Archaea: Difference between revisions
TimVickers (talk | contribs) |
Interactions with other organisms |
||
| Line 153: | Line 153: | ||
==Interactions with other organisms== |
==Interactions with other organisms== |
||
Many [[methanogen]]ic archaea are [[symbiosis|symbionts]] found in the digestive tracts of animals, such as [[ruminant]]s and [[termite]]s. In humans, the formate-consuming methanogen ''[[Methanobrevibacter smithii]]'' is by far the most common archaean, and this species can make up one in ten of all the prokaryotes in the intestines.<ref>{{cite journal |author=Eckburg PB, Bik EM, Bernstein CN, ''et al'' |title=Diversity of the human intestinal microbial flora |journal=Science (journal) |volume=308 |issue=5728 |pages=1635–8 |year=2005 |month=June |pmid=15831718 |pmc=1395357 |doi=10.1126/science.1110591 |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=15831718}}</ref> Further associations with other organisms are now being found, with the discovery that the marine archaean ''Cenarchaeum symbiosum'' is a symbiont of the [[sponge]] ''Axinella mexicana''.<ref name=Preston1996>{{cite journal |authors=Preston, C.M.; Wu, K.Y.; Molinski, T.F.; Delong, E.F. | year = 1996 | title = A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum gen. nov., sp. nov. | journal = Proc Natl Acad Sci USA | volume = 93 | issue = 13 | pages = 6241-6246 | url = http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=39006}}</ref> As of 2007, no clear examples of archaeal [[pathogen]]s are known,<ref>{{cite journal |author=Eckburg P, Lepp P, Relman D |title=Archaea and their potential role in human disease |journal=Infect Immun |volume=71 |issue=2 |pages=591–6 |year=2003 |pmid=12540534 |doi=10.1128/IAI.71.2.591-596.2003}}</ref><ref>{{cite journal |author=Cavicchioli R, Curmi P, Saunders N, Thomas T |title=Pathogenic archaea: do they exist? |journal=Bioessays |volume=25 |issue=11 |pages=1119–28 |year=2003 |pmid=14579252 |doi=10.1002/bies.10354}}</ref> although a relationship has been proposed between the presence of some methanogens and human [[periodontal disease]].<ref>{{cite journal |author=Lepp P, Brinig M, Ouverney C, Palm K, Armitage G, Relman D |title=Methanogenic Archaea and human periodontal disease |journal=Proc Natl Acad Sci U S A |volume=101 |issue=16 |pages=6176–81 |year=2004 |pmid=15067114 |doi=10.1073/pnas.0308766101}}</ref> |
Many [[methanogen]]ic archaea are [[symbiosis|symbionts]] found in the digestive tracts of animals, such as [[ruminant]]s and [[termite]]s. In humans, the formate-consuming methanogen ''[[Methanobrevibacter smithii]]'' is by far the most common archaean, and this species can make up one in ten of all the prokaryotes in the intestines.<ref>{{cite journal |author=Eckburg PB, Bik EM, Bernstein CN, ''et al'' |title=Diversity of the human intestinal microbial flora |journal=Science (journal) |volume=308 |issue=5728 |pages=1635–8 |year=2005 |month=June |pmid=15831718 |pmc=1395357 |doi=10.1126/science.1110591 |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=15831718}}</ref> Further associations with other organisms are now being found, with the discovery that the marine archaean ''Cenarchaeum symbiosum'' is a symbiont of the [[sponge]] ''Axinella mexicana''.<ref name=Preston1996>{{cite journal |authors=Preston, C.M.; Wu, K.Y.; Molinski, T.F.; Delong, E.F. | year = 1996 | title = A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum gen. nov., sp. nov. | journal = Proc Natl Acad Sci USA | volume = 93 | issue = 13 | pages = 6241-6246 | url = http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=39006}}</ref> In these marines environments, Archaea and [[Bacteria]] life forms coexist as oceanic microbial communities, or [[Plankton]]. On these communities, Archaea make up to 60% of present biomass, and take the same percentage of dissolved [[amino acids]], showing that both groups of microorganisms coexist in this ecosystem <ref name=Ouverney2000>{{cite journal |authors=Ouverney, Cleber C.; Fuhrman, Jed A. | year = 2000 | title = Marine Planktonic Archaea Take Up Amino Acids | journal = Applied and Environmental Microbiology| volume = 66 | issue = 11| pages = 4829-4833 | url = http://aem.asm.org/cgi/content/abstract/66/11/4829}}</ref>. As of 2007, no clear examples of archaeal [[pathogen]]s are known,<ref>{{cite journal |author=Eckburg P, Lepp P, Relman D |title=Archaea and their potential role in human disease |journal=Infect Immun |volume=71 |issue=2 |pages=591–6 |year=2003 |pmid=12540534 |doi=10.1128/IAI.71.2.591-596.2003}}</ref><ref>{{cite journal |author=Cavicchioli R, Curmi P, Saunders N, Thomas T |title=Pathogenic archaea: do they exist? |journal=Bioessays |volume=25 |issue=11 |pages=1119–28 |year=2003 |pmid=14579252 |doi=10.1002/bies.10354}}</ref> although a relationship has been proposed between the presence of some methanogens and human [[periodontal disease]].<ref>{{cite journal |author=Lepp P, Brinig M, Ouverney C, Palm K, Armitage G, Relman D |title=Methanogenic Archaea and human periodontal disease |journal=Proc Natl Acad Sci U S A |volume=101 |issue=16 |pages=6176–81 |year=2004 |pmid=15067114 |doi=10.1073/pnas.0308766101}}</ref> |
||
==Significance in technology and industry== |
==Significance in technology and industry== |
||
Revision as of 04:37, 24 June 2008
| Archaea Temporal range: Paleoarchean - Recent
| |
|---|---|
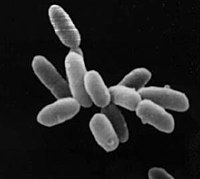
| |
| Halobacteria sp. strain NRC-1, each cell about 5 μm in length. | |
| Scientific classification | |
| Superdomain: | |
| Domain: | Archaea |
| Phyla | |
|
Crenarchaeota | |
The Archaea are a group of prokaryotic, single-celled microorganisms. In this they are similar to bacteria, but these two groups evolved differently and are classified as different domains in the three-domain system. Originally these organisms were named archaebacteria.[1] However, this term has not been favored since the three-domain system became popular.
Although there is still uncertainty in the phylogeny, Archaea, Eukaryota and Bacteria were introduced as the fundamental classifications in what would later become the three-domain system by Carl Woese in 1977. As prokaryotes, archaea are also classified in kingdom Monera in the traditional five-kingdom Linnaean taxonomy. While their prokaryotic cell structure is similar to Bacteria, the genes of Archaea and several of their metabolic pathways are more closely related to those of eukaryotes. One way to account for this is to group archaeans and eukaryotes together in the clade Neomura, which might have arisen from gram-positive bacteria. On the other hand, other studies have suggested that Archaea may instead be the most ancient lineage in the world, with bacteria and eukaryotes diverging from this group.[2]
Archaea were originally described in extreme environments, but have since been found in all habitats and may contribute up to 20% of total biomass.[3] These cells are particularly common in the oceans, and the archaea in plankton may be one of the most abundant groups of organisms on the planet.[4] A single individual or species from this domain is called an archaeon (sometimes spelled "archeon"),[5] while the adjectival form is archaeal or archaean. The etymology is Ancient Greek, from ἀρχαία meaning "ancient things".
Discovery
Early in the 20th century, prokaryotes were regarded as a single group of organisms and classified based on their biochemistry, morphology and metabolism. For example, microbiologists tried to classify microorganisms based on the substances they consume, their shapes, and the structures of their cell walls.[6] However, a new approach was proposed in 1965,[7] and microbiologists began to examine the sequences of the genes in these organisms and use this genetic information to work out which prokaryotes are genuinely related to each other: this is known as phylogenetics.

Archaea were identified as a separate group of prokaryotes in 1977 by Carl Woese and George E. Fox due to their separation from other prokaryotes in phylogenetic trees that were based on the sequences of ribosomal RNA (rRNA) genes.[8] These two groups were originally named the Archaebacteria and Eubacteria and treated as kingdoms or subkingdoms, which Woese and Fox termed Urkingdoms. Woese argued that this group of prokaryotes represented a fundamentally different branch of living things. He later renamed the two groups of prokaryotes Archaea and Bacteria to emphasize this, and argued that together with Eukarya they compose three Domains of living organisms.[9] At first, only the methanogens were placed in this new domain, but gradually microbiologists realized that the archaea are a large and diverse group of organisms.
Initially, archaea were thought of as extremophiles that existed only in apparently-inhospitable habitats, such as hot springs and salt lakes, but in the late 20th century it became increasingly clear that archaea are in fact widely distributed in nature and are common inhabitants of much less extreme habitats, such as soils and oceans.[10] This new appreciation of the importance and ubiquity of archaea came mostly from the increasing application of molecular biology techniques that could detect prokaryotes in samples of water or soil from their nucleic acids alone, avoiding the need to find ways to culture the organisms in the laboratory.[11][12]
Origin and early evolution
The Archaea should not be confused with the geological term Archean eon, also known as the Archeozoic era. This refers to the primordial period of earth history when prokaryotes were the only cellular organisms living on the planet.[13][14] Probable fossils of these ancient cells have been dated to almost 3.5 billion years ago,[15] and the remains of lipids that may be either archaean or eukaryotic have been detected in shales dating from 2.7 billion years ago.[16] It is not practical to visually identify fossils as belonging to the Archaea. Chemical fossils, in the form of isoprene compounds, are preferable because such compounds do not occur in other groups of organisms and are therefore diagnostic of the Archaea. The first report of such isoprenoid residues in the geologic record was published in 1979 from the Miocene-age Messel oil shale of Germany.[17] Since that time, additional discoveries around the world dating back to the Precambrian have been published. The oldest known traces of isoprene residues come from the Isua district of west Greenland, which include sediments formed 3.8 billion years old, the oldest on Earth.[18]
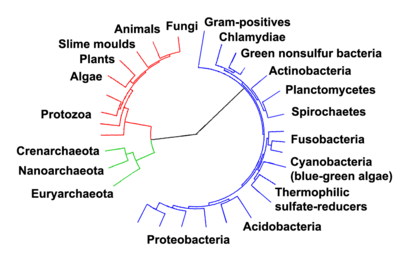
Woese argued that the bacteria, archaea, and eukaryotes each represent a primary line of descent that diverged early on from an ancestral progenote or colony or organisms with poorly developed genetic machinery.[20][21] Now these groups are usually treated as domains, each comprising several kingdoms. A few biologists, however, have argued that the archaebacteria and eukaryotes arose from specialized eubacteria.[22] One possibility is that the last common ancestor of the bacteria and archaea may have been a thermophile, which raises the possibility that lower temperatures are "extreme environments" in archaeal terms, and organisms that live in cooler environments appeared later in the history of life on Earth.[23]
The relationship between archaea and eukaryotes remains an important problem. Aside from the similarities noted above, many genetic trees group the two together, with some analyses even suggesting that eukaryotes have a closer relationship to the archaeal phylum Euryarchaeota than the relationship between the Euryarchaeota and the phylum Crenarchaeota: although the shared similarities in the cell structure of the archaea might suggest otherwise. However, the discovery of archaean-like genes in certain bacteria, such as Thermotoga maritima, makes these relationships difficult to determine, since horizontal gene transfer has occurred.[24]
Some have suggested that eukaryotes arose through fusion of an archaean and eubacterium, which became the nucleus and cytoplasm; this accounts for various genetic similarities but runs into difficulties explaining cell structure.[25] However, a recent large scale phylogenetic analysis of the structure of proteins encoded in almost 200 completely sequenced genomes showed that the origin of Archaea is much more ancient and that the archaeal lineage may represent the most ancient that exists on earth.[2] In fact, the study suggests that the ancestor of all life had a proteome with a rather complex collection of protein structures, many of which are now widely spread in modern metabolism.
Classification
The classification of archaea, and of prokaryotes in general, is a rapidly-moving and contentious field. These classification systems aim to organize archaea into groups of organisms that share structural features and common ancestors.[26] This follows the classification of other organisms, with a popular definition of a species in animals being a set of actually or potentially interbreeding populations that are reproductively isolated from other such groups.[27]
However, efforts to classify prokaryotes such as archaea into species are complicated by the fact that they are asexual and show high levels of horizontal gene transfer between lineages. The area is controversial, with for example, some arguing that in groups such as the genus Ferroplasma, related archaea form population clusters that can be seen as species.[28]

On the other hand, studies in Halorubrum found significant genetic exchange between such population clusters.[29] Such results have led to the argument that prokaryotic species are points within an interconnected net of gene transfer events, rather than parts of a standard phylogenetic tree.[30]
The current state of knowledge on archaean diversity is fragmentary.[31] Estimates of the total number of phylum-level lineages in the archaea range from 18 to 23, of which only 8 phyla have representatives that have been grown in culture and studied directly. Many of these hypothetical groups are known from only a single rRNA sequence, indicating that the vast majority of the diversity among these organisms remains completely unknown.[32] This problem of how to study and classify an uncultured microbial majority is common across all prokaryotes.[33]
Most of the culturable and well-investigated species of archaea are members of two main phyla, the Euryarchaeota and Crenarchaeota. Other groups have been tentatively created, such as the peculiar species Nanoarchaeum equitans that was discovered in 2003 has been given its own phylum, the Nanoarchaeota;[34] and the phylum Korarchaeota that contains a small group of thermophilic species, which are most closely related to the Crenarchaeota.[35] Other recently-detected species of archaea cannot be easily classified within any of these groups, such as the Archaeal Richmond Mine Acidophilic Nanoorganisms (ARMAN), which were discovered in 2006.[36]
Morphology and physiology
Size and shape

Individual archaeans range from 0.1 micrometres (μm) to over 15 μm in diameter, and occur in various shapes, commonly as spheres, rods, spirals or plates.[37] Other morphologies in the Crenarchaeota include irregularly-shaped lobed cells in Sulfolobus, thin needle-like filaments that are less than half a micometre in diameter in Thermofilum, and almost perfectly rectangular rods in Thermoproteus and Pyrobaculum.[38] Recently, even a species of flat, square archaea called Haloquadra walsbyi that lives in hypersaline pools has been discovered.[39] These unusual shapes are probably maintained both by their cell walls and a prokaryotic cytoskeleton, but in contrast to bacteria, these cellular structures are poorly understood in archaea.[40] However, proteins related to the cytoskeleton components of other organisms have been identified in the archaea,[41] and filaments have been observed within these cells.[42]
Some species of archaea form aggregates or filaments of cells up to 200 μm in length,[37] and these organisms can be prominent members of the communities of microbes that make up biofilms.[43] A particularly elaborate form of multicellular colonies are produced by archaea in the genus Pyrodictium. Here, the cells produce arrays of long, thin hollow tubes called cannulae, that stick out from the cells' surfaces and connect them together into a dense bush-like colony.[44] The function of these cannulae is not known, but they may allow the cells to communicate or exchange nutrients with their neighbors.[45]
Comparison of archaea with other cells
Archaea are similar to other prokaryotes in many aspects of their cell structure and metabolism, but other characteristics set the archaea apart. In overall structure the archaea are similar to gram-positive bacteria, as most have a single plasma membrane and cell wall, and lack a periplasmic space; the exception to this general rule is Ignicoccus, which possess a particularly large periplasm that contains membrane-bound vesicles and is enclosed by an outer membrane.[46]
Like bacteria and eukaryotes, archaea possess glycerol-based phospholipids called ether lipids.[47] However, three features of archaeal lipids are highly unusual:[48]

- The archaeal lipids are unique because the stereochemistry of the glycerol is the reverse of that found in bacterial and eukaryotic lipids - the glycerol components of these lipids are mirror images of each other. In archaea, acyl chains are attached to the sn-2 and sn-3 positions of the glycerol, while bacterial and eucaryotic lipids have acyl chains at the sn-1 and sn-2 positions. This is strong evidence for a different biosynthetic pathway.
- Most bacteria and eukaryotes have membranes composed mainly of glycerol-ester lipids, whereas archaea have membranes composed of glycerol-ether lipids. Even when bacteria have ether-linked lipids, the stereochemistry of the glycerol is the bacterial form. These differences may be an adaptation on the part of archaea to hyperthermophily. However, it is worth noting that even mesophilic archaea have ether-linked lipids.
- Archaeal lipids are based upon the isoprenoid sidechain. Only the archaea incorporate these compounds into their cellular lipids, frequently as C-20 (four monomers) or C-40 (eight monomers) side-chains. In some archaea, the C-40 isoprenoid side-chain is long enough to span the membrane, forming a monolayer for a cell membrane with glycerol phosphate moieties on both ends. Although dramatic, this adaptation is most common in the extremely thermophilic archaea.
Cell wall and flagella
Most archaea possess a cell wall, with the exceptions being Thermoplasma and Ferroplasma.[49] Although not unique, archaeal cell walls are unusual. For instance, in most archaea they are made of surface-layer proteins, which form what is called an S-layer.[50] S-layers are also found in some bacteria, where they serve as the sole cell wall component in the Planctomyces, or an outer layer in many organisms with peptidoglycan. With the exception of one group of methanogens, archaea lack a peptidoglycan wall (and in the case of the exception, the peptidoglycan is very different from the type found in bacteria).[51]
Archaea also have flagella, and while this similar to bacterial flagella in that they are rotatory motors driven by a proton gradient, that are notably different in their composition and development.[52] The bacterial flagellum is a modified type III secretion system, while archaeal flagella appear to have evolved from to the bacterial type IV pili.[53] In contrast to the bacterial flagellum, where filament proteins move up a central pore and are added to the tip of the filament, archaeal filaments appear to be synthesized by adding subunits to their base.[54]
Metabolism
Archaea exhibit a variety of different types of metabolism. Some are lithotrophs, such as nitrifiers, methanogens and anaerobic methane oxidisers.[5] Oxygen-generating photosynthesis does not occur, but several groups of archaea are phototrophs that use sunlight to power ATP synthesis directly.[55] Many basic metabolic pathways are shared between archaea and bacteria, with archaea that grow on complex organic compounds (the chemoorganotrophs) having a modified form of glycolysis (the Entner-Doudoroff Pathway) and either a complete or partial citric acid cycle.[56] These similarities with other organisms probably reflect the early evolution of carbohydrate metabolism in the history of life.[57]
| Nutritional types in archaeal metabolism | |||
|---|---|---|---|
| Nutritional type | Source of energy | Source of carbon | Examples |
| Phototrophs | Sunlight | Organic compounds | Halobacteria |
| Lithotrophs | Inorganic compounds | Organic compounds or carbon fixation | Ferroglobus, Methanobacteria or Pyrolobus |
| Heterotrophs | Organic compounds | Organic compounds or carbon fixation | Pyrococcus, Sulfolobus or Methanosarcinales |
Some Euryarchaeota are methanogens, which produce methane gas as a waste product in anaerobic environments such as swamps. This form of metabolism evolved early, and the first free-living organism may have been a methanogen.[58] A common reaction in these organisms is to use carbon dioxide as an electron acceptor to oxidize hydrogen, as shown below:

Methanogenesis involves a range of unique coenzymes, such as coenzyme M and methanofuran,[60] and generates ATP through chemiosmosis.[55] Other organic compounds such as alcohols, acetic acid or formic acid can also be used as electron acceptors by methanogens. These reactions are common in the gut. Acetic acid can also be broken down into methane and carbon dioxide directly, by acetotrophic archaea. These acetotrophs are archaea in the order Methanosarcinales, and can dominate the microbial communities involved in biogas production.[61]
Phototrophic archaea use light to produce chemical energy, in the form of ATP, but there are no known archaea that carry out photosynthesis and use light for energy as well as fixing carbon dioxide.[62] In the Halobacteria light-activated ion pumps like bacteriorhodopsin and halorhodopsin generate ion gradients, which are converted into adenosine triphosphate (ATP).[37] The structure and function of these light-driven pumps has been studied in great detail, which has revealed that their ability to move ions across membranes depends on light-driven changes in the structure of a retinol cofactor buried in the center of the protein.[63]
Some archaea are autotrophs that can fix carbon dioxide. This can involve either a highly-modified form of the Calvin cycle,[64] or a recently-discovered metabolic pathway called the 3-hydroxypropionate/4-hydroxybutyrate cycle.[65] The Crenarchaeota also use the reverse Krebs cycle and the Euryarchaeota also use the reductive acetyl CoA Pathway.[66] In these organisms carbon-fixation reactions are powered by lithotrophic metabolism, which is the use of inorganic compounds as energy sources. These are extremely diverse, and range from the oxidation of ammonia by the Nitrosopumilales in anammox metabolism,[67][68] to the oxidation of hydrogen sulfide or elemental sulfur by species of Sulfolobus, using either oxygen or metal ions as electron acceptors.[55] These archaea produce sulphuric acid as a waste product and the growth of these organisms in abandoned mines can cause serious environmental damage through acid mine drainage.[69]
Reproduction
Archaea reproduce asexually by binary or multiple fission, fragmentation, or budding; meiosis does not occur, so if a species of archaea exists in more than one form, these will all have the same number of chromosomes (they have the same karyotype).[37] Cell division is controlled in the archaea as part of a complex cell cycle where the cell's chromosome is replicated, the two daughter chromosomes are separated, and the cell then divides.[70] The details of the archaeal cell cycle have only been investigated in the genus Sulfolobus, but here it has characters that are similar to both bacterial and eukaryotic systems: with the chromosomes being replicated from multiple starting-points (origins of replication) using DNA polymerases that are similar to the equivalent eukaryotic enzymes.[71] However, the proteins that direct cell division, such as the protein FtsZ, which forms a contracting ring around the cell, and the components of the septum that is constructed across the center of the cell, appear to be closer to their bacterial equivalents.[70]
Spores, such as the endospores made by some bacteria, are not formed in any of the known archaea.[72] Some species of Haloarchaea can undergo phenotypic switching and grow as several different types of cell, including thick-walled structures that are resistant to osmotic shock and allow the archaea to survive in water at low concentrations of salt, but these are not reproductive structures and may instead help them disperse to new habitats.[73]
Genetics
Archaea are similar to bacteria in that they usually have a single circular chromosome.[74] These range in size from 5,751,492 base pairs in Methanosarcina acetivorans,[75] the largest archaean genome sequenced to date, to the tiny 490,885 base-pair genome of Nanoarchaeum equitans, which is the smallest microbial genome known and may contain only 537 protein-encoding genes.[76] Plasmids are also found in archaea, and can spread between cells by physical contact, in a process that may be similar to bacterial conjugation.[77][78] Archaeal plasmids are increasingly important as genetic tools and allow the performance of genetic studies in archaea.[79]

As with the bacteriophages that infect bacteria, viruses exist that replicate within archaea: these are double-stranded DNA viruses that appear to be unrelated to any other form of virus and can have a variety of unusual shapes, with some resembling bottles, hooked rods, or teardrops.[81] These viruses have been studied in most detail in the thermophilic archaea, particularly the orders Sulfolobales and Thermoproteale.[82] Defenses against these viruses may involve RNA interference from repetitive DNA sequences within archaean genomes that are related to the genes of the viruses.[83][84]
Archaea are genetically distinct from other organisms, with up to 15% of the proteins encoded by any one archaeal genome being unique to the Archaea, although most of these unique genes have no known function.[85] Of the remainder of the genes that are unique to archaea and do have an identified function, most are involved in methanogenesis. The genes that are shared between archaea, bacteria and eukaryotes form a common core of cell function, relating mostly to transcription, translation, and nucleotide metabolism.[86] Other characteristic features of archaean genomes are the organization of genes of related function, such as enzymes catalysing steps in the same metabolic pathway, into novel operons, and large differences in tRNA genes and their aminoacyl tRNA synthetases.[86]
Transcription and translation in archaea are more similar to that in eukaryotes than in bacteria, with both the RNA polymerase II and the ribosomes of archaea sharing both subunits and sequence similarity with their equivalents in eukaryotes.[74] The function and interactions of the archaeal RNA polymerase in transcription also seems to be related to that of eukaryotes, with similar assemblies of proteins (the general transcription factors) directing the binding of the RNA polymerase to a gene's promoter. However, many other archaean transcription factors are similar to those seen in bacteria.[87]
Habitats
Multiple archaeans are extremophiles, and some would say this is their ecological niche.[5] They can survive high temperatures, often above 100 °C, as found in geysers, black smokers, and oil wells. Some are found in very cold habitats and others in highly saline, acidic, or alkaline water. Mesophiles favor milder conditions in marshland, sewage and soil.

Archaea are commonly placed into three physiological groups. These are the halophiles, thermophiles and acidophiles. These groups are not necessarily comprehensive or monophyletic, nor even mutually exclusive. Nonetheless, they are a useful starting point for ecological studies. Halophiles, including the genus Halobacterium, live in extremely saline environments and start outnumbering their bacterial counterparts at salinities greater than 20-25%.[5] Thermophiles live in places that have high temperatures, such as hot springs. Where optimal growth occurs at greater than 80 °C, the archaeon is a hyperthermophyle, and the highest recorded temperature survived was 121 °C. Although thermophilic bacteria predominate at some high temperatures, archaea generally have the edge when acidity exceeds pH 5. One of the most extreme archaean acidophiles is Picrophilus torridus, which can grow at pH 0, which is equivalent to thriving in 1.2 Molar sulfuric acid.[88]
Recently, several studies have shown that archaea exist not only in mesophilic and thermophilic environments but are also present, sometimes in high numbers, at low temperatures as well. It is increasingly becoming recognised that methanogens are commonly present in low-temperature environments such as cold sediments. Perhaps even more significant are the large numbers of archaea found throughout most of the world's oceans, a predominantly cold environment. These archaea, which belong to several deeply branching lineages unrelated to those previously known, can be present in extremely high numbers (up to 40% of the microbial biomass) although almost none have been isolated in pure culture.[89] Currently we have little information regarding the physiology of these organisms, meaning that their effects on global biogeochemical cycles remain unknown. One recent study has shown, however, that one group of marine Crenarchaeota are capable of nitrification, a trait previously unknown among the archaea.[90]
Interactions with other organisms
Many methanogenic archaea are symbionts found in the digestive tracts of animals, such as ruminants and termites. In humans, the formate-consuming methanogen Methanobrevibacter smithii is by far the most common archaean, and this species can make up one in ten of all the prokaryotes in the intestines.[91] Further associations with other organisms are now being found, with the discovery that the marine archaean Cenarchaeum symbiosum is a symbiont of the sponge Axinella mexicana.[92] In these marines environments, Archaea and Bacteria life forms coexist as oceanic microbial communities, or Plankton. On these communities, Archaea make up to 60% of present biomass, and take the same percentage of dissolved amino acids, showing that both groups of microorganisms coexist in this ecosystem [93]. As of 2007, no clear examples of archaeal pathogens are known,[94][95] although a relationship has been proposed between the presence of some methanogens and human periodontal disease.[96]
Significance in technology and industry
Extremophile archaea, particularly organisms that are resistant to heat, or extremes of acidity and alkalinity, are a source of enzymes that can function under these harsh conditions.[97][98] These enzymes have a wide range of uses. For example, thermostable DNA polymerases, such as the Pfu DNA polymerase from Pyrococcus furiosus, have revolutionized molecular biology by allowing the polymerase chain reaction to be used as a simple and rapid technique for cloning DNA. In industry, amylases, galactosidases and pullulanases in other species of Pyrococcus that function at over 100 °C allow food processing at high temperatures, such as the production of low lactose milk and whey.[99] Enzymes from these thermophilic archaea also tend to be very stable in organic solvents, so can be used in a broad range of environmentally-friendly processes in green chemistry that synthesize organic compounds.[98]
In contrast to the range of applications of archaean enzymes, the use of the organisms themselves in biotechnology are more restricted. However, methanogenic archaea are a vital part of sewage treatment, since they are part of the community of microorganisms that carry out anaerobic digestion and produce biogas.[100] Acidophillic archaea also show promise in the extraction of metals such as gold, cobalt and copper from ores in mineral processing.[101]
A new class of potentially useful antibiotics have been discovered in archaea. A few of these archaeocins have been characterized, but hundreds more are believed to exist, especially within Haloarchaea and Sulfobolous.[102] These compounds are important since they are different in structure to bacterial antibiotics, so they may have novel modes of action. In addition, they may allow the creation of new selectable markers for use in archaeal molecular biology. The discovery of new archaeocins depends on successful recovery and cultivation of new species of archaea from the environment.[103]
See also
References
- ^ Howland, John L. (2000). The Surprising Archaea: Discovering Another Domain of Life. Oxford: Oxford University Press. p. 32. ISBN 0-19-511183-4.
- ^ a b Wang M, Yafremava LS, Caetano-Anollés D, Mittenthal JE, Caetano-Anollés G (2007). "Reductive evolution of architectural repertoires in proteomes and the birth of the tripartite world". Genome Res. 17 (11): 1572–85. doi:10.1101/gr.6454307. PMID 17908824.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ DeLong EF, Pace NR (2001). "Environmental diversity of bacteria and archaea". Syst. Biol. 50 (4): 470–8. doi:10.1080/106351501750435040. PMID 12116647.
- ^ Karner MB, DeLong EF, Karl DM (2001). "Archaeal dominance in the mesopelagic zone of the Pacific Ocean". Nature. 409 (6819): 507–10. doi:10.1038/35054051. PMID 11206545.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Valentine DL (2007). "Adaptations to energy stress dictate the ecology and evolution of the Archaea". Nat. Rev. Microbiol. 5 (4): 316–23. doi:10.1038/nrmicro1619. PMID 17334387.
- ^ Staley JT (2006). "The bacterial species dilemma and the genomic-phylogenetic species concept". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 361 (1475): 1899–909. doi:10.1098/rstb.2006.1914. PMID 17062409.
- ^ Zuckerkandl E, Pauling L (1965). "Molecules as documents of evolutionary history". J. Theor. Biol. 8 (2): 357–66. doi:10.1016/0022-5193(65)90083-4. PMID 5876245.
- ^ Woese C, Fox G (1977). "Phylogenetic structure of the prokaryotic domain: the primary kingdoms". Proc Natl Acad Sci USA. 74 (11): 5088–90. doi:10.1073/pnas.74.11.5088. PMID 270744.
- ^ Woese CR, Kandler O, Wheelis ML (1990). "Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya". Proc. Natl. Acad. Sci. U.S.A. 87 (12): 4576–9. doi:10.1073/pnas.87.12.4576. PMID 2112744.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ DeLong EF (1998). "Everything in moderation: archaea as 'non-extremophiles'". Curr. Opin. Genet. Dev. 8 (6): 649–54. doi:10.1016/S0959-437X(98)80032-4. PMID 9914204.
- ^ Theron J, Cloete TE (2000). "Molecular techniques for determining microbial diversity and community structure in natural environments". Crit. Rev. Microbiol. 26 (1): 37–57. doi:10.1080/10408410091154174. PMID 10782339.
- ^ Schmidt TM (2006). "The maturing of microbial ecology" (PDF). Int. Microbiol. 9 (3): 217–23. PMID 17061212.
- ^ Altermann W, Kazmierczak J (2003). "Archean microfossils: a reappraisal of early life on Earth". Res Microbiol. 154 (9): 611–7. doi:10.1016/j.resmic.2003.08.006. PMID 14596897.
- ^ Cavalier-Smith T (2006). "Cell evolution and Earth history: stasis and revolution" (PDF). Philos Trans R Soc Lond B Biol Sci. 361 (1470): 969–1006. doi:10.1098/rstb.2006.1842. PMID 16754610.
- ^ Schopf J (2006). "Fossil evidence of Archaean life" (PDF). Philos Trans R Soc Lond B Biol Sci. 361 (1470): 869–85. doi:10.1098/rstb.2006.1834. PMID 16754604.
- ^ Brocks JJ, Logan GA, Buick R, Summons RE (1999). "Archean molecular fossils and the early rise of eukaryotes". Science. 285 (5430): 1033–6. doi:10.1126/science.285.5430.1033. PMID 10446042.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Michaelis, W. (1979). "Molecular fossils of Archaebacteria in kerogen". Naturwissenschaften. 66 (8): 420–422.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hahn, Jürgen (1986). "Traces of Archaebacteria in ancient sediments". System Applied Microbiology. 7 (Archaebacteria '85 Proceedings): 178–183.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P (2006). "Toward automatic reconstruction of a highly resolved tree of life". Science. 311 (5765): 1283–7. doi:10.1126/science.1123061. PMID 16513982.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Woese CR, Gupta R (1981). "Are archaebacteria merely derived 'prokaryotes'?". Nature. 289 (5793): 95–6. doi:10.1038/289095a0. PMID 6161309.
- ^ Woese C (1998). "The universal ancestor". Proc. Natl. Acad. Sci. U.S.A. 95 (12): 6854–9. doi:10.1073/pnas.95.12.6854. PMID 9618502.
- ^ Gupta RS (2000). "The natural evolutionary relationships among prokaryotes". Crit. Rev. Microbiol. 26 (2): 111–31. doi:10.1080/10408410091154219. PMID 10890353.
- ^ Gribaldo S, Brochier-Armanet C (2006). "The origin and evolution of Archaea: a state of the art". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 361 (1470): 1007–22. doi:10.1098/rstb.2006.1841. PMID 16754611.
- ^ Nelson KE, Clayton RA, Gill SR; et al. (1999). "Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima". Nature. 399 (6734): 323–9. doi:10.1038/20601. PMID 10360571.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Lake JA. (1988). "Origin of the eukaryotic nucleus determined by rate-invariant analysis of rRNA sequences". Nature. 331 (6152): 184–6. doi:10.1038/331184a0. PMID 3340165.
- ^ Gevers D, Dawyndt P, Vandamme P; et al. (2006). "Stepping stones towards a new prokaryotic taxonomy". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 361 (1475): 1911–6. doi:10.1098/rstb.2006.1915. PMID 17062410.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ de Queiroz K (2005). "Ernst Mayr and the modern concept of species". Proc. Natl. Acad. Sci. U.S.A. 102 Suppl 1: 6600–7. doi:10.1073/pnas.0502030102. PMID 15851674.
- ^ Eppley JM, Tyson GW, Getz WM, Banfield JF (2007). "Genetic exchange across a species boundary in the archaeal genus ferroplasma". Genetics. 177 (1): 407–16. doi:10.1534/genetics.107.072892. PMID 17603112.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Papke RT, Zhaxybayeva O, Feil EJ, Sommerfeld K, Muise D, Doolittle WF (2007). "Searching for species in haloarchaea". Proc. Natl. Acad. Sci. U.S.A. 104 (35): 14092–7. doi:10.1073/pnas.0706358104. PMID 17715057.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kunin V, Goldovsky L, Darzentas N, Ouzounis CA (2005). "The net of life: reconstructing the microbial phylogenetic network". Genome Res. 15 (7): 954–9. doi:10.1101/gr.3666505. PMID 15965028.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Robertson CE, Harris JK, Spear JR, Pace NR (2005). "Phylogenetic diversity and ecology of environmental Archaea". Curr. Opin. Microbiol. 8 (6): 638–42. PMID 16236543.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hugenholtz P (2002). "Exploring prokaryotic diversity in the genomic era". Genome Biol. 3 (2): REVIEWS0003. doi:10.1186/gb-2002-3-2-reviews0003. PMID 11864374.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rappé MS, Giovannoni SJ (2003). "The uncultured microbial majority". Annu. Rev. Microbiol. 57: 369–94. doi:10.1146/annurev.micro.57.030502.090759. PMID 14527284.
- ^ Huber H, Hohn MJ, Rachel R, Fuchs T, Wimmer VC, Stetter KO. (2002). "A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont". Nature. 417 (6884): 27–8. doi:10.1038/417063a. PMID 11986665.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Barns SM, Delwiche CF, Palmer JD, Pace NR (1996). "Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences". Proc. Natl. Acad. Sci. U.S.A. 93 (17): 9188–93. doi:10.1073/pnas.93.17.9188. PMID 8799176.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Baker, B.J., Tyson, G.W., Webb, R.I., Flanagan, J., Hugenholtz, P. and Banfield, J.F. (2006). "Lineages of acidophilic Archaea revealed by community genomic analysis. Science". Science. 314 (6884): 1933–1935. doi:10.1126/science.1132690. PMID 17185602.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Krieg, Noel (2005). Bergey’s Manual® of Systematic Bacteriology. USA: Springer. pp. 21–26. ISBN 978-0-387-24143-2.
- ^ Barns, Sue and Burggraf, Siegfried. (1997) Crenarchaeota. Version 01 January 1997. in The Tree of Life Web Project
- ^ Walsby AE (2005). "Archaea with square cells". Trends Microbiol. 13 (5): 193–5. doi:10.1016/j.tim.2005.03.002. PMID 15866034.
- ^ Hixon WG, Searcy DG (1993). "Cytoskeleton in the archaebacterium Thermoplasma acidophilum? Viscosity increase in soluble extracts". BioSystems. 29 (2–3): 151–60. doi:10.1016/0303-2647(93)90091-P. PMID 8374067.
- ^ Hara F, Yamashiro K, Nemoto N; et al. (2007). "An actin homolog of the archaeon Thermoplasma acidophilum that retains the ancient characteristics of eukaryotic actin". J. Bacteriol. 189 (5): 2039–45. doi:10.1128/JB.01454-06. PMID 17189356.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Trent JD, Kagawa HK, Yaoi T, Olle E, Zaluzec NJ (1997). "Chaperonin filaments: the archaeal cytoskeleton?". Proc. Natl. Acad. Sci. U.S.A. 94 (10): 5383–8. doi:10.1073/pnas.94.10.5383. PMID 9144246.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hall-Stoodley L, Costerton JW, Stoodley P (2004). "Bacterial biofilms: from the natural environment to infectious diseases". Nat. Rev. Microbiol. 2 (2): 95–108. doi:10.1038/nrmicro821. PMID 15040259.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nickell S, Hegerl R, Baumeister W, Rachel R (2003). "Pyrodictium cannulae enter the periplasmic space but do not enter the cytoplasm, as revealed by cryo-electron tomography". J. Struct. Biol. 141 (1): 34–42. doi:10.1016/S1047-8477(02)00581-6. PMID 12576018.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Horn C, Paulmann B, Kerlen G, Junker N, Huber H (1999). "In vivo observation of cell division of anaerobic hyperthermophiles by using a high-intensity dark-field microscope". J. Bacteriol. 181 (16): 5114–8. doi:10.1073/pnas.241636498v1. PMID 10438790.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rachel R, Wyschkony I, Riehl S, Huber H (2002). "The ultrastructure of Ignicoccus: evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon" (PDF). Archaea. 1 (1): 9–18. PMID 15803654.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ De Rosa M, Gambacorta A, Gliozzi A (1986). "Structure, biosynthesis, and physicochemical properties of archaebacterial lipids". Microbiol. Rev. 50 (1): 70–80. PMID 3083222.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Koga Y, Morii H (2007). "Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations". Microbiol. Mol. Biol. Rev. 71 (1): 97–120. doi:10.1128/MMBR.00033-06. PMID 17347520.
- ^ Golyshina OV, Pivovarova TA, Karavaiko GI; et al. (2000). "Ferroplasma acidiphilum gen. nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. nov., comprising a distinct lineage of the Archaea". Int. J. Syst. Evol. Microbiol. 50 Pt 3: 997–1006. PMID 10843038.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sára M, Sleytr UB (2000). "S-Layer proteins". J. Bacteriol. 182 (4): 859–68. doi:10.1128/JB.182.4.859-868.2000. PMID 10648507.
- ^ Howland, John L. (2000) The Surprising Archaea: Discovering Another domain of Life, pages 69-71. (Oxford: Oxford University Press). ISBN 0-19-511183-4.
- ^ Thomas NA, Bardy SL, Jarrell KF (2001). "The archaeal flagellum: a different kind of prokaryotic motility structure". FEMS Microbiol. Rev. 25 (2): 147–74. doi:10.1111/j.1574-6976.2001.tb00575.x. PMID 11250034.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ng SY, Chaban B, Jarrell KF (2006). "Archaeal flagella, bacterial flagella and type IV pili: a comparison of genes and posttranslational modifications". J. Mol. Microbiol. Biotechnol. 11 (3–5): 167–91. doi:10.1159/000094053. PMID 16983194.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bardy SL, Ng SY, Jarrell KF (2003). "Prokaryotic motility structures". Microbiology (Reading, Engl.). 149 (Pt 2): 295–304. PMID 12624192.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Schäfer G, Engelhard M, Müller V (1999). "Bioenergetics of the Archaea". Microbiol. Mol. Biol. Rev. 63 (3): 570–620. PMC 103747. PMID 10477309.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Zillig W (1991). "Comparative biochemistry of Archaea and Bacteria". Curr. Opin. Genet. Dev. 1 (4): 544–51. PMID 1822288.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Romano A, Conway T (1996). "Evolution of carbohydrate metabolic pathways". Res Microbiol. 147 (6–7): 448–55. doi:10.1016/0923-2508(96)83998-2. PMID 9084754.
- ^ Koch A (1998). "How did bacteria come to be?". Adv Microb Physiol. 40: 353–99. PMID 9889982.
- ^ Based on PDB 1FBB
- ^ DiMarco AA, Bobik TA, Wolfe RS (1990). "Unusual coenzymes of methanogenesis". Annu. Rev. Biochem. 59: 355–94. doi:10.1146/annurev.bi.59.070190.002035. PMID 2115763.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Klocke M, Nettmann E, Bergmann I; et al. (2008). "Characterization of the methanogenic Archaea within two-phase biogas reactor systems operated with plant biomass". Syst. Appl. Microbiol. doi:10.1016/j.syapm.2008.02.003. PMID 18501543.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bryant DA, Frigaard NU (2006). "Prokaryotic photosynthesis and phototrophy illuminated". Trends Microbiol. 14 (11): 488–96. doi:10.1016/j.tim.2006.09.001. PMID 16997562.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Lanyi JK (2004). "Bacteriorhodopsin". Annu. Rev. Physiol. 66: 665–88. doi:10.1146/annurev.physiol.66.032102.150049. PMID 14977418.
- ^ Mueller-Cajar O, Badger MR (2007). "New roads lead to Rubisco in archaebacteria". Bioessays. 29 (8): 722–4. doi:10.1002/bies.20616. PMID 17621634.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Berg IA, Kockelkorn D, Buckel W, Fuchs G (2007). "A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea". Science (journal). 318 (5857): 1782–6. doi:10.1126/science.1149976. PMID 18079405.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Thauer RK (2007). "Microbiology. A fifth pathway of carbon fixation". Science (journal). 318 (5857): 1732–3. doi:10.1126/science.1152209. PMID 18079388.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Francis CA, Beman JM, Kuypers MM (2007). "New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation". ISME J. 1 (1): 19–27. doi:10.1038/ismej.2007.8. PMID 18043610.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005). "Isolation of an autotrophic ammonia-oxidizing marine archaeon". Nature. 437 (7058): 543–6. doi:10.1038/nature03911. PMID 16177789.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Brock TD, Gustafson J (1976). "Ferric iron reduction by sulfur- and iron-oxidizing bacteria". Appl. Environ. Microbiol. 32 (4): 567–71. PMC 170307. PMID 825043.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Bernander R (1998). "Archaea and the cell cycle". Mol. Microbiol. 29 (4): 955–61. doi:10.1046/j.1365-2958.1998.00956.x. PMID 9767564.
- ^ Kelman LM, Kelman Z (2004). "Multiple origins of replication in archaea". Trends Microbiol. 12 (9): 399–401. doi:10.1016/j.tim.2004.07.001. PMID 153371581.
{{cite journal}}: Check|pmid=value (help) - ^ Onyenwoke RU, Brill JA, Farahi K, Wiegel J (2004). "Sporulation genes in members of the low G+C Gram-type-positive phylogenetic branch ( Firmicutes)". Arch. Microbiol. 182 (2–3): 182–92. doi:10.1007/s00203-004-0696-y. PMID 15340788.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kostrikina NA, Zvyagintseva IS, Duda VI. (1991). "Cytological peculiarities of some extremely halophilic soil archaeobacteria". Arch. Microbiol. 156 (5): 344–49. doi:10.1007/BF00248708.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Allers T, Mevarech M (2005). "Archaeal genetics - the third way". Nat. Rev. Genet. 6 (1): 58–73. doi:10.1038/nrg1504. PMID 15630422.
- ^ Baliga NS, Bonneau R, Facciotti MT; et al. (2004). "Genome sequence of Haloarcula marismortui: a halophilic archaeon from the Dead Sea". Genome Res. 14 (11): 2221–34. doi:10.1101/gr.2700304. PMID 15520287.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Waters E, Hohn MJ, Ahel I; et al. (2003). "The genome of Nanoarchaeum equitans: insights into early archaeal evolution and derived parasitism". Proc. Natl. Acad. Sci. U.S.A. 100 (22): 12984–8. doi:10.1073/pnas.1735403100. PMID 14566062.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Schleper C, Holz I, Janekovic D, Murphy J, Zillig W (1995). "A multicopy plasmid of the extremely thermophilic archaeon Sulfolobus effects its transfer to recipients by mating". J. Bacteriol. 177 (15): 4417–26. PMID 7635827.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sota M; Top EM (2008). "Horizontal Gene Transfer Mediated by Plasmids". Plasmids: Current Research and Future Trends. Caister Academic Press. ISBN 978-1-904455-35-6.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Lipps G (2008). "Archaeal Plasmids". Plasmids: Current Research and Future Trends. Caister Academic Press. ISBN 978-1-904455-35-6.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Xiang X, Chen L, Huang X, Luo Y, She Q, Huang L (2005). "Sulfolobus tengchongensis spindle-shaped virus STSV1: virus-host interactions and genomic features". J. Virol. 79 (14): 8677–86. doi:10.1128/JVI.79.14.8677-8686.2005. PMID 15994761.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Prangishvili D, Forterre P, Garrett RA (2006). "Viruses of the Archaea: a unifying view". Nat. Rev. Microbiol. 4 (11): 837–48. doi:10.1038/nrmicro1527. PMID 17041631.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Prangishvili D, Garrett RA (2004). "Exceptionally diverse morphotypes and genomes of crenarchaeal hyperthermophilic viruses". Biochem. Soc. Trans. 32 (Pt 2): 204–8. doi:10.1042/BST0320204. PMID 15046572.
- ^ Mojica FJ, Díez-Villaseñor C, García-Martínez J, Soria E (2005). "Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements". J. Mol. Evol. 60 (2): 174–82. doi:10.1007/s00239-004-0046-3. PMID 15791728.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Makarova KS, Grishin NV, Shabalina SA, Wolf YI, Koonin EV (2006). "A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action". Biol. Direct. 1: 7. doi:10.1186/1745-6150-1-7. PMID 16545108.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Graham DE, Overbeek R, Olsen GJ, Woese CR (2000). "An archaeal genomic signature". Proc. Natl. Acad. Sci. U.S.A. 97 (7): 3304–8. doi:10.1073/pnas.050564797. PMID 10716711.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Gaasterland T (1999). "Archaeal genomics". Curr. Opin. Microbiol. 2 (5): 542–7. doi:10.1016/S1369-5274(99)00014-4. PMID 10508726.
- ^ Aravind L, Koonin EV (1999). "DNA-binding proteins and evolution of transcription regulation in the archaea". Nucleic Acids Res. 27 (23): 4658–70. doi:10.1093/nar/27.23.4658. PMID 10556324.
- ^ Ciaramella M, Napoli A, Rossi M (2005). "Another extreme genome: how to live at pH 0". Trends Microbiol. 13 (2): 49–51. doi:10.1016/j.tim.2004.12.001. PMID 15680761.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Giovannoni SJ, Stingl U. (2005). "Molecular diversity and ecology of microbial plankton". Nature. 427 (7057): 343–8. doi:10.1038/nature04158. PMID 16163344.
- ^ Konneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA. (2005). "Isolation of an autotrophic ammonia-oxidizing marine archaeon". Nature. 437 (7057): 543–6. doi:10.1038/nature03911. PMID 16177789.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eckburg PB, Bik EM, Bernstein CN; et al. (2005). "Diversity of the human intestinal microbial flora". Science (journal). 308 (5728): 1635–8. doi:10.1126/science.1110591. PMC 1395357. PMID 15831718.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum gen. nov., sp. nov". Proc Natl Acad Sci USA. 93 (13): 6241–6246. 1996.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Marine Planktonic Archaea Take Up Amino Acids". Applied and Environmental Microbiology. 66 (11): 4829–4833. 2000.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Eckburg P, Lepp P, Relman D (2003). "Archaea and their potential role in human disease". Infect Immun. 71 (2): 591–6. doi:10.1128/IAI.71.2.591-596.2003. PMID 12540534.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cavicchioli R, Curmi P, Saunders N, Thomas T (2003). "Pathogenic archaea: do they exist?". Bioessays. 25 (11): 1119–28. doi:10.1002/bies.10354. PMID 14579252.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lepp P, Brinig M, Ouverney C, Palm K, Armitage G, Relman D (2004). "Methanogenic Archaea and human periodontal disease". Proc Natl Acad Sci U S A. 101 (16): 6176–81. doi:10.1073/pnas.0308766101. PMID 15067114.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Breithaupt H (2001). "The hunt for living gold. The search for organisms in extreme environments yields useful enzymes for industry". EMBO Rep. 2 (11): 968–71. doi:10.1093/embo-reports/kve238. PMID 11713183.
- ^ a b Egorova K, Antranikian G (2005). "Industrial relevance of thermophilic Archaea". Curr. Opin. Microbiol. 8 (6): 649–55. PMID 16257257.
- ^ Synowiecki J, Grzybowska B, Zdziebło A (2006). "Sources, properties and suitability of new thermostable enzymes in food processing". Crit Rev Food Sci Nutr. 46 (3): 197–205. doi:10.1080/10408690590957296. PMID 16527752.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schiraldi C, Giuliano M, De Rosa M (2002). "Perspectives on biotechnological applications of archaea" (PDF). Archaea. 1 (2): 75–86. PMID 15803645.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Norris PR, Burton NP, Foulis NA (2000). "Acidophiles in bioreactor mineral processing". Extremophiles. 4 (2): 71–6. doi:10.1007/s007920050139. PMID 10805560.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ O'Connor EM, Shand RF (2002). "Halocins and sulfolobicins: the emerging story of archaeal protein and peptide antibiotics". J. Ind. Microbiol. Biotechnol. 28 (1): 23–31. doi:10.1038/sj/jim/7000190. PMID 11938468.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Shand RF; Leyva KJ (2008). Archaea: New Models for Prokaryotic Biology. Caister Academic Press.
{{cite book}}: CS1 maint: multiple names: authors list (link)
Further reading
- Howland, John L. (2000). The Surprising Archaea: Discovering Another Domain of Life. Oxford: Oxford University Press. ISBN 0-19-511183-4.
- Martinko JM, Madigan MT (2005). Brock Biology of Microorganisms (11th ed. ed.). Englewood Cliffs, N.J: Prentice Hall. ISBN 0-13-144329-1.
{{cite book}}:|edition=has extra text (help) - Garrett RA, Klenk H (2005). Archaea: Evolution, Physiology and Molecular Biology. WileyBlackwell. ISBN 1-40-514404-1.
- Cavicchioli R (2007). Archaea: Molecular and Cellular Biology. American Society for Microbiology. ISBN 1-55-581391-7.
- Blum P (editor). (2008). Archaea: New Models for Prokaryotic Biology. Caister Academic Press. ISBN 978-1-904455-27-1 .
{{cite book}}:|author=has generic name (help) - Lipps G (2008). "Archaeal Plasmids". Plasmids: Current Research and Future Trends. Caister Academic Press. ISBN 978-1-904455-35-6.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)
External links
General
- ArchaeaWeb - by UNSW - Information about Archaea
- Introduction to the Archaea, ecology, systematics and morphology
- Oceans of Archaea - E.F. DeLong, ASM News, 2003
Classification
- NCBI taxonomy parge on Archaea
- Tree of Life illustration showing how Archaea relates to other lifeforms
- Shotgun sequencing finds nanoorganisms - discovery of the ARMAN group of archaea
Genomics
- Browse any completed archaeal genome at UCSC
- Comparative Analysis of Archaeal Genomes (at DOE's IMG system)

