = 1064874 | doi = 10.1186/ar1447 }}</ref> and [[neurodegeneration]] in [[motor neuron disease]]s.<ref>{{cite journal | authors = Cookson MR, Shaw PJ | title = Oxidative stress and motor neurone disease | journal = Brain Pathology | volume = 9 | issue = 1 | pages = 165–86 | date = January 1999 | pmid = 9989458 | doi = 10.1111/j.1750-3639.1999.tb00217.x }}</ref> In many of these cases, it is unclear if oxidants trigger the disease, or if they are produced as a secondary consequence of the disease and from general tissue damage;<ref name="emfafb" /> One case in which this link is particularly well understood is the role of oxidative stress in [[cardiovascular disease]]. Here, [[low density lipoprotein]] (LDL) oxidation appears to trigger the process of [[atherosclerosis#Atherogenesis|atherogenesis]], which results in [[atherosclerosis]], and finally cardiovascular disease.<ref>{{cite journal | authors = Van Gaal LF, Mertens IL, De Block CE | title = Mechanisms linking obesity with cardiovascular disease | journal = Nature | volume = 444 | issue = 7121 | pages = 875–80 | date = December 2006 | pmid = 17167476 | doi = 10.1038/nature05487 | bibcode = 2006Natur.444..875V }}</ref><ref>{{cite journal | authors = Aviram M | title = Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases | journal = Free Radical Research | volume = 33 Suppl | pages = S85–97 | date = November 2000 | pmid = 11191279 }}</ref> |
||
Oxidative damage in DNA can cause cancer. Several antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, glutathione S-transferase etc. protect DNA from oxidative stress. It has been proposed that polymorphisms in these enzymes are associated with DNA damage and subsequently the individual's risk of cancer susceptibility.<ref>{{cite journal | |
Oxidative damage in DNA can cause cancer. Several antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, glutathione S-transferase etc. protect DNA from oxidative stress. It has been proposed that polymorphisms in these enzymes are associated with DNA damage and subsequently the individual's risk of cancer susceptibility.<ref>{{cite journal |authors=Khan MA, Tania M, Zhang D, Chen H |title=Antioxidant enzymes and cancer |journal=Chin J Cancer Res |volume=22 |issue=2 |pages=87–92 |year=2010 |url=http://www.springerlink.com/content/4h2277984v0t180k/ |doi=10.1007/s11670-010-0087-7}}</ref> |
||
A [[Calorie restriction|low calorie diet]] extends median and [[Maximum life span|maximum lifespan]] in many animals. This effect may involve a reduction in oxidative stress.<ref>{{cite journal | |
A [[Calorie restriction|low calorie diet]] extends median and [[Maximum life span|maximum lifespan]] in many animals. This effect may involve a reduction in oxidative stress.<ref>{{cite journal | authors = López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R | title = Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 103 | issue = 6 | pages = 1768–1773 | date = February 2006 | pmid = 16446459 | pmc = 1413655 | doi = 10.1073/pnas.0510452103 | bibcode = 2006PNAS..103.1768L }}</ref> While there is some evidence to support the role of oxidative stress in aging in model organisms such as ''[[Drosophila melanogaster]]'' and ''[[Caenorhabditis elegans]]'',<ref>{{cite journal | authors = Larsen PL | title = Aging and resistance to oxidative damage in Caenorhabditis elegans | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 90 | issue = 19 | pages = 8905–9 | date = October 1993 | pmid = 8415630 | pmc = 47469 | doi = 10.1073/pnas.90.19.8905 | bibcode = 1993PNAS...90.8905L }}</ref><ref>{{cite journal | authors = Helfand SL, Rogina B | title = Genetics of aging in the fruit fly, Drosophila melanogaster | journal = Annual Review of Genetics | volume = 37 | pages = 329–48 | year = 2003 | pmid = 14616064 | doi = 10.1146/annurev.genet.37.040103.095211 }}</ref> the evidence in mammals is less clear.<ref name="hdanrt">{{cite journal | authors = Sohal RS, Mockett RJ, Orr WC | title = Mechanisms of aging: an appraisal of the oxidative stress hypothesis | journal = Free Radical Biology & Medicine | volume = 33 | issue = 5 | pages = 575–86 | date = September 2002 | pmid = 12208343 | doi = 10.1016/S0891-5849(02)00886-9 }}</ref><ref name="Sohal R 2002 37–44">{{cite journal | authors = Sohal RS | title = Role of oxidative stress and protein oxidation in the aging process | journal = Free Radical Biology & Medicine | volume = 33 | issue = 1 | pages = 37–44 | date = July 2002 | pmid = 12086680 | doi = 10.1016/S0891-5849(02)00856-0 }}</ref><ref name="Rattan S 2006 1230–8">{{cite journal | authors = Rattan SI | title = Theories of biological aging: genes, proteins, and free radicals | journal = Free Radical Research | volume = 40 | issue = 12 | pages = 1230–8 | date = December 2006 | pmid = 17090411 | doi = 10.1080/10715760600911303 | url = http://www.link-age.eu/Freeradicalresearch-Rattan.pdf }}</ref> Indeed, a 2009 review of experiments in mice concluded that almost all manipulations of antioxidant systems had no effect on aging.<ref>{{cite journal | authors = Pérez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A | title = Is the oxidative stress theory of aging dead? | journal = Biochimica et Biophysica Acta | volume = 1790 | issue = 10 | pages = 1005–1014 | date = October 2009 | pmid = 19524016 | pmc = 2789432 | doi = 10.1016/j.bbagen.2009.06.003 | url = http://www.sciencedirect.com/science/article/B6T1W-4WH2KYY-3/2/3b2909c65fa19256ae2436cb8c143471 }}</ref> |
||
== Uses in technology == |
== Uses in technology == |
||
=== Food preservatives === |
=== Food preservatives === |
||
Antioxidants are used as [[food additive]]s to help [[preservative|guard against food deterioration]]. Exposure to oxygen and sunlight are the two main factors in the oxidation of food, so food is preserved by keeping in the dark and sealing it in containers or even coating it in wax, as with cucumbers. However, as oxygen is also important for plant [[Respiration (physiology)|respiration]], storing plant materials in [[Anaerobic organism#Metabolism|anaerobic]] conditions produces unpleasant flavors and unappealing colors.<ref>{{cite journal | |
Antioxidants are used as [[food additive]]s to help [[preservative|guard against food deterioration]]. Exposure to oxygen and sunlight are the two main factors in the oxidation of food, so food is preserved by keeping in the dark and sealing it in containers or even coating it in wax, as with cucumbers. However, as oxygen is also important for plant [[Respiration (physiology)|respiration]], storing plant materials in [[Anaerobic organism#Metabolism|anaerobic]] conditions produces unpleasant flavors and unappealing colors.<ref>{{cite journal | authors = Kader AA, Zagory D, Kerbel EL | title = Modified atmosphere packaging of fruits and vegetables | journal = Critical Reviews in Food Science and Nutrition | volume = 28 | issue = 1 | pages = 1–30 | year = 1989 | pmid = 2647417 | doi = 10.1080/10408398909527490 }}</ref> Consequently, packaging of fresh fruits and vegetables contains an ~8% oxygen atmosphere. Antioxidants are an especially important class of preservatives as, unlike bacterial or [[fungus|fungal]] spoilage, oxidation reactions still occur relatively rapidly in frozen or refrigerated food.<ref>{{cite journal | authors = Zallen EM, Hitchcock MJ, Goertz GE | title = Chilled food systems. Effects of chilled holding on quality of beef loaves | journal = Journal of the American Dietetic Association | volume = 67 | issue = 6 | pages = 552–7 | date = December 1975 | pmid = 1184900 }}</ref> These preservatives include natural antioxidants such as ascorbic acid (AA, E300) and tocopherols (E306), as well as synthetic antioxidants such as [[propyl gallate]] (PG, E310), [[tert-Butylhydroquinone|tertiary butylhydroquinone]] (TBHQ), [[butylated hydroxyanisole]] (BHA, E320) and [[butylated hydroxytoluene]] (BHT, E321).<ref>{{cite journal | authors = Iverson F | title = Phenolic antioxidants: Health Protection Branch studies on butylated hydroxyanisole | journal = Cancer Letters | volume = 93 | issue = 1 | pages = 49–54 | date = June 1995 | pmid = 7600543 | doi = 10.1016/0304-3835(95)03787-W }}</ref><ref>{{cite web|title=E number index |publisher=UK food guide|url=http://www.ukfoodguide.net/enumeric.htm#antioxidants |accessdate=5 March 2007| archiveurl= https://web.archive.org/web/20070304151341/http://www.ukfoodguide.net/enumeric.htm| archivedate= 4 March 2007 | deadurl= no}}</ref> |
||
The most common molecules attacked by oxidation are unsaturated fats; oxidation causes them to turn [[rancidification|rancid]].<ref>{{cite journal | |
The most common molecules attacked by oxidation are unsaturated fats; oxidation causes them to turn [[rancidification|rancid]].<ref>{{cite journal | authors = Robards K, Kerr AF, Patsalides E | title = Rancidity and its measurement in edible oils and snack foods. A review | journal = The Analyst | volume = 113 | issue = 2 | pages = 213–24 | date = February 1988 | pmid = 3288002 | doi = 10.1039/an9881300213 | bibcode = 1988Ana...113..213R }}</ref> Since oxidized lipids are often discolored and usually have unpleasant tastes such as metallic or [[sulfur]]ous flavors, it is important to avoid oxidation in fat-rich foods. Thus, these foods are rarely preserved by drying; instead, they are preserved by [[Smoking (cooking technique)|smoking]], [[salting (food)|salting]] or [[fermentation (food)|fermenting]]. Even less fatty foods such as fruits are sprayed with sulfurous antioxidants prior to air drying. Oxidation is often catalyzed by metals, which is why fats such as butter should never be wrapped in [[aluminium foil]] or kept in metal containers. Some fatty foods such as olive oil are partially protected from oxidation by their natural content of antioxidants, but remain sensitive to photooxidation.<ref>{{cite journal | authors = Del Carlo M, Sacchetti G, Di Mattia C, Compagnone D, Mastrocola D, Liberatore L, Cichelli A | title = Contribution of the phenolic fraction to the antioxidant activity and oxidative stability of olive oil | journal = Journal of Agricultural and Food Chemistry | volume = 52 | issue = 13 | pages = 4072–9 | date = June 2004 | pmid = 15212450 | doi = 10.1021/jf049806z }}</ref> Antioxidant preservatives are also added to fat based cosmetics such as lipstick and [[moisturizer]]s to prevent rancidity. |
||
=== Industrial uses === |
=== Industrial uses === |
||
| Line 232: | Line 232: | ||
[[Image:Vegetarian diet.jpg|upright|thumb|Fruits and vegetables are good sources of antioxidant vitamins C and E]] |
[[Image:Vegetarian diet.jpg|upright|thumb|Fruits and vegetables are good sources of antioxidant vitamins C and E]] |
||
Antioxidant vitamins are found in vegetables, fruits, eggs, legumes and nuts. Vitamins A, C, and E can be destroyed by long-term storage or prolonged cooking.<ref>{{cite journal | |
Antioxidant vitamins are found in vegetables, fruits, eggs, legumes and nuts. Vitamins A, C, and E can be destroyed by long-term storage or prolonged cooking.<ref>{{cite journal | authors = Rodriguez-Amaya DB | title = Food carotenoids: analysis, composition and alterations during storage and processing of foods | journal = Forum of Nutrition | volume = 56 | pages = 35–7 | year = 2003 | pmid = 15806788 }}</ref> The effects of cooking and food processing are complex, as these processes can also increase the [[bioavailability]] of antioxidants, such as some carotenoids in vegetables.<ref>{{cite journal | authors = Maiani G, Castón MJ, Catasta G, Toti E, Cambrodón IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M, Böhm V, Mayer-Miebach E, Behsnilian D, Schlemmer U | title = Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans | journal = Molecular Nutrition & Food Research | volume = 53 Suppl 2 | pages = S194–218 | date = September 2009 | pmid = 19035552 | doi = 10.1002/mnfr.200800053 | url = https://openagrar.bmel-forschung.de/receive/import_mods_00002107 }}</ref> Processed food contains fewer antioxidant vitamins than fresh and uncooked foods, as preparation exposes food to heat and oxygen.<ref>{{cite journal | authors = Henry CJ, Heppell N | title = Nutritional losses and gains during processing: future problems and issues | journal = The Proceedings of the Nutrition Society | volume = 61 | issue = 1 | pages = 145–8 | date = February 2002 | pmid = 12002789 | doi = 10.1079/PNS2001142 | url = http://journals.cambridge.org/production/action/cjoGetFulltext?fulltextid=804076 }}</ref> |
||
{| class="wikitable" style="margin-left: auto; margin-right: auto;" |
{| class="wikitable" style="margin-left: auto; margin-right: auto;" |
||
|- |
|- |
||
!Antioxidant vitamins |
!Antioxidant vitamins |
||
!Foods containing high levels of antioxidant vitamins<ref name="Beecher" /><ref>{{cite web|title=Antioxidants and Cancer Prevention: Fact Sheet |publisher=National Cancer Institute|url=http://www.cancer.gov/cancertopics/factsheet/antioxidantsprevention|accessdate=27 February 2007| archiveurl= https://web.archive.org/web/20070304120554/http://www.cancer.gov/cancertopics/factsheet/antioxidantsprevention| archivedate= 4 March 2007 | deadurl= no}}</ref><ref>{{cite journal | |
!Foods containing high levels of antioxidant vitamins<ref name="Beecher" /><ref>{{cite web|title=Antioxidants and Cancer Prevention: Fact Sheet |publisher=National Cancer Institute|url=http://www.cancer.gov/cancertopics/factsheet/antioxidantsprevention|accessdate=27 February 2007| archiveurl= https://web.archive.org/web/20070304120554/http://www.cancer.gov/cancertopics/factsheet/antioxidantsprevention| archivedate= 4 March 2007 | deadurl= no}}</ref><ref>{{cite journal | authors = Ortega R | title = Importance of functional foods in the Mediterranean diet | journal = Public Health Nutrition | volume = 9 | issue = 8A | pages = 1136–40 | date = December 2006 | pmid = 17378953 | doi = 10.1017/S1368980007668530 }}</ref> |
||
|- |
|- |
||
| [[Vitamin C]] (ascorbic acid) |
| [[Vitamin C]] (ascorbic acid) |
||
| Line 249: | Line 249: | ||
|} |
|} |
||
Other antioxidants are not obtained from the diet, but instead are made in the body. For example, [[ubiquinol]] (coenzyme Q) is poorly absorbed from the gut and is made through the [[mevalonate pathway]].<ref name="Turunen" /> Another example is [[glutathione]], which is made from amino acids. As any glutathione in the gut is broken down to free cysteine, [[glycine]] and [[glutamic acid]] before being absorbed, even large oral intake has little effect on the concentration of glutathione in the body.<ref>{{cite journal | |
Other antioxidants are not obtained from the diet, but instead are made in the body. For example, [[ubiquinol]] (coenzyme Q) is poorly absorbed from the gut and is made through the [[mevalonate pathway]].<ref name="Turunen" /> Another example is [[glutathione]], which is made from amino acids. As any glutathione in the gut is broken down to free cysteine, [[glycine]] and [[glutamic acid]] before being absorbed, even large oral intake has little effect on the concentration of glutathione in the body.<ref>{{cite journal | authors = Witschi A, Reddy S, Stofer B, Lauterburg BH | title = The systemic availability of oral glutathione | journal = European Journal of Clinical Pharmacology | volume = 43 | issue = 6 | pages = 667–9 | year = 1992 | pmid = 1362956 | doi = 10.1007/BF02284971 }}</ref><ref>{{cite journal | authors = Flagg EW, Coates RJ, Eley JW, Jones DP, Gunter EW, Byers TE, Block GS, Greenberg RS | title = Dietary glutathione intake in humans and the relationship between intake and plasma total glutathione level | journal = Nutrition and Cancer | volume = 21 | issue = 1 | pages = 33–46 | year = 1994 | pmid = 8183721 | doi = 10.1080/01635589409514302 }}</ref> Although large amounts of sulfur-containing amino acids such as [[acetylcysteine]] can increase glutathione,<ref name=Dodd>{{cite journal | authors = Dodd S, Dean O, Copolov DL, Malhi GS, Berk M | title = N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility | journal = Expert Opinion on Biological Therapy | volume = 8 | issue = 12 | pages = 1955–62 | date = December 2008 | pmid = 18990082 | doi = 10.1517/14728220802517901 }}</ref> no evidence exists that eating high levels of these glutathione precursors is beneficial for healthy adults.<ref>{{cite journal | authors = van de Poll MC, Dejong CH, Soeters PB | title = Adequate range for sulfur-containing amino acids and biomarkers for their excess: lessons from enteral and parenteral nutrition | journal = The Journal of Nutrition | volume = 136 | issue = 6 Suppl | pages = 1694S–1700S | date = June 2006 | pmid = 16702341 | doi = 10.1093/jn/136.6.1694S }}</ref> |
||
===Measurement and invalidation of ORAC=== |
===Measurement and invalidation of ORAC=== |
||
Measurement of antioxidant content in food is not a straightforward process, as antioxidants collectively are a diverse group of compounds with different reactivities to various reactive oxygen species. In [[food science]], the [[oxygen radical absorbance capacity]] (ORAC) was once an industry standard for antioxidant strength of whole foods, juices and food additives.<ref>{{cite journal | |
Measurement of antioxidant content in food is not a straightforward process, as antioxidants collectively are a diverse group of compounds with different reactivities to various reactive oxygen species. In [[food science]], the [[oxygen radical absorbance capacity]] (ORAC) was once an industry standard for antioxidant strength of whole foods, juices and food additives.<ref>{{cite journal | authors = Cao G, Alessio HM, Cutler RG | title = Oxygen-radical absorbance capacity assay for antioxidants | journal = Free Radical Biology & Medicine | volume = 14 | issue = 3 | pages = 303–11 | date = March 1993 | pmid = 8458588 | doi = 10.1016/0891-5849(93)90027-R | url = https://zenodo.org/record/1258621/files/article.pdf | format = Submitted manuscript }}</ref><ref>{{cite journal | authors = Ou B, Hampsch-Woodill M, Prior RL | title = Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe | journal = Journal of Agricultural and Food Chemistry | volume = 49 | issue = 10 | pages = 4619–26 | date = October 2001 | pmid = 11599998 | doi = 10.1021/jf010586o }}</ref> However, the [[United States Department of Agriculture]] withdrew these ratings in 2012 as biologically invalid, stating that no physiological proof ''[[in vivo]]'' existed to support the [[free-radical theory]] or roles for ingested [[phytochemical]]s, especially for [[polyphenol]]s.<ref name=USDAx>{{cite web |url=http://www.ars.usda.gov/services/docs.htm?docid=15866 |title=Withdrawn: Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2 (2010) |date=16 May 2012 |work= |publisher=United States Department of Agriculture, Agricultural Research Service |accessdate=13 June 2012}}</ref> Consequently, the ORAC method, derived only from ''in vitro'' experiments, is no longer considered relevant to human diets or [[biology]]. |
||
Alternative ''in vitro'' measurements of antioxidant content in foods include the [[Folin-Ciocalteu reagent]], and the [[Trolox equivalent antioxidant capacity]] assay.<ref>{{cite journal | |
Alternative ''in vitro'' measurements of antioxidant content in foods include the [[Folin-Ciocalteu reagent]], and the [[Trolox equivalent antioxidant capacity]] assay.<ref>{{cite journal | authors = Prior RL, Wu X, Schaich K | title = Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements | journal = Journal of Agricultural and Food Chemistry | volume = 53 | issue = 10 | pages = 4290–302 | date = May 2005 | pmid = 15884874 | doi = 10.1021/jf0502698 | url = https://naldc.nal.usda.gov/Policy.pdf | accessdate = 24 October 2017 | archive-url = https://web.archive.org/web/20161229203509/https://naldc.nal.usda.gov/Policy.pdf | archive-date = 29 December 2016 | deadurl = yes | df = dmy-all }}</ref> |
||
== History == |
== History == |
||
As part of their adaptation from marine life, terrestrial plants began producing non-marine antioxidants such as [[ascorbic acid]] ([[vitamin C]]), [[polyphenol]]s and [[tocopherol]]s. The evolution of [[angiosperm]] plants between 50 and 200 million years ago resulted in the development of many antioxidant pigments – particularly during the [[Jurassic]] period – as chemical defences against [[reactive oxygen species]] that are byproducts of [[photosynthesis]].<ref>{{cite journal | |
As part of their adaptation from marine life, terrestrial plants began producing non-marine antioxidants such as [[ascorbic acid]] ([[vitamin C]]), [[polyphenol]]s and [[tocopherol]]s. The evolution of [[angiosperm]] plants between 50 and 200 million years ago resulted in the development of many antioxidant pigments – particularly during the [[Jurassic]] period – as chemical defences against [[reactive oxygen species]] that are byproducts of [[photosynthesis]].<ref>{{cite journal | authors = Benzie IF | title = Evolution of dietary antioxidants | journal = Comparative Biochemistry and Physiology A | volume = 136 | issue = 1 | pages = 113–26 | date = September 2003 | pmid = 14527634 | doi = 10.1016/S1095-6433(02)00368-9 | url = http://hdl.handle.net/10397/34754 }}</ref> Originally, the term antioxidant specifically referred to a chemical that prevented the consumption of oxygen. In the late 19th and early 20th centuries, extensive study concentrated on the use of antioxidants in important industrial processes, such as the prevention of metal [[corrosion]], the [[sulfur vulcanization|vulcanization]] of rubber, and the [[polymerization]] of fuels in the [[fouling]] of [[internal combustion engine]]s.<ref>{{cite journal | authors = Mattill HA | title = Antioxidants | journal = Annual Review of Biochemistry | volume = 16 | pages = 177–92 | year = 1947 | pmid = 20259061 | doi = 10.1146/annurev.bi.16.070147.001141 }}</ref> |
||
Early research on the role of antioxidants in biology focused on their use in preventing the oxidation of [[unsaturated fat]]s, which is the cause of [[rancidity]].<ref>{{cite journal | |
Early research on the role of antioxidants in biology focused on their use in preventing the oxidation of [[unsaturated fat]]s, which is the cause of [[rancidity]].<ref>{{cite journal | authors = German JB | title = Food processing and lipid oxidation | volume = 459 | pages = 23–50 | year = 1999 | pmid = 10335367 | doi = 10.1007/978-1-4615-4853-9_3 | isbn = 978-0-306-46051-7 | series = Advances in Experimental Medicine and Biology }}</ref> Antioxidant activity could be measured simply by placing the fat in a closed container with oxygen and measuring the rate of oxygen consumption. However, it was the identification of [[Vitamin A|vitamins A]], [[Vitamin C|C]], and [[Vitamin E|E]] as antioxidants that revolutionized the field and led to the realization of the importance of antioxidants in the biochemistry of [[Organism|living organisms]].<ref>{{cite journal | authors = Jacob RA | title = Three eras of vitamin C discovery | journal = Sub-Cellular Biochemistry | volume = 25 | pages = 1–16 | year = 1996 | pmid = 8821966 | doi = 10.1007/978-1-4613-0325-1_1 | isbn = 978-1-4613-7998-0 | series = Subcellular Biochemistry | url = https://books.google.com/books?id=kZdtMAEACAAJ }}</ref><ref>{{cite journal | authors = Knight JA | title = Free radicals: their history and current status in aging and disease | journal = Annals of Clinical and Laboratory Science | volume = 28 | issue = 6 | pages = 331–46 | year = 1998 | pmid = 9846200 }}</ref> The possible [[mechanisms of action]] of antioxidants were first explored when it was recognized that a substance with anti-oxidative activity is likely to be one that is itself readily oxidized.<ref>{{cite journal |last1=Moureu|first1=Charles |last2=Dufraisse|first2=Charles |year=1922 |title=Sur l'autoxydation: Les antioxygènes|journal=Comptes Rendus des Séances et Mémoires de la Société de Biologie |volume=86 |pages=321–322|language=French | name-list-format = vanc }}</ref> Research into how [[vitamin E]] prevents the process of [[lipid peroxidation]] led to the identification of antioxidants as reducing agents that prevent oxidative reactions, often by [[scavenger (chemistry)|scavenging]] [[reactive oxygen species]] before they can damage cells.<ref>{{cite journal | authors = Wolf G | title = The discovery of the antioxidant function of vitamin E: the contribution of Henry A. Mattill | journal = The Journal of Nutrition | volume = 135 | issue = 3 | pages = 363–6 | date = March 2005 | pmid = 15735064 | url = http://jn.nutrition.org/content/135/3/363.long | doi = 10.1093/jn/135.3.363 }}</ref> |
||
== References == |
== References == |
||
Revision as of 21:53, 23 July 2018
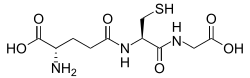
Antioxidants are compounds that inhibit the oxidation. Oxidation is a chemical reaction that can produce free radicals, thereby leading to chain reactions that may damage the cells of organisms. Antioxidants such as thiols or ascorbic acid (vitamin C) terminate these chain reactions. To balance the oxidative state, plants and animals maintain complex systems of overlapping antioxidants, such as glutathione and enzymes (e.g., catalase and superoxide dismutase), produced internally, or the dietary antioxidants vitamin A, vitamin C, and vitamin E.
The term "antioxidant" is mostly used for two entirely different groups of substances: industrial chemicals that are added to products to prevent oxidation, and naturally occurring compounds that are present in foods and tissue. The former, industrial antioxidants, have diverse uses: acting as preservatives in food and cosmetics, and being oxidation-inhibitors in fuels.[1]
Importantly, antioxidant dietary supplements have not yet been shown to improve health in humans, or to be effective at preventing disease.[2] Supplements of beta-carotene, vitamin A, and vitamin E have no effect on mortality rate[3][4] or cancer risk.[5][6] Additionally, supplementation with selenium or vitamin E do not reduce the risk of cardiovascular disease.[7][8]
Health effects
Relation to diet
Although certain levels of antioxidant vitamins in the diet are required for good health, there is still considerable debate on whether antioxidant-rich foods or supplements have anti-disease activity. Moreover, if they are actually beneficial, it is unknown which antioxidants are health-promoting in the diet and in what amounts beyond typical dietary intake.[9][10][11] Some authors dispute the hypothesis that antioxidant vitamins could prevent chronic diseases,[9][12] and others maintain such that hypothesis is unproven and misguided.[13]
Polyphenols, which often have antioxidant properties in vitro, are not necessarily antioxidants in vivo due to extensive metabolism following digestion.[14] In many polyphenols the catechol group acts as an electron acceptor and is therefore responsible for the antioxidant activity.[15] However, this catechol group undergoes extensive metabolism upon uptake in the human body, for example by catechol-O-methyl transferase, and is therefore no longer able to act as an electron acceptor. Many polyphenols may have non-antioxidant roles in minute concentrations that affect cell-to-cell signaling, receptor sensitivity, inflammatory enzyme activity or gene regulation.[16][17]
Although dietary antioxidants have been investigated for potential effects on neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis,[18][19] these studies have been inconclusive.[20][21][22]
Drug candidates
Tirilazad is an antioxidant steroid derivative that inhibits the lipid peroxidation that is believed to play a key role in neuronal death in stroke and head injury. It demonstrated activity in animal models of stroke,[23] but human trials demonstrated no effect on mortality or other outcomes in subarachnoid haemorrhage[24] and worsened results in ischemic stroke.[25]
Similarly, the designed antioxidant NXY-059 exhibited efficacy in animal models, but failed to improve stroke outcomes in a clinical trial.[26] As of November 2014, other antioxidants are being studied as potential neuroprotectants.[27]
Common pharmaceuticals (and supplements) with antioxidant properties may interfere with the efficacy of certain anticancer medication and radiation.[28][29]
A 2016 systematic review examined antioxidant medications, such as allopurinol and acetylcysteine, as add on treatment for schizophrenia.[30] Evidence was insufficient to determine benefits and there was potential for adverse effects.[30]
Adverse effects

Relatively strong reducing acids can have antinutrient effects by binding to dietary minerals such as iron and zinc in the gastrointestinal tract and preventing them from being absorbed.[31] Notable examples are oxalic acid, tannins and phytic acid, which are high in plant-based diets.[32] Calcium and iron deficiencies are not uncommon in diets in developing countries where less meat is eaten and there is high consumption of phytic acid from beans and unleavened whole grain bread.[33]
| Foods | Reducing acid present |
|---|---|
| Cocoa bean and chocolate, spinach, turnip and rhubarb.[34] | Oxalic acid |
| Whole grains, maize, legumes.[35] | Phytic acid |
| Tea, beans, cabbage.[34][36] | Tannins |
Nonpolar antioxidants such as eugenol—a major component of oil of cloves—have toxicity limits that can be exceeded with the misuse of undiluted essential oils.[37] Toxicity associated with high doses of water-soluble antioxidants such as ascorbic acid are less of a concern, as these compounds can be excreted rapidly in urine.[38] More seriously, very high doses of some antioxidants may have harmful long-term effects. The beta-carotene and Retinol Efficacy Trial (CARET) study of lung cancer patients found that smokers given supplements containing beta-carotene and vitamin A had increased rates of lung cancer.[39] Subsequent studies confirmed these adverse effects.[40]
These harmful effects may also be seen in non-smokers, as one meta-analysis including data from approximately 230,000 patients showed that β-carotene, vitamin A or vitamin E supplementation is associated with increased mortality, but saw no significant effect from vitamin C.[41] No health risk was seen when all the randomized controlled studies were examined together, but an increase in mortality was detected when only high-quality and low-bias risk trials were examined separately.[42] As the majority of these low-bias trials dealt with either elderly people, or people with disease, these results may not apply to the general population.[43] This meta-analysis was later repeated and extended by the same authors, with the new analysis published by the Cochrane Collaboration; this analysis confirmed the previous results.[42] These two publications are consistent with some previous meta-analyzes that also suggested that Vitamin E supplementation increased mortality,[44] and that antioxidant supplements increased the risk of colon cancer.[45] Beta-carotene may also increase lung cancer.[45][46] Overall, the large number of clinical trials carried out on antioxidant supplements suggest that either these products have no effect on health, or that they cause a small increase in mortality in elderly or vulnerable populations.[9][10][41]
While antioxidant supplementation is widely used in attempts to prevent the development of cancer, antioxidants may interfere with cancer treatments,[47] since the environment of cancer cells causes high levels of oxidative stress, making these cells more susceptible to the further oxidative stress induced by treatments. As a result, by reducing the redox stress in cancer cells, antioxidant supplements (and pharmaceuticals) could decrease the effectiveness of radiotherapy and chemotherapy.[28][48][49] On the other hand, other reviews have suggested that antioxidants could reduce side effects or increase survival times.[50][51]
Oxidative challenge in biology

A paradox in metabolism is that, while the vast majority of complex life on Earth requires oxygen for its existence, oxygen is a highly reactive molecule that damages living organisms by producing reactive oxygen species.[52] Consequently, organisms contain a complex network of antioxidant metabolites and enzymes that work together to prevent oxidative damage to cellular components such as DNA, proteins and lipids.[53][54] In general, antioxidant systems either prevent these reactive species from being formed, or remove them before they can damage vital components of the cell.[52][53] However, reactive oxygen species also have useful cellular functions, such as redox signaling. Thus, the function of antioxidant systems is not to remove oxidants entirely, but instead to keep them at an optimum level.[55]
The reactive oxygen species produced in cells include hydrogen peroxide (H2O2), hypochlorous acid (HClO), and free radicals such as the hydroxyl radical (·OH) and the superoxide anion (O2−).[56] The hydroxyl radical is particularly unstable and will react rapidly and non-specifically with most biological molecules. This species is produced from hydrogen peroxide in metal-catalyzed redox reactions such as the Fenton reaction.[57] These oxidants can damage cells by starting chemical chain reactions such as lipid peroxidation, or by oxidizing DNA or proteins.[53] Damage to DNA can cause mutations and possibly cancer, if not reversed by DNA repair mechanisms,[58][59] while damage to proteins causes enzyme inhibition, denaturation and protein degradation.[60]
The use of oxygen as part of the process for generating metabolic energy produces reactive oxygen species.[61] In this process, the superoxide anion is produced as a by-product of several steps in the electron transport chain.[62] Particularly important is the reduction of coenzyme Q in complex III, since a highly reactive free radical is formed as an intermediate (Q·−). This unstable intermediate can lead to electron "leakage", when electrons jump directly to oxygen and form the superoxide anion, instead of moving through the normal series of well-controlled reactions of the electron transport chain.[63] Peroxide is also produced from the oxidation of reduced flavoproteins, such as complex I.[64] However, although these enzymes can produce oxidants, the relative importance of the electron transfer chain to other processes that generate peroxide is unclear.[65][66] In plants, algae, and cyanobacteria, reactive oxygen species are also produced during photosynthesis,[67] particularly under conditions of high light intensity.[68] This effect is partly offset by the involvement of carotenoids in photoinhibition, and in algae and cyanobacteria, by large amount of iodide and selenium,[69] which involves these antioxidants reacting with over-reduced forms of the photosynthetic reaction centres to prevent the production of reactive oxygen species.[70][71]
Examples of bioactive antioxidant compounds
Antioxidants are classified into two broad divisions, depending on whether they are soluble in water (hydrophilic) or in lipids (lipophilic). In general, water-soluble antioxidants react with oxidants in the cell cytosol and the blood plasma, while lipid-soluble antioxidants protect cell membranes from lipid peroxidation.[53] These compounds may be synthesized in the body or obtained from the diet.[54] The different antioxidants are present at a wide range of concentrations in body fluids and tissues, with some such as glutathione or ubiquinone mostly present within cells, while others such as uric acid are more evenly distributed (see table below). Some antioxidants are only found in a few organisms and these compounds can be important in pathogens and can be virulence factors.[72]
The relative importance and interactions between these different antioxidants is a very complex question, with the various antioxidant compounds and antioxidant enzyme systems having synergistic and interdependent effects on one another.[73][74] The action of one antioxidant may therefore depend on the proper function of other members of the antioxidant system.[54] The amount of protection provided by any one antioxidant will also depend on its concentration, its reactivity towards the particular reactive oxygen species being considered, and the status of the antioxidants with which it interacts.[54]
Some compounds contribute to antioxidant defense by chelating transition metals and preventing them from catalyzing the production of free radicals in the cell. Particularly important is the ability to sequester iron, which is the function of iron-binding proteins such as transferrin and ferritin.[66] Selenium and zinc are commonly referred to as antioxidant nutrients, but these chemical elements have no antioxidant action themselves and are instead required for the activity of some antioxidant enzymes, as is discussed below.
| Antioxidant | Solubility | Concentration in human serum (μM) | Concentration in liver tissue (μmol/kg) |
|---|---|---|---|
| Ascorbic acid (vitamin C) | Water | 50–60[75] | 260 (human)[76] |
| Glutathione | Water | 4[77] | 6,400 (human)[76] |
| Lipoic acid | Water | 0.1–0.7[78] | 4–5 (rat)[79] |
| Uric acid | Water | 200–400[80] | 1,600 (human)[76] |
| Carotenes | Lipid | β-carotene: 0.5–1[81] | 5 (human, total carotenoids)[83] |
| α-Tocopherol (vitamin E) | Lipid | 10–40[82] | 50 (human)[76] |
| Ubiquinol (coenzyme Q) | Lipid | 5[84] | 200 (human)[85] |
Uric acid
Uric acid is by far the highest concentration antioxidant in human blood. Uric acid (UA) is an antioxidant oxypurine produced from xanthine by the enzyme xanthine oxidase, and is an intermediate product of purine metabolism.[86] In almost all land animals, urate oxidase further catalyzes the oxidation of uric acid to allantoin,[87] but in humans and most higher primates, the urate oxidase gene is nonfunctional, so that UA is not further broken down.[87][88] The evolutionary reasons for this loss of urate conversion to allantoin remain the topic of active speculation.[89][90] The antioxidant effects of uric acid have led researchers to suggest this mutation was beneficial to early primates and humans.[90][91] Studies of high altitude acclimatization support the hypothesis that urate acts as an antioxidant by mitigating the oxidative stress caused by high-altitude hypoxia.[92]
Uric acid has the highest concentration of any blood antioxidant[80] and provides over half of the total antioxidant capacity of human serum.[93] Uric acid's antioxidant activities are also complex, given that it does not react with some oxidants, such as superoxide, but does act against peroxynitrite,[94] peroxides, and hypochlorous acid.[86] Concerns over elevated UA's contribution to gout must be considered as one of many risk factors.[95] By itself, UA-related risk of gout at high levels (415–530 μmol/L) is only 0.5% per year with an increase to 4.5% per year at UA supersaturation levels (535+ μmol/L).[96] Many of these aforementioned studies determined UA's antioxidant actions within normal physiological levels,[92][94] and some found antioxidant activity at levels as high as 285 μmol/L.[97]
Vitamin C
Ascorbic acid or "vitamin C" is a monosaccharide oxidation-reduction (redox) catalyst found in both animals and plants. As one of the enzymes needed to make ascorbic acid has been lost by mutation during primate evolution, humans must obtain it from the diet; it is therefore a vitamin.[98] Most other animals are able to produce this compound in their bodies and do not require it in their diets.[99] Ascorbic acid is required for the conversion of the procollagen to collagen by oxidizing proline residues to hydroxyproline. In other cells, it is maintained in its reduced form by reaction with glutathione, which can be catalysed by protein disulfide isomerase and glutaredoxins.[100][101] Ascorbic acid is a redox catalyst which can reduce, and thereby neutralize, reactive oxygen species such as hydrogen peroxide.[102] In addition to its direct antioxidant effects, ascorbic acid is also a substrate for the redox enzyme ascorbate peroxidase, a function that is particularly important in stress resistance in plants.[103] Ascorbic acid is present at high levels in all parts of plants and can reach concentrations of 20 millimolar in chloroplasts.[104]
Glutathione
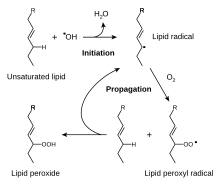
Glutathione is a cysteine-containing peptide found in most forms of aerobic life.[105] It is not required in the diet and is instead synthesized in cells from its constituent amino acids.[106] Glutathione has antioxidant properties since the thiol group in its cysteine moiety is a reducing agent and can be reversibly oxidized and reduced. In cells, glutathione is maintained in the reduced form by the enzyme glutathione reductase and in turn reduces other metabolites and enzyme systems, such as ascorbate in the glutathione-ascorbate cycle, glutathione peroxidases and glutaredoxins, as well as reacting directly with oxidants.[100] Due to its high concentration and its central role in maintaining the cell's redox state, glutathione is one of the most important cellular antioxidants.[105] In some organisms glutathione is replaced by other thiols, such as by mycothiol in the Actinomycetes, bacillithiol in some Gram-positive bacteria,[107][108] or by trypanothione in the Kinetoplastids.[109][110]
Vitamin E
Vitamin E is the collective name for a set of eight related tocopherols and tocotrienols, which are fat-soluble vitamins with antioxidant properties.[111][112] Of these, α-tocopherol has been most studied as it has the highest bioavailability, with the body preferentially absorbing and metabolising this form.[113]
It has been claimed that the α-tocopherol form is the most important lipid-soluble antioxidant, and that it protects membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction.[111][114] This removes the free radical intermediates and prevents the propagation reaction from continuing. This reaction produces oxidised α-tocopheroxyl radicals that can be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol or ubiquinol.[115] This is in line with findings showing that α-tocopherol, but not water-soluble antioxidants, efficiently protects glutathione peroxidase 4 (GPX4)-deficient cells from cell death.[116] GPx4 is the only known enzyme that efficiently reduces lipid-hydroperoxides within biological membranes.
However, the roles and importance of the various forms of vitamin E are presently unclear,[117][118] and it has even been suggested that the most important function of α-tocopherol is as a signaling molecule, with this molecule having no significant role in antioxidant metabolism.[119][120] The functions of the other forms of vitamin E are even less well understood, although γ-tocopherol is a nucleophile that may react with electrophilic mutagens,[113] and tocotrienols may be important in protecting neurons from damage.[121]
Pro-oxidant activities
Antioxidants that are reducing agents can also act as pro-oxidants. For example, vitamin C has antioxidant activity when it reduces oxidizing substances such as hydrogen peroxide,[122] however, it will also reduce metal ions that generate free radicals through the Fenton reaction.[57][123]
- 2 Fe3+ + Ascorbate → 2 Fe2+ + Dehydroascorbate
- 2 Fe2+ + 2 H2O2 → 2 Fe3+ + 2 OH· + 2 OH−
The relative importance of the antioxidant and pro-oxidant activities of antioxidants is an area of current research, but vitamin C, which exerts its effects as a vitamin by oxidizing polypeptides, appears to have a mostly antioxidant action in the human body.[123] However, less data is available for other dietary antioxidants, such as vitamin E,[124] or the polyphenols.[125][126] Likewise, the pathogenesis of diseases involving hyperuricemia likely involve uric acid's direct and indirect pro-oxidant properties.
That is, paradoxically, agents which are normally considered antioxidants can act as conditional pro-oxidants and actually increase oxidative stress. Besides ascorbate, medically important conditional pro-oxidants include uric acid and sulfhydryl amino acids such as homocysteine. Typically, this involves some transition-series metal such as copper or iron as catalyst. The potential role of the pro-oxidant role of uric acid in (e.g.) atherosclerosis and ischemic stroke is considered above. Another example is the postulated role of homocysteine in atherosclerosis.
Enzyme systems
As with the chemical antioxidants, cells are protected against oxidative stress by an interacting network of antioxidant enzymes.[52][53] Here, the superoxide released by processes such as oxidative phosphorylation is first converted to hydrogen peroxide and then further reduced to give water. This detoxification pathway is the result of multiple enzymes, with superoxide dismutases catalysing the first step and then catalases and various peroxidases removing hydrogen peroxide. As with antioxidant metabolites, the contributions of these enzymes to antioxidant defenses can be hard to separate from one another, but the generation of transgenic mice lacking just one antioxidant enzyme can be informative.[127]
Superoxide dismutase, catalase, and peroxiredoxins
Superoxide dismutases (SODs) are a class of closely related enzymes that catalyze the breakdown of the superoxide anion into oxygen and hydrogen peroxide.[128][129] SOD enzymes are present in almost all aerobic cells and in extracellular fluids.[130] Superoxide dismutase enzymes contain metal ion cofactors that, depending on the isozyme, can be copper, zinc, manganese or iron. In humans, the copper/zinc SOD is present in the cytosol, while manganese SOD is present in the mitochondrion.[129] There also exists a third form of SOD in extracellular fluids, which contains copper and zinc in its active sites.[131] The mitochondrial isozyme seems to be the most biologically important of these three, since mice lacking this enzyme die soon after birth.[132] In contrast, the mice lacking copper/zinc SOD (Sod1) are viable but have numerous pathologies and a reduced lifespan (see article on superoxide), while mice without the extracellular SOD have minimal defects (sensitive to hyperoxia).[127][133] In plants, SOD isozymes are present in the cytosol and mitochondria, with an iron SOD found in chloroplasts that is absent from vertebrates and yeast.[134]
Catalases are enzymes that catalyse the conversion of hydrogen peroxide to water and oxygen, using either an iron or manganese cofactor.[135][136] This protein is localized to peroxisomes in most eukaryotic cells.[137] Catalase is an unusual enzyme since, although hydrogen peroxide is its only substrate, it follows a ping-pong mechanism. Here, its cofactor is oxidised by one molecule of hydrogen peroxide and then regenerated by transferring the bound oxygen to a second molecule of substrate.[138] Despite its apparent importance in hydrogen peroxide removal, humans with genetic deficiency of catalase — "acatalasemia" — or mice genetically engineered to lack catalase completely, suffer few ill effects.[139][140]

Peroxiredoxins are peroxidases that catalyze the reduction of hydrogen peroxide, organic hydroperoxides, as well as peroxynitrite.[142] They are divided into three classes: typical 2-cysteine peroxiredoxins; atypical 2-cysteine peroxiredoxins; and 1-cysteine peroxiredoxins.[143] These enzymes share the same basic catalytic mechanism, in which a redox-active cysteine (the peroxidatic cysteine) in the active site is oxidized to a sulfenic acid by the peroxide substrate.[144] Over-oxidation of this cysteine residue in peroxiredoxins inactivates these enzymes, but this can be reversed by the action of sulfiredoxin.[145] Peroxiredoxins seem to be important in antioxidant metabolism, as mice lacking peroxiredoxin 1 or 2 have shortened lifespan and suffer from hemolytic anaemia, while plants use peroxiredoxins to remove hydrogen peroxide generated in chloroplasts.[146][147][148]
Thioredoxin and glutathione systems
The thioredoxin system contains the 12-kDa protein thioredoxin and its companion thioredoxin reductase.[149] Proteins related to thioredoxin are present in all sequenced organisms. Plants, such as Arabidopsis thaliana, have a particularly great diversity of isoforms.[150] The active site of thioredoxin consists of two neighboring cysteines, as part of a highly conserved CXXC motif, that can cycle between an active dithiol form (reduced) and an oxidized disulfide form. In its active state, thioredoxin acts as an efficient reducing agent, scavenging reactive oxygen species and maintaining other proteins in their reduced state.[151] After being oxidized, the active thioredoxin is regenerated by the action of thioredoxin reductase, using NADPH as an electron donor.[152]
The glutathione system includes glutathione, glutathione reductase, glutathione peroxidases, and glutathione S-transferases.[105] This system is found in animals, plants and microorganisms.[105][153] Glutathione peroxidase is an enzyme containing four selenium-cofactors that catalyzes the breakdown of hydrogen peroxide and organic hydroperoxides. There are at least four different glutathione peroxidase isozymes in animals.[154] Glutathione peroxidase 1 is the most abundant and is a very efficient scavenger of hydrogen peroxide, while glutathione peroxidase 4 is most active with lipid hydroperoxides. Surprisingly, glutathione peroxidase 1 is dispensable, as mice lacking this enzyme have normal lifespans,[155] but they are hypersensitive to induced oxidative stress.[156] In addition, the glutathione S-transferases show high activity with lipid peroxides.[157] These enzymes are at particularly high levels in the liver and also serve in detoxification metabolism.[158]
Oxidative stress in disease
Oxidative stress is thought to contribute to the development of a wide range of diseases including Alzheimer's disease,[159][160] Parkinson's disease,[161] the pathologies caused by diabetes,[162][163] rheumatoid arthritis,[164] and neurodegeneration in motor neuron diseases.[165] In many of these cases, it is unclear if oxidants trigger the disease, or if they are produced as a secondary consequence of the disease and from general tissue damage;[56] One case in which this link is particularly well understood is the role of oxidative stress in cardiovascular disease. Here, low density lipoprotein (LDL) oxidation appears to trigger the process of atherogenesis, which results in atherosclerosis, and finally cardiovascular disease.[166][167]
Oxidative damage in DNA can cause cancer. Several antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, glutathione S-transferase etc. protect DNA from oxidative stress. It has been proposed that polymorphisms in these enzymes are associated with DNA damage and subsequently the individual's risk of cancer susceptibility.[168]
A low calorie diet extends median and maximum lifespan in many animals. This effect may involve a reduction in oxidative stress.[169] While there is some evidence to support the role of oxidative stress in aging in model organisms such as Drosophila melanogaster and Caenorhabditis elegans,[170][171] the evidence in mammals is less clear.[172][173][174] Indeed, a 2009 review of experiments in mice concluded that almost all manipulations of antioxidant systems had no effect on aging.[175]
Uses in technology
Food preservatives
Antioxidants are used as food additives to help guard against food deterioration. Exposure to oxygen and sunlight are the two main factors in the oxidation of food, so food is preserved by keeping in the dark and sealing it in containers or even coating it in wax, as with cucumbers. However, as oxygen is also important for plant respiration, storing plant materials in anaerobic conditions produces unpleasant flavors and unappealing colors.[176] Consequently, packaging of fresh fruits and vegetables contains an ~8% oxygen atmosphere. Antioxidants are an especially important class of preservatives as, unlike bacterial or fungal spoilage, oxidation reactions still occur relatively rapidly in frozen or refrigerated food.[177] These preservatives include natural antioxidants such as ascorbic acid (AA, E300) and tocopherols (E306), as well as synthetic antioxidants such as propyl gallate (PG, E310), tertiary butylhydroquinone (TBHQ), butylated hydroxyanisole (BHA, E320) and butylated hydroxytoluene (BHT, E321).[178][179]
The most common molecules attacked by oxidation are unsaturated fats; oxidation causes them to turn rancid.[180] Since oxidized lipids are often discolored and usually have unpleasant tastes such as metallic or sulfurous flavors, it is important to avoid oxidation in fat-rich foods. Thus, these foods are rarely preserved by drying; instead, they are preserved by smoking, salting or fermenting. Even less fatty foods such as fruits are sprayed with sulfurous antioxidants prior to air drying. Oxidation is often catalyzed by metals, which is why fats such as butter should never be wrapped in aluminium foil or kept in metal containers. Some fatty foods such as olive oil are partially protected from oxidation by their natural content of antioxidants, but remain sensitive to photooxidation.[181] Antioxidant preservatives are also added to fat based cosmetics such as lipstick and moisturizers to prevent rancidity.
Industrial uses

Antioxidants are frequently added to industrial products. A common use is as stabilizers in fuels and lubricants to prevent oxidation, and in gasolines to prevent the polymerization that leads to the formation of engine-fouling residues.[182] In 2014, the worldwide market for natural and synthetic antioxidants was US $2.25 billion with a forecast of growth to $3.25 billion by 2020.[183]
Antioxidant polymer stabilizers are widely used to prevent the degradation of polymers such as rubbers, plastics and adhesives that causes a loss of strength and flexibility in these materials.[184] Polymers containing double bonds in their main chains, such as natural rubber and polybutadiene, are especially susceptible to oxidation and ozonolysis. They can be protected by antiozonants. Solid polymer products start to crack on exposed surfaces as the material degrades and the chains break. The mode of cracking varies between oxygen and ozone attack, the former causing a "crazy paving" effect, while ozone attack produces deeper cracks aligned at right angles to the tensile strain in the product. Oxidation and UV degradation are also frequently linked, mainly because UV radiation creates free radicals by bond breakage. The free radicals then react with oxygen to produce peroxy radicals which cause yet further damage, often in a chain reaction. Other polymers susceptible to oxidation include polypropylene and polyethylene. The former is more sensitive owing to the presence of secondary carbon atoms present in every repeat unit. Attack occurs at this point because the free radical formed is more stable than one formed on a primary carbon atom. Oxidation of polyethylene tends to occur at weak links in the chain, such as branch points in low-density polyethylene.
| Fuel additive | Components[185] | Applications[185] |
|---|---|---|
| AO-22 | N,N'-di-2-butyl-1,4-phenylenediamine | Turbine oils, transformer oils, hydraulic fluids, waxes, and greases |
| AO-24 | N,N'-di-2-butyl-1,4-phenylenediamine | Low-temperature oils |
| AO-29 | 2,6-di-tert-butyl-4-methylphenol | Turbine oils, transformer oils, hydraulic fluids, waxes, greases, and gasolines |
| AO-30 | 2,4-dimethyl-6-tert-butylphenol | Jet fuels and gasolines, including aviation gasolines |
| AO-31 | 2,4-dimethyl-6-tert-butylphenol | Jet fuels and gasolines, including aviation gasolines |
| AO-32 | 2,4-dimethyl-6-tert-butylphenol and 2,6-di-tert-butyl-4-methylphenol | Jet fuels and gasolines, including aviation gasolines |
| AO-37 | 2,6-di-tert-butylphenol | Jet fuels and gasolines, widely approved for aviation fuels |
Levels in food

Antioxidant vitamins are found in vegetables, fruits, eggs, legumes and nuts. Vitamins A, C, and E can be destroyed by long-term storage or prolonged cooking.[186] The effects of cooking and food processing are complex, as these processes can also increase the bioavailability of antioxidants, such as some carotenoids in vegetables.[187] Processed food contains fewer antioxidant vitamins than fresh and uncooked foods, as preparation exposes food to heat and oxygen.[188]
| Antioxidant vitamins | Foods containing high levels of antioxidant vitamins[36][189][190] |
|---|---|
| Vitamin C (ascorbic acid) | Fresh or frozen fruits and vegetables |
| Vitamin E (tocopherols, tocotrienols) | Vegetable oils, nuts, and seeds |
| Carotenoids (carotenes as provitamin A) | Fruit, vegetables and eggs |
Other antioxidants are not obtained from the diet, but instead are made in the body. For example, ubiquinol (coenzyme Q) is poorly absorbed from the gut and is made through the mevalonate pathway.[85] Another example is glutathione, which is made from amino acids. As any glutathione in the gut is broken down to free cysteine, glycine and glutamic acid before being absorbed, even large oral intake has little effect on the concentration of glutathione in the body.[191][192] Although large amounts of sulfur-containing amino acids such as acetylcysteine can increase glutathione,[193] no evidence exists that eating high levels of these glutathione precursors is beneficial for healthy adults.[194]
Measurement and invalidation of ORAC
Measurement of antioxidant content in food is not a straightforward process, as antioxidants collectively are a diverse group of compounds with different reactivities to various reactive oxygen species. In food science, the oxygen radical absorbance capacity (ORAC) was once an industry standard for antioxidant strength of whole foods, juices and food additives.[195][196] However, the United States Department of Agriculture withdrew these ratings in 2012 as biologically invalid, stating that no physiological proof in vivo existed to support the free-radical theory or roles for ingested phytochemicals, especially for polyphenols.[197] Consequently, the ORAC method, derived only from in vitro experiments, is no longer considered relevant to human diets or biology.
Alternative in vitro measurements of antioxidant content in foods include the Folin-Ciocalteu reagent, and the Trolox equivalent antioxidant capacity assay.[198]
History
As part of their adaptation from marine life, terrestrial plants began producing non-marine antioxidants such as ascorbic acid (vitamin C), polyphenols and tocopherols. The evolution of angiosperm plants between 50 and 200 million years ago resulted in the development of many antioxidant pigments – particularly during the Jurassic period – as chemical defences against reactive oxygen species that are byproducts of photosynthesis.[199] Originally, the term antioxidant specifically referred to a chemical that prevented the consumption of oxygen. In the late 19th and early 20th centuries, extensive study concentrated on the use of antioxidants in important industrial processes, such as the prevention of metal corrosion, the vulcanization of rubber, and the polymerization of fuels in the fouling of internal combustion engines.[200]
Early research on the role of antioxidants in biology focused on their use in preventing the oxidation of unsaturated fats, which is the cause of rancidity.[201] Antioxidant activity could be measured simply by placing the fat in a closed container with oxygen and measuring the rate of oxygen consumption. However, it was the identification of vitamins A, C, and E as antioxidants that revolutionized the field and led to the realization of the importance of antioxidants in the biochemistry of living organisms.[202][203] The possible mechanisms of action of antioxidants were first explored when it was recognized that a substance with anti-oxidative activity is likely to be one that is itself readily oxidized.[204] Research into how vitamin E prevents the process of lipid peroxidation led to the identification of antioxidants as reducing agents that prevent oxidative reactions, often by scavenging reactive oxygen species before they can damage cells.[205]
References
- ^ Dabelstein, Werner; Reglitzky, Arno; Schütze, Andrea; Reders, Klaus (2007). "Automotive Fuels". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a16_719.pub2. ISBN 978-3-527-30673-2.
{{cite encyclopedia}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Antioxidants: In Depth". NCCIH. Retrieved 20 June 2018.
- ^ "Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm?". PLoS ONE. 8 (9): e74558. 2013. Bibcode:2013PLoSO...874558B. doi:10.1371/journal.pone.0074558. PMC 3765487. PMID 24040282.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ "Vitamin E and all-cause mortality: a meta-analysis". Current Aging Science. 4 (2): 158–70. July 2011. doi:10.2174/1874609811104020158. PMC 4030744. PMID 21235492.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Drugs for preventing lung cancer in healthy people". The Cochrane Database of Systematic Reviews. 10: CD002141. 2012. doi:10.1002/14651858.CD002141.pub2. PMID 23076895.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials". Nutrition and Cancer. 62 (6): 719–27. 2010. doi:10.1080/01635581.2010.494335. PMID 20661819.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Selenium supplementation for the primary prevention of cardiovascular disease" (Full text). The Cochrane Database of Systematic Reviews. 1 (1): CD009671. 2013. doi:10.1002/14651858.CD009671.pub2. PMC 4176632. PMID 23440843.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease" (Full text). Journal of General Internal Medicine. 19 (4): 380–9. April 2004. doi:10.1111/j.1525-1497.2004.30090.x. PMC 1492195. PMID 15061748.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c "A review of the epidemiological evidence for the 'antioxidant hypothesis'". Public Health Nutrition. 7 (3): 407–22. May 2004. doi:10.1079/PHN2003543. PMID 15153272.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "The key role of micronutrients". Clinical Nutrition. 25 (1): 1–13. February 2006. doi:10.1016/j.clnu.2005.11.006. PMID 16376462.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Micronutrients: dietary intake v. supplement use". The Proceedings of the Nutrition Society. 64 (4): 543–53. November 2005. doi:10.1079/PNS2005464. PMID 16313697.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. World Cancer Research Fund (2007). ISBN 978-0-9722522-2-5.
- ^ "Cancer chemoprevention: a radical perspective". Free Radical Biology & Medicine. 45 (2): 97–110. July 2008. doi:10.1016/j.freeradbiomed.2008.04.004. PMID 18454943.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Flavonoids". Linus Pauling Institute, Oregon State University, Corvallis. 2016. Retrieved 24 July 2016.
- ^ Csepregi, K; Neugart, S; Schreiner, M; Hideg, Éva (2016). "Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols". Molecules. 21 (2): 208. doi:10.3390/molecules21020208. PMID 26867192.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Flavonoids: antioxidants or signalling molecules?". Free Radical Biology & Medicine. 36 (7): 838–49. April 2004. doi:10.1016/j.freeradbiomed.2004.01.001. PMID 15019969.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Regulation of cellular signals from nutritional molecules: a specific role for phytochemicals, beyond antioxidant activity". Free Radical Biology & Medicine. 45 (9): 1205–16. November 2008. doi:10.1016/j.freeradbiomed.2008.08.001. PMID 18762244.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis". Current Drug Targets. CNS and Neurological Disorders. 2 (2): 95–107. April 2003. doi:10.2174/1568007033482959. PMID 12769802.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Role of oxidative stress and antioxidants in neurodegenerative diseases". Nutritional Neuroscience. 5 (5): 291–309. October 2002. doi:10.1080/1028415021000033767. PMID 12385592.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Dietary antioxidants, cognitive function and dementia--a systematic review". Plant Foods for Human Nutrition. 68 (3): 279–92. September 2013. doi:10.1007/s11130-013-0370-0. PMID 23881465.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Vitamin A and carotenoids and the risk of Parkinson's disease: a systematic review and meta-analysis". Neuroepidemiology. 42 (1): 25–38. 2014. doi:10.1159/000355849. PMID 24356061.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer's disease" (Accepted manuscript). Journal of Alzheimer's Disease. 29 (4): 711–26. 2012. doi:10.3233/JAD-2012-111853. PMC 3727637. PMID 22366772.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke". Stroke: A Journal of Cerebral Circulation. 38 (2): 388–94. February 2007. doi:10.1161/01.STR.0000254462.75851.22. PMID 17204689.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Tirilazad for aneurysmal subarachnoid haemorrhage". The Cochrane Database of Systematic Reviews (2): CD006778. 2010. doi:10.1002/14651858.CD006778.pub2. PMID 20166088.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Tirilazad for acute ischaemic stroke". The Cochrane Database of Systematic Reviews (4): CD002087. 2001. doi:10.1002/14651858.CD002087. PMID 11687138.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Effects of NXY-059 in experimental stroke: an individual animal meta-analysis". British Journal of Pharmacology. 157 (7): 1157–71. August 2009. doi:10.1111/j.1476-5381.2009.00196.x. PMC 2743834. PMID 19422398.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers". Current Drug Targets. CNS and Neurological Disorders. 4 (2): 109–18. April 2005. doi:10.2174/1568007053544156. PMID 15857295.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Potential interactions of prescription and over-the-counter medications having antioxidant capabilities with radiation and chemotherapy". International Journal of Cancer. 137 (11): 2525–33. September 2014. doi:10.1002/ijc.29208. PMID 25220632.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Use of antioxidants during chemotherapy and radiotherapy should be avoided". CA: A Cancer Journal for Clinicians. 55 (5): 319–21. 2005. doi:10.3322/canjclin.55.5.319. PMID 16166076.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b Magalhães, P; Dean, O; Andreazza, A (2016). "Antioxidant treatments for schizophrenia". Cochrane Database of Systematic Reviews. 1: CD008919.pub2. doi:10.1002/14651858.CD008919.pub2.
- ^ "Influence of vegetable protein sources on trace element and mineral bioavailability". The Journal of Nutrition. 133 (9): 2973S–7S. September 2003. doi:10.1093/jn/133.9.2973S. PMID 12949395.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Bioavailability of iron, zinc, and other trace minerals from vegetarian diets". The American Journal of Clinical Nutrition. 78 (3 Suppl): 633S–639S. September 2003. doi:10.1093/ajcn/78.3.633S. PMID 12936958.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Improving the bioavailability of nutrients in plant foods at the household level". The Proceedings of the Nutrition Society. 65 (2): 160–8. May 2006. doi:10.1079/PNS2006489. PMID 16672077.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Effect of blanching on the content of antinutritional factors in selected vegetables". Plant Foods for Human Nutrition. 47 (4): 361–7. June 1995. doi:10.1007/BF01088275. PMID 8577655.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Bioavailability of minerals in legumes". The British Journal of Nutrition. 88 Suppl 3 (Suppl 3): S281–5. December 2002. doi:10.1079/BJN/2002718. PMID 12498628.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Overview of dietary flavonoids: nomenclature, occurrence and intake". The Journal of Nutrition. 133 (10): 3248S–3254S. October 2003. doi:10.1093/jn/133.10.3248S. PMID 14519822.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells". Cell Proliferation. 39 (4): 241–8. August 2006. doi:10.1111/j.1365-2184.2006.00384.x. PMID 16872360.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Absorption of large, single, oral intakes of ascorbic acid". International Journal for Vitamin and Nutrition Research. 50 (3): 309–14. 1980. PMID 7429760.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial" (PDF). Journal of the National Cancer Institute. 88 (21): 1550–9. November 1996. doi:10.1093/jnci/88.21.1550. PMID 8901853.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Beta-carotene and lung cancer: a case study". The American Journal of Clinical Nutrition. 69 (6): 1345S–50S. June 1999. doi:10.1093/ajcn/69.6.1345S. PMID 10359235.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis". JAMA. 297 (8): 842–57. February 2007. doi:10.1001/jama.297.8.842. PMID 17327526.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases" (Submitted manuscript). The Cochrane Database of Systematic Reviews. 3 (3): CD007176. 14 March 2012. doi:10.1002/14651858.CD007176.pub2. PMID 22419320.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Study Citing Antioxidant Vitamin Risks Based On Flawed Methodology, Experts Argue News release from Oregon State University published on ScienceDaily. Retrieved 19 April 2007
- ^ "Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality". Annals of Internal Medicine. 142 (1): 37–46. January 2005. doi:10.7326/0003-4819-142-1-200501040-00110. PMID 15537682.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Meta-analysis: antioxidant supplements for primary and secondary prevention of colorectal adenoma". Alimentary Pharmacology & Therapeutics. 24 (2): 281–91. July 2006. doi:10.1111/j.1365-2036.2006.02970.x. PMID 16842454.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Drugs for preventing lung cancer in healthy people". The Cochrane Database of Systematic Reviews. 10: CD002141. 17 October 2012. doi:10.1002/14651858.CD002141.pub2. PMID 23076895.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Reactive oxygen species in cancer cells: live by the sword, die by the sword". Cancer Cell. 10 (3): 175–6. September 2006. doi:10.1016/j.ccr.2006.08.015. PMID 16959608.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "The antioxidant conundrum in cancer". Cancer Research. 63 (15): 4295–8. August 2003. PMID 12907593.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy?". Journal of the National Cancer Institute. 100 (11): 773–83. June 2008. doi:10.1093/jnci/djn148. PMID 18505970.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials". International Journal of Cancer. 123 (6): 1227–39. September 2008. doi:10.1002/ijc.23754. PMID 18623084.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials". Cancer Treatment Reviews. 33 (5): 407–18. August 2007. doi:10.1016/j.ctrv.2007.01.005. PMID 17367938.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c "Oxidative stress: the paradox of aerobic life". Biochemical Society Symposium. 61: 1–31. 1995. doi:10.1042/bss0610001. PMID 8660387.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d e "Oxidative stress: oxidants and antioxidants". Experimental Physiology. 82 (2): 291–5. March 1997. doi:10.1113/expphysiol.1997.sp004024. PMID 9129943.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d "The antioxidants and pro-antioxidants network: an overview". Current Pharmaceutical Design. 10 (14): 1677–94. 2004. doi:10.2174/1381612043384655. PMID 15134565.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Cell signaling. H2O2, a necessary evil for cell signaling". Science. 312 (5782): 1882–3. June 2006. doi:10.1126/science.1130481. PMID 16809515.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Free radicals and antioxidants in normal physiological functions and human disease". The International Journal of Biochemistry & Cell Biology. 39 (1): 44–84. 2007. doi:10.1016/j.biocel.2006.07.001. PMID 16978905.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Oxidative mechanisms in the toxicity of metal ions" (Submitted manuscript). Free Radical Biology & Medicine. 18 (2): 321–36. February 1995. doi:10.1016/0891-5849(94)00159-H. PMID 7744317.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids". Biological Chemistry. 387 (4): 373–9. April 2006. doi:10.1515/BC.2006.050. PMID 16606334.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Role of oxygen radicals in DNA damage and cancer incidence". Molecular and Cellular Biochemistry. 266 (1–2): 37–56. November 2004. doi:10.1023/B:MCBI.0000049134.69131.89. PMID 15646026.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Protein oxidation and aging" (Submitted manuscript). Science. 257 (5074): 1220–4. August 1992. Bibcode:1992Sci...257.1220S. doi:10.1126/science.1355616. PMID 1355616.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Mitochondria, oxygen free radicals, disease and ageing". Trends in Biochemical Sciences. 25 (10): 502–8. October 2000. doi:10.1016/S0968-0004(00)01674-1. PMID 11050436.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology". IUBMB Life. 52 (3–5): 159–64. 2001. doi:10.1080/15216540152845957. PMID 11798028.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Oxidants, oxidative stress and the biology of ageing". Nature. 408 (6809): 239–47. November 2000. Bibcode:2000Natur.408..239F. doi:10.1038/35041687. PMID 11089981.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "The production of reactive oxygen species by complex I". Biochemical Society Transactions. 36 (Pt 5): 976–80. October 2008. doi:10.1042/BST0360976. PMID 18793173.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Are respiratory enzymes the primary sources of intracellular hydrogen peroxide?". The Journal of Biological Chemistry. 279 (47): 48742–50. November 2004. doi:10.1074/jbc.M408754200. PMID 15361522.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ a b "Pathways of oxidative damage". Annual Review of Microbiology. 57: 395–418. 2003. doi:10.1146/annurev.micro.57.030502.090938. PMID 14527285.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Antioxidants in photosynthesis and human nutrition". Science. 298 (5601): 2149–53. December 2002. Bibcode:2002Sci...298.2149D. doi:10.1126/science.1078002. PMID 12481128.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Singlet oxygen production in photosynthesis". Journal of Experimental Botany. 56 (411): 337–46. January 2005. CiteSeerX 10.1.1.327.9651. doi:10.1093/jxb/erh237. PMID 15310815.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Kupper, F. C.; Carpenter, L. J.; McFiggans, G. B.; Palmer, C. J.; Waite, T. J.; Boneberg, E.-M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; Potin, P.; Butler, A.; Luther, G. W.; Kroneck, P. M. H.; Meyer-Klaucke, W.; Feiters, M. C. (2008). "Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry". Proceedings of the National Academy of Sciences. 105 (19): 6954–6958. Bibcode:2008PNAS..105.6954K. doi:10.1073/pnas.0709959105. ISSN 0027-8424. PMC 2383960. PMID 18458346.
- ^ "Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation". EMBO Reports. 6 (7): 629–34. July 2005. doi:10.1038/sj.embor.7400460. PMC 1369118. PMID 15995679.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Water-soluble carotenoid proteins of cyanobacteria" (Submitted manuscript). Archives of Biochemistry and Biophysics. 430 (1): 2–9. October 2004. doi:10.1016/j.abb.2004.03.018. PMID 15325905.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Role of oxidants in microbial pathophysiology". Clinical Microbiology Reviews. 10 (1): 1–18. January 1997. PMC 172912. PMID 8993856.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Intracellular antioxidants: from chemical to biochemical mechanisms". Food and Chemical Toxicology. 37 (9–10): 949–62. 1999. doi:10.1016/S0278-6915(99)00090-3. PMID 10541450.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Strategies of antioxidant defense". European Journal of Biochemistry / FEBS. 215 (2): 213–9. July 1993. doi:10.1111/j.1432-1033.1993.tb18025.x. PMID 7688300.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Interrelation of vitamin C, infection, haemostatic factors, and cardiovascular disease". BMJ. 310 (6994): 1559–63. June 1995. doi:10.1136/bmj.310.6994.1559. PMC 2549940. PMID 7787643.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d "Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols". Archives of Biochemistry and Biophysics. 388 (2): 261–6. April 2001. doi:10.1006/abbi.2001.2292. PMID 11368163.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Serum glutathione in adolescent males predicts parental coronary heart disease" (PDF). Circulation. 100 (22): 2244–7. November 1999. doi:10.1161/01.CIR.100.22.2244. PMID 10577998.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "HPLC-methods for determination of lipoic acid and its reduced form in human plasma". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 30 (11): 511–2. November 1992. PMID 1490813.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Assay of protein-bound lipoic acid in tissues by a new enzymatic method". Analytical Biochemistry. 258 (2): 299–304. May 1998. doi:10.1006/abio.1998.2615. PMID 9570844.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Uric acid and oxidative stress". Current Pharmaceutical Design. 11 (32): 4145–51. 2005. doi:10.2174/138161205774913255. PMID 16375736.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake". The American Journal of Clinical Nutrition. 76 (1): 172–9. July 2002. doi:10.1093/ajcn/76.1.172. PMID 12081831.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Retinol, alpha-tocopherol, lutein/zeaxanthin, beta-cryptoxanthin, lycopene, alpha-carotene, trans-beta-carotene, and four retinyl esters in serum determined simultaneously by reversed-phase HPLC with multiwavelength detection". Clinical Chemistry. 40 (3): 411–6. March 1994. PMID 8131277.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "cis-trans isomers of lycopene and beta-carotene in human serum and tissues". Archives of Biochemistry and Biophysics. 294 (1): 173–7. April 1992. doi:10.1016/0003-9861(92)90153-N. PMID 1550343.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Serum coenzyme Q10 concentrations in healthy men supplemented with 30 mg or 100 mg coenzyme Q10 for two months in a randomised controlled study". BioFactors. 18 (1–4): 185–93. 2003. doi:10.1002/biof.5520180221. PMID 14695934.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Metabolism and function of coenzyme Q". Biochimica et Biophysica Acta. 1660 (1–2): 171–99. January 2004. doi:10.1016/j.bbamem.2003.11.012. PMID 14757233.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease". Clinical and Experimental Nephrology. 9 (3): 195–205. September 2005. doi:10.1007/s10157-005-0368-5. PMID 16189627.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Urate oxidase: primary structure and evolutionary implications". Proceedings of the National Academy of Sciences of the United States of America. 86 (23): 9412–6. December 1989. Bibcode:1989PNAS...86.9412W. doi:10.1073/pnas.86.23.9412. PMC 298506. PMID 2594778.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Two independent mutational events in the loss of urate oxidase during hominoid evolution". Journal of Molecular Evolution. 34 (1): 78–84. January 1992. Bibcode:1992JMolE..34...78W. doi:10.1007/BF00163854. PMID 1556746.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Uric acid and evolution". Rheumatology. 49 (11): 2010–5. November 2010. doi:10.1093/rheumatology/keq204. PMID 20627967.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity". Hypertension. 40 (3): 355–60. September 2002. doi:10.1161/01.HYP.0000028589.66335.AA. PMID 12215479.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Theodore E. Woodward award. The evolution of obesity: insights from the mid-Miocene". Transactions of the American Clinical and Climatological Association. 121: 295–305, discussion 305–8. 2010. PMC 2917125. PMID 20697570.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Endogenous urate production augments plasma antioxidant capacity in healthy lowland subjects exposed to high altitude". Chest. 131 (5): 1473–8. May 2007. doi:10.1378/chest.06-2235. PMID 17494796.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Towards the physiological function of uric acid". Free Radical Biology & Medicine. 14 (6): 615–31. June 1993. doi:10.1016/0891-5849(93)90143-I. PMID 8325534.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Uric acid: the oxidant-antioxidant paradox" (Accepted manuscript). Nucleosides, Nucleotides & Nucleic Acids. 27 (6): 608–19. June 2008. doi:10.1080/15257770802138558. PMC 2895915. PMID 18600514.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Gout: an update". American Family Physician. 76 (6): 801–8. September 2007. PMID 17910294.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study". The American Journal of Medicine. 82 (3): 421–6. March 1987. doi:10.1016/0002-9343(87)90441-4. PMID 3826098.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Effect of short-term ketogenic diet on redox status of human blood". Rejuvenation Research. 10 (4): 435–40. December 2007. doi:10.1089/rej.2007.0540. PMID 17663642.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "L-ascorbic acid biosynthesis". Vitamins and Hormones. Vitamins & Hormones. 61: 241–66. 2001. doi:10.1016/S0083-6729(01)61008-2. ISBN 978-0-12-709861-6. PMID 11153268.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Vitamin C. Biosynthesis, recycling and degradation in mammals". The FEBS Journal. 274 (1): 1–22. January 2007. doi:10.1111/j.1742-4658.2006.05607.x. PMID 17222174.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Glutathione-ascorbic acid antioxidant system in animals". The Journal of Biological Chemistry. 269 (13): 9397–400. April 1994. PMID 8144521.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity". The Journal of Biological Chemistry. 265 (26): 15361–4. September 1990. PMID 2394726.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Vitamin C as an antioxidant: evaluation of its role in disease prevention". Journal of the American College of Nutrition. 22 (1): 18–35. February 2003. doi:10.1080/07315724.2003.10719272. PMID 12569111.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Regulation and function of ascorbate peroxidase isoenzymes". Journal of Experimental Botany. 53 (372): 1305–19. May 2002. doi:10.1093/jexbot/53.372.1305. PMID 11997377.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Ascorbic acid in plants: biosynthesis and function". Critical Reviews in Biochemistry and Molecular Biology. 35 (4): 291–314. 2000. doi:10.1080/10409230008984166. PMID 11005203.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d "Glutathione". Annual Review of Biochemistry. 52: 711–60. 1983. doi:10.1146/annurev.bi.52.070183.003431. PMID 6137189.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Glutathione metabolism and its selective modification". The Journal of Biological Chemistry. 263 (33): 17205–8. November 1988. PMID 3053703.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Biosynthesis and functions of bacillithiol, a major low-molecular-weight thiol in Bacilli". Proceedings of the National Academy of Sciences of the United States of America. 107 (14): 6482–6. April 2010. Bibcode:2010PNAS..107.6482G. doi:10.1073/pnas.1000928107. PMC 2851989. PMID 20308541.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Bacillithiol is an antioxidant thiol produced in Bacilli". Nature Chemical Biology. 5 (9): 625–627. September 2009. doi:10.1038/nchembio.189. PMC 3510479. PMID 19578333.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Novel thiols of prokaryotes". Annual Review of Microbiology. 55: 333–56. 2001. doi:10.1146/annurev.micro.55.1.333. PMID 11544359.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Metabolism and functions of trypanothione in the Kinetoplastida". Annual Review of Microbiology. 46: 695–729. 1992. doi:10.1146/annurev.mi.46.100192.003403. PMID 1444271.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Vitamin E: action, metabolism and perspectives". Journal of Physiology and Biochemistry. 57 (2): 43–56. March 2001. doi:10.1007/BF03179812. PMID 11579997.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling". The Journal of Nutrition. 131 (2): 369S–73S. February 2001. doi:10.1093/jn/131.2.369S. PMID 11160563.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Vitamin E: function and metabolism". FASEB Journal. 13 (10): 1145–55. July 1999. CiteSeerX 10.1.1.337.5276. doi:10.1096/fasebj.13.10.1145. PMID 10385606.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ "Vitamin E, antioxidant and nothing more" (Accepted manuscript). Free Radical Biology & Medicine. 43 (1): 4–15. July 2007. doi:10.1016/j.freeradbiomed.2007.03.024. PMC 2040110. PMID 17561088.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Vitamin E and its function in membranes". Progress in Lipid Research. 38 (4): 309–36. July 1999. doi:10.1016/S0163-7827(99)00008-9. PMID 10793887.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death". Cell Metabolism. 8 (3): 237–48. September 2008. doi:10.1016/j.cmet.2008.07.005. PMID 18762024.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Is vitamin E an antioxidant, a regulator of signal transduction and gene expression, or a 'junk' food? Comments on the two accompanying papers: "Molecular mechanism of alpha-tocopherol action" by A. Azzi and "Vitamin E, antioxidant and nothing more" by M. Traber and J. Atkinson". Free Radical Biology & Medicine. 43 (1): 2–3. July 2007. doi:10.1016/j.freeradbiomed.2007.05.016. PMID 17561087.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Tocopherols and tocotrienols in membranes: a critical review". Free Radical Biology & Medicine. 44 (5): 739–64. March 2008. doi:10.1016/j.freeradbiomed.2007.11.010. PMID 18160049.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Molecular mechanism of alpha-tocopherol action". Free Radical Biology & Medicine. 43 (1): 16–21. July 2007. doi:10.1016/j.freeradbiomed.2007.03.013. PMID 17561089.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Non-antioxidant activities of vitamin E". Current Medicinal Chemistry. 11 (9): 1113–33. May 2004. doi:10.2174/0929867043365332. PMID 15134510. Archived from the original on 6 October 2011.
{{cite journal}}: Unknown parameter|authors=ignored (help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Tocotrienols: Vitamin E beyond tocopherols" (Accepted manuscript). Life Sciences. 78 (18): 2088–98. March 2006. doi:10.1016/j.lfs.2005.12.001. PMC 1790869. PMID 16458936.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C". Free Radical Research. 39 (7): 671–86. July 2005. doi:10.1080/10715760500104025. PMID 16036346.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Does vitamin C act as a pro-oxidant under physiological conditions?". FASEB Journal. 13 (9): 1007–24. June 1999. PMID 10336883.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Chemistry and biology of vitamin E". Molecular Nutrition & Food Research. 49 (1): 7–30. January 2005. doi:10.1002/mnfr.200400049. PMID 15580660.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies?". Archives of Biochemistry and Biophysics. 476 (2): 107–112. August 2008. doi:10.1016/j.abb.2008.01.028. PMID 18284912.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis)". Experimental Gerontology. 45 (6): 410–418. June 2010. doi:10.1016/j.exger.2010.03.014. PMID 20350594.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "The nature of antioxidant defense mechanisms: a lesson from transgenic studies" (Full text). Environmental Health Perspectives. 106 Suppl 5 (Suppl 5): 1219–28. October 1998. doi:10.2307/3433989. JSTOR 3433989. PMC 1533365. PMID 9788901.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression". Free Radical Biology & Medicine. 33 (3): 337–49. August 2002. doi:10.1016/S0891-5849(02)00905-X. PMID 12126755.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Aspects of the structure, function, and applications of superoxide dismutase". CRC Critical Reviews in Biochemistry. 22 (2): 111–80. 1987. doi:10.3109/10409238709083738. PMID 3315461.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Superoxide dismutases and their impact upon human health". Molecular Aspects of Medicine. 26 (4–5): 340–52. 2005. doi:10.1016/j.mam.2005.07.006. PMID 16099495.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Extracellular superoxide dismutase". The International Journal of Biochemistry & Cell Biology. 37 (12): 2466–71. December 2005. doi:10.1016/j.biocel.2005.06.012. PMID 16087389.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase". Nature Genetics. 18 (2): 159–63. February 1998. doi:10.1038/ng0298-159. PMID 9462746.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury". Nature Genetics. 13 (1): 43–7. May 1996. doi:10.1038/ng0596-43. PMID 8673102.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "The regulation and function of tobacco superoxide dismutases". Free Radical Biology & Medicine. 23 (3): 515–20. 1997. doi:10.1016/S0891-5849(97)00112-3. PMID 9214590.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Diversity of structures and properties among catalases" (Submitted manuscript). Cellular and Molecular Life Sciences. 61 (2): 192–208. January 2004. doi:10.1007/s00018-003-3206-5. PMID 14745498.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis". Progress in Biophysics and Molecular Biology. 72 (1): 19–66. 1999. doi:10.1016/S0079-6107(98)00058-3. PMID 10446501.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Metabolism of oxygen radicals in peroxisomes and cellular implications". Free Radical Biology & Medicine. 13 (5): 557–80. November 1992. doi:10.1016/0891-5849(92)90150-F. PMID 1334030.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Mechanisms of compound I formation in heme peroxidases". Journal of Inorganic Biochemistry. 91 (1): 27–34. July 2002. doi:10.1016/S0162-0134(02)00390-2. PMID 12121759.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Direct evidence for catalase as the predominant H2O2 -removing enzyme in human erythrocytes". Blood. 90 (12): 4973–8. December 1997. PMID 9389716.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Acatalasemia". Human Genetics. 86 (4): 331–40. February 1991. doi:10.1007/BF00201829. PMID 1999334.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Analysis of the link between enzymatic activity and oligomeric state in AhpC, a bacterial peroxiredoxin" (Accepted manuscript). Biochemistry. 44 (31): 10583–92. 2005. doi:10.1021/bi050448i. PMC 3832347. PMID 16060667.
{{cite journal}}: Unknown parameter|authors=ignored (help) PDB 1YEX - ^ "Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling". Free Radical Biology & Medicine. 38 (12): 1543–52. June 2005. doi:10.1016/j.freeradbiomed.2005.02.026. PMID 15917183.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Structure, mechanism and regulation of peroxiredoxins". Trends in Biochemical Sciences. 28 (1): 32–40. January 2003. doi:10.1016/S0968-0004(02)00003-8. PMID 12517450.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation". Biochemistry. 38 (47): 15407–16. November 1999. doi:10.1021/bi992025k. PMID 10569923.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "The peroxiredoxin repair proteins". Sub-Cellular Biochemistry. Subcellular Biochemistry. 44: 115–41. 2007. doi:10.1007/978-1-4020-6051-9_6. ISBN 978-1-4020-6050-2. PMC 2391273. PMID 18084892.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression" (Full text). Nature. 424 (6948): 561–5. July 2003. Bibcode:2003Natur.424..561N. doi:10.1038/nature01819. PMID 12891360.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice". Blood. 101 (12): 5033–8. June 2003. doi:10.1182/blood-2002-08-2548. PMID 12586629.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "The function of peroxiredoxins in plant organelle redox metabolism". Journal of Experimental Botany. 57 (8): 1697–709. 2006. doi:10.1093/jxb/erj160. PMID 16606633.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Reactive oxygen species, antioxidants, and the mammalian thioredoxin system". Free Radical Biology & Medicine. 31 (11): 1287–312. December 2001. doi:10.1016/S0891-5849(01)00724-9. PMID 11728801.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Plant thioredoxins are key actors in the oxidative stress response". Trends in Plant Science. 11 (7): 329–34. July 2006. doi:10.1016/j.tplants.2006.05.005. PMID 16782394.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Physiological functions of thioredoxin and thioredoxin reductase". European Journal of Biochemistry / FEBS. 267 (20): 6102–9. October 2000. doi:10.1046/j.1432-1327.2000.01701.x. PMID 11012661.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Thioredoxin reductase". The Biochemical Journal. 346 (1): 1–8. February 2000. doi:10.1042/0264-6021:3460001. PMC 1220815. PMID 10657232.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Manipulation of glutathione metabolism in transgenic plants". Biochemical Society Transactions. 24 (2): 465–9. May 1996. doi:10.1042/bst0240465. PMID 8736785.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Tissue-specific functions of individual glutathione peroxidases". Free Radical Biology & Medicine. 27 (9–10): 951–65. November 1999. doi:10.1016/S0891-5849(99)00173-2. PMID 10569628.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia". The Journal of Biological Chemistry. 272 (26): 16644–51. June 1997. doi:10.1074/jbc.272.26.16644. PMID 9195979.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ "Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide". The Journal of Biological Chemistry. 273 (35): 22528–36. August 1998. doi:10.1074/jbc.273.35.22528. PMID 9712879.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ "Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis". Antioxidants & Redox Signaling. 6 (2): 289–300. April 2004. doi:10.1089/152308604322899350. PMID 15025930.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Glutathione transferases". Annual Review of Pharmacology and Toxicology. 45: 51–88. 2005. doi:10.1146/annurev.pharmtox.45.120403.095857. PMID 15822171.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Oxidative stress and Alzheimer disease". The American Journal of Clinical Nutrition. 71 (2): 621S–629S. February 2000. doi:10.1093/ajcn/71.2.621s. PMID 10681270.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Involvement of oxidative stress in Alzheimer disease". Journal of Neuropathology and Experimental Neurology. 65 (7): 631–41. July 2006. doi:10.1097/01.jnen.0000228136.58062.bf. PMID 16825950.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Understanding the molecular causes of Parkinson's disease". Trends in Molecular Medicine. 12 (11): 521–8. November 2006. doi:10.1016/j.molmed.2006.09.007. PMID 17027339.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Lipid peroxidation in diabetes mellitus". Antioxidants & Redox Signaling. 7 (1–2): 256–68. 2005. doi:10.1089/ars.2005.7.256. PMID 15650413.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Oxidative stress and diabetic vascular complications". Diabetes Care. 19 (3): 257–67. March 1996. doi:10.2337/diacare.19.3.257. PMID 8742574.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Oxidation in rheumatoid arthritis". Arthritis Research & Therapy. 6 (6): 265–78. 2004. doi:10.1186/ar1447. PMC 1064874. PMID 15535839.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ "Oxidative stress and motor neurone disease". Brain Pathology. 9 (1): 165–86. January 1999. doi:10.1111/j.1750-3639.1999.tb00217.x. PMID 9989458.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Mechanisms linking obesity with cardiovascular disease". Nature. 444 (7121): 875–80. December 2006. Bibcode:2006Natur.444..875V. doi:10.1038/nature05487. PMID 17167476.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases". Free Radical Research. 33 Suppl: S85–97. November 2000. PMID 11191279.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Antioxidant enzymes and cancer". Chin J Cancer Res. 22 (2): 87–92. 2010. doi:10.1007/s11670-010-0087-7.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency". Proceedings of the National Academy of Sciences of the United States of America. 103 (6): 1768–1773. February 2006. Bibcode:2006PNAS..103.1768L. doi:10.1073/pnas.0510452103. PMC 1413655. PMID 16446459.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Aging and resistance to oxidative damage in Caenorhabditis elegans". Proceedings of the National Academy of Sciences of the United States of America. 90 (19): 8905–9. October 1993. Bibcode:1993PNAS...90.8905L. doi:10.1073/pnas.90.19.8905. PMC 47469. PMID 8415630.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Genetics of aging in the fruit fly, Drosophila melanogaster". Annual Review of Genetics. 37: 329–48. 2003. doi:10.1146/annurev.genet.37.040103.095211. PMID 14616064.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Mechanisms of aging: an appraisal of the oxidative stress hypothesis". Free Radical Biology & Medicine. 33 (5): 575–86. September 2002. doi:10.1016/S0891-5849(02)00886-9. PMID 12208343.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Role of oxidative stress and protein oxidation in the aging process". Free Radical Biology & Medicine. 33 (1): 37–44. July 2002. doi:10.1016/S0891-5849(02)00856-0. PMID 12086680.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Theories of biological aging: genes, proteins, and free radicals" (PDF). Free Radical Research. 40 (12): 1230–8. December 2006. doi:10.1080/10715760600911303. PMID 17090411.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Is the oxidative stress theory of aging dead?". Biochimica et Biophysica Acta. 1790 (10): 1005–1014. October 2009. doi:10.1016/j.bbagen.2009.06.003. PMC 2789432. PMID 19524016.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Modified atmosphere packaging of fruits and vegetables". Critical Reviews in Food Science and Nutrition. 28 (1): 1–30. 1989. doi:10.1080/10408398909527490. PMID 2647417.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Chilled food systems. Effects of chilled holding on quality of beef loaves". Journal of the American Dietetic Association. 67 (6): 552–7. December 1975. PMID 1184900.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Phenolic antioxidants: Health Protection Branch studies on butylated hydroxyanisole". Cancer Letters. 93 (1): 49–54. June 1995. doi:10.1016/0304-3835(95)03787-W. PMID 7600543.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "E number index". UK food guide. Archived from the original on 4 March 2007. Retrieved 5 March 2007.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Rancidity and its measurement in edible oils and snack foods. A review". The Analyst. 113 (2): 213–24. February 1988. Bibcode:1988Ana...113..213R. doi:10.1039/an9881300213. PMID 3288002.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Contribution of the phenolic fraction to the antioxidant activity and oxidative stability of olive oil". Journal of Agricultural and Food Chemistry. 52 (13): 4072–9. June 2004. doi:10.1021/jf049806z. PMID 15212450.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Boozer, Charles E.; Hammond, George S.; Hamilton, Chester E.; Sen, Jyotirindra N. (1955). "Air Oxidation of Hydrocarbons.1II. The Stoichiometry and Fate of Inhibitors in Benzene and Chlorobenzene". Journal of the American Chemical Society. 77 (12): 3233–7. Bibcode:1955JAChS..77.1678G. doi:10.1021/ja01617a026.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Global Antioxidants (Natural and Synthetic) Market Poised to Surge From USD 2.25 Billion in 2014 to USD 3.25 Billion by 2020, Growing at 5.5% CAGR". GlobalNewswire, El Segundo, CA. 19 January 2016. Retrieved 30 January 2017.
- ^ "Why use Antioxidants?". SpecialChem Adhesives. Archived from the original on 11 February 2007. Retrieved 27 February 2007.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b "Fuel antioxidants". Innospec Chemicals. Archived from the original on 15 October 2006. Retrieved 27 February 2007.
- ^ "Food carotenoids: analysis, composition and alterations during storage and processing of foods". Forum of Nutrition. 56: 35–7. 2003. PMID 15806788.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans". Molecular Nutrition & Food Research. 53 Suppl 2: S194–218. September 2009. doi:10.1002/mnfr.200800053. PMID 19035552.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Nutritional losses and gains during processing: future problems and issues". The Proceedings of the Nutrition Society. 61 (1): 145–8. February 2002. doi:10.1079/PNS2001142. PMID 12002789.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Antioxidants and Cancer Prevention: Fact Sheet". National Cancer Institute. Archived from the original on 4 March 2007. Retrieved 27 February 2007.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Importance of functional foods in the Mediterranean diet". Public Health Nutrition. 9 (8A): 1136–40. December 2006. doi:10.1017/S1368980007668530. PMID 17378953.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "The systemic availability of oral glutathione". European Journal of Clinical Pharmacology. 43 (6): 667–9. 1992. doi:10.1007/BF02284971. PMID 1362956.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Dietary glutathione intake in humans and the relationship between intake and plasma total glutathione level". Nutrition and Cancer. 21 (1): 33–46. 1994. doi:10.1080/01635589409514302. PMID 8183721.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility". Expert Opinion on Biological Therapy. 8 (12): 1955–62. December 2008. doi:10.1517/14728220802517901. PMID 18990082.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Adequate range for sulfur-containing amino acids and biomarkers for their excess: lessons from enteral and parenteral nutrition". The Journal of Nutrition. 136 (6 Suppl): 1694S–1700S. June 2006. doi:10.1093/jn/136.6.1694S. PMID 16702341.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Oxygen-radical absorbance capacity assay for antioxidants" (Submitted manuscript). Free Radical Biology & Medicine. 14 (3): 303–11. March 1993. doi:10.1016/0891-5849(93)90027-R. PMID 8458588.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe". Journal of Agricultural and Food Chemistry. 49 (10): 4619–26. October 2001. doi:10.1021/jf010586o. PMID 11599998.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Withdrawn: Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2 (2010)". United States Department of Agriculture, Agricultural Research Service. 16 May 2012. Retrieved 13 June 2012.
- ^ "Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements" (PDF). Journal of Agricultural and Food Chemistry. 53 (10): 4290–302. May 2005. doi:10.1021/jf0502698. PMID 15884874. Archived from the original (PDF) on 29 December 2016. Retrieved 24 October 2017.
{{cite journal}}: Unknown parameter|authors=ignored (help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Evolution of dietary antioxidants". Comparative Biochemistry and Physiology A. 136 (1): 113–26. September 2003. doi:10.1016/S1095-6433(02)00368-9. PMID 14527634.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Antioxidants". Annual Review of Biochemistry. 16: 177–92. 1947. doi:10.1146/annurev.bi.16.070147.001141. PMID 20259061.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Food processing and lipid oxidation". Advances in Experimental Medicine and Biology. 459. 1999: 23–50. doi:10.1007/978-1-4615-4853-9_3. ISBN 978-0-306-46051-7. PMID 10335367.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|authors=ignored (help) - ^ "Three eras of vitamin C discovery". Sub-Cellular Biochemistry. Subcellular Biochemistry. 25: 1–16. 1996. doi:10.1007/978-1-4613-0325-1_1. ISBN 978-1-4613-7998-0. PMID 8821966.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Free radicals: their history and current status in aging and disease". Annals of Clinical and Laboratory Science. 28 (6): 331–46. 1998. PMID 9846200.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Moureu, Charles; Dufraisse, Charles (1922). "Sur l'autoxydation: Les antioxygènes". Comptes Rendus des Séances et Mémoires de la Société de Biologie (in French). 86: 321–322.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "The discovery of the antioxidant function of vitamin E: the contribution of Henry A. Mattill". The Journal of Nutrition. 135 (3): 363–6. March 2005. doi:10.1093/jn/135.3.363. PMID 15735064.
{{cite journal}}: Unknown parameter|authors=ignored (help)
Further reading
- Nick Lane Oxygen: The Molecule That Made the World (Oxford University Press, 2003) ISBN 0-19-860783-0
- Barry Halliwell and John M.C. Gutteridge Free Radicals in Biology and Medicine (Oxford University Press, 2007) ISBN 0-19-856869-X
- Jan Pokorny, Nelly Yanishlieva and Michael H. Gordon Antioxidants in Food: Practical Applications (CRC Press Inc, 2001) ISBN 0-8493-1222-1
External links
 Media related to Antioxidants at Wikimedia Commons
Media related to Antioxidants at Wikimedia Commons

![{\displaystyle {\ce {{\underset {Oxygen}{O2}}->{\underset {Superoxide}{*O2^{-}}}->[{{} \atop {\ce {Superoxide \atop dismutase}}}]{\underset {Hydrogen \atop peroxide}{H2O2}}->[{{} \atop {\ce {Peroxidases \atop catalase}}}]{\underset {Water}{H2O}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9afb7e4b0bc072f286b00e47a3d28967be8d6689)