Trans fat regulation: Difference between revisions
No edit summary |
→History: removed editorial exclamation; geez |
||
| Line 96: | Line 96: | ||
}}</ref> |
}}</ref> |
||
In January 2007, faced with the prospect of an outright ban on the sale of their product, Crisco was reformulated to meet the United States [[Food and Drug Administration (United States)|Food and Drug Administration]] definition of "zero grams trans fats per serving" (that is less than one gram per tablespoon, or up to 7% by weight |
In January 2007, faced with the prospect of an outright ban on the sale of their product, Crisco was reformulated to meet the United States [[Food and Drug Administration (United States)|Food and Drug Administration]] definition of "zero grams trans fats per serving" (that is less than one gram per tablespoon, or up to 7% by weight)<ref name="21CFR" /><ref name="FDA05" /><ref name="Newswise" /><ref name="Shock" /> by boosting the saturation and then cutting the resulting solid with oils. A [[University of Guelph]] research group has found a way to mix oils (such as olive, soybean and canola), water, [[monoglycerides]] and fatty acids to form a "cooking fat" that acts the same way as trans and saturated fats.<ref>{{cite web|url=http://www.cbc.ca/health/fitness-blog/2007/01/trans_fats_headed_for_the_exit.html|title=CBC ''Trans Fats Headed for the Exit''}}</ref><ref>{{cite web|url=http://www.rsc.org/Publishing/ChemScience/Volume/2007/02/trans_fat_free_future.asp|title=''Trans fat free future''}}</ref> |
||
==Chemistry== |
==Chemistry== |
||
Revision as of 03:19, 6 June 2010
| Types of fats in food |
|---|
| Components |
| Manufactured fats |
Trans fat is the common name for unsaturated fat with trans-isomer fatty acid(s). Trans fats may be monounsaturated or polyunsaturated but never saturated.
Unsaturated fat is a fat molecule containing one or more double bonds between the carbon atoms. Since the carbons are double-bonded to each other, there are fewer bonds connected to hydrogen, so there are fewer hydrogen atoms, hence "unsaturated". Cis and trans are terms that refer to the arrangement of chains of carbon atoms across the double bond. In the cis arrangement, the chains are on the same side of the double bond, resulting in a kink. In the trans arrangement, the chains are on opposite sides of the double bond, and the chain is straight.
Each fat molecule has three hydrocarbon chains. The process of hydrogenation adds hydrogen atoms to cis-unsaturated fats, eliminating double bonds and making them into partially or completely saturated fats. These more-completely saturated fats have a higher melting point, which makes them more attractive for baking, and the saturation extends their shelf-life. However, partial hydrogenation converts a part of cis-isomers into trans-unsaturated fats instead of hydrogenating them completely. Complete hydrogenation converts the fat into a saturated "hard" fat. Alternatively, hard fats can be softened by cutting with cis fats such as vegetable oil, or by transesterification with cis fats into fats with cis unsaturated and saturated hydrocarbon chains.
Trans fats occur also naturally, although to a limited extent: vaccenyl and conjugated linoleyl (CLA) containing trans fats occur naturally in trace amounts in meat and dairy products from ruminants, although the latter also constitutes a cis fat.
Unlike other dietary fats, trans fats are not essential, and they do not promote good health.[1] The consumption of trans fats increases the risk of coronary heart disease[2] by raising levels of "bad" LDL cholesterol and lowering levels of "good" HDL cholesterol.[3] Health authorities worldwide recommend that consumption of trans fat be reduced to trace amounts. Trans fats from partially hydrogenated oils are more harmful than naturally occurring oils.[4]
History
The examples and perspective in this article may not represent a worldwide view of the subject. |

Nobel laureate Paul Sabatier worked in the late 1890s to develop the chemistry of hydrogenation which enabled the margarine, oil hydrogenation, and synthetic methanol industries.[5] While Sabatier only considered hydrogenation of vapors, the German chemist Wilhelm Normann showed in 1901 that liquid oils could be hydrogenated, and patented the process in 1902.[6][7][8] During the years 1905 – 1910 Normann built a fat hardening facility in the Herford company. At the same time the invention was extended to a large scale plant in Warrington, England, at Joseph Crosfield & Sons, Limited. It took only two years until the hardened fat could be successfully produced in the plant in Warrington, commencing production in the autumn of 1909. The initial year's production totalled nearly 3,000 tonnes.[9] In 1909, Procter & Gamble acquired the US rights to the Normann patent;[10] in 1911, they began marketing the first hydrogenated shortening, Crisco (composed largely of partially hydrogenated cottonseed oil). Further success came from the marketing technique of giving away free cookbooks in which every recipe called for Crisco.
Normann's hydrogenation process made it possible to stabilize affordable whale oil or fish oil for human consumption, a practice kept secret to avoid consumer distaste.[11]
Prior to 1910, dietary fats primarily consisted of butterfat, beef tallow, and lard. During Napoleon’s reign in France in the early 1800s, a type of margarine was invented to feed the troops using tallow and buttermilk; it did not gain acceptance in the U.S. In the early 1900s, soybeans began to be imported into the U.S. as a source of protein; soybean oil was a by-product. What to do with that oil became an issue. At the same time, there was not enough butterfat available for consumers. The method of hydrogenating fat and turning a liquid fat into a solid one had been discovered, and now the ingredients (soybeans) and the “need” (shortage of butter) were there. Later, the means for storage, the refrigerator, was a factor in trans fat development. The fat industry found that hydrogenated fats provided some special features to margarines, which unlike butter, allowed margarine to be taken out of the refrigerator and immediately spread on a slice of bread. By some minor changes to the chemical composition of hydrogenated fat, they also found such hydrogenated fat provided superior baking properties compared to lard. Margarine made from hydrogenated soybean oil began to replace butterfat. Hydrogenated fat such as Crisco and Spry, sold in England, began to replace lard in the baking of bread, pies, cookies, and cakes in 1920.[12]
In the 1940s Dr Catherine Kousmine researched the effects of trans fats on cancer.
Production of hydrogenated fats increased steadily until the 1960s as processed vegetable fats replaced animal fats in the US and other western countries. At first, the argument was a financial one due to lower costs; however, advocates also said that the unsaturated trans fats of margarine were healthier than the saturated fats of butter.[13]
There were suggestions in the scientific literature as early as 1988 that trans fats could be a cause of the large increase in coronary artery disease.[13][14] In 1994, it was estimated that trans fats caused 20,000 deaths annually in the US from heart disease.[15]
In January 2007, faced with the prospect of an outright ban on the sale of their product, Crisco was reformulated to meet the United States Food and Drug Administration definition of "zero grams trans fats per serving" (that is less than one gram per tablespoon, or up to 7% by weight)[16][17][18][19] by boosting the saturation and then cutting the resulting solid with oils. A University of Guelph research group has found a way to mix oils (such as olive, soybean and canola), water, monoglycerides and fatty acids to form a "cooking fat" that acts the same way as trans and saturated fats.[20][21]
Chemistry
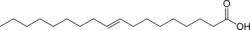
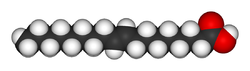
Chemically, trans fat refers to a lipid molecule that contains one or more double bonds in trans geometric configuration. A double bond may exhibit one of two possible configurations; trans or cis. In trans configuration, the carbon chain extends from opposite sides of the double bond, rendering a straighter molecule, whereas in cis configuration, the carbon chain extends from the same side of the double bond, rendering a bent molecule.
| Trans (Elaidic acid) | Cis (Oleic acid) | Saturated (Stearic acid) |
|---|---|---|
| Elaidic acid is the principal trans unsaturated fatty acid often found in partially hydrogenated vegetable oils.[22] | Oleic acid is a cis unsaturated fatty acid that comprises 55–80% of olive oil.[23] | Stearic acid is a saturated fatty acid found in animal fats and is the intended product in full hydrogenation. Stearic acid is neither cis nor trans because it has no double bonds. |
 |
 |
File:Octadecanoic acid (stearic).png |
 |
||
| These fatty acids are geometric isomers (structurally identical except for the arrangement of the double bond). | This fatty acid contains no double bond and is not isomeric with the previous two. | |
Fatty acids are characterized as either saturated or unsaturated based on the presence of double bonds in its structure. If the molecule contains no double bonds, it is said to be saturated; otherwise, it is unsaturated to some degree.[24][25]
Only unsaturated fats can be trans fats. Saturated fatty acids are never trans fats because they have no double bonds, and therefore cannot display a trans- configuration. Moreover, lipids containing a triple bond (but no double bonds) cannot be trans fats because a triple bond can only assume one configuration.
Carbon atoms are tetravalent, forming four covalent bonds with other atoms, while hydrogen atoms bond with only one other atom. In saturated fatty acids, each carbon atom is connected to its two neighbour carbon atoms as well as two hydrogen atoms. In unsaturated fatty acids the carbon atoms that are missing a hydrogen atom are joined by double bonds rather than single bonds so that each carbon atom participates in four bonds.

Hydrogenation of an unsaturated fatty acid refers to the addition of hydrogen atoms to the acid, causing double bonds to become single ones as carbon atoms acquire new hydrogen partners (to maintain four bonds per carbon atom). Full hydrogenation results in a molecule containing the maximum amount of hydrogen (in other words the conversion of an unsaturated fatty acid into a saturated one). Partial hydrogenation results in the addition of hydrogen atoms at some of the empty positions, with a corresponding reduction in the number of double bonds. Commercial hydrogenation is typically partial in order to obtain a malleable mixture of fats that is solid at room temperature, but melts upon baking (or consumption).
In most naturally occurring unsaturated fatty acids, the hydrogen atoms are on the same side of the double bonds of the carbon chain (cis configuration — meaning "on the same side" in Latin). However, partial hydrogenation reconfigures most of the double bonds that do not become chemically saturated, twisting them so that the hydrogen atoms end up on different sides of the chain. This type of configuration is called trans, which means "across" in Latin.[26] The trans conformation is the lower energy form, and is favored when catalytically equilibriated as a side reaction in hydrogenation.
The same molecule, containing the same number of atoms, with a double bond in the same location, can be either a trans or a cis fatty acid depending on the conformation of the double bond. For example, oleic acid and elaidic acid are both unsaturated fatty acids with the chemical formula C9H17C9H17O2.[27] They both have a double bond located midway along the carbon chain. It is the conformation of this bond that sets them apart. The conformation has implications for the physical-chemical properties of the molecule. The trans configuration is straighter, while the cis configuration is noticeably kinked as can be seen from the following three-dimensional representation.
The trans fatty acid elaidic acid has different chemical and physical properties owing to the slightly different bond configuration. Notably, it has a much higher melting point, 45 °C rather than oleic acid's 13.4 °C, due to the ability of the trans molecules to pack more tightly, forming a solid that is more difficult to break apart.[27] This notably means that it is a solid at human body temperatures.
In food production, the goal is not to simply change the configuration of double bonds while maintaining the same ratios of hydrogen to carbon. Instead, the goal is to decrease the number of double bonds and increase the amount of hydrogen in the fatty acid. This changes the consistency of the fatty acid and makes it less prone to rancidity (in which free radicals attack double bonds). Production of trans fatty acids is therefore a side-effect of partial hydrogenation.
Catalytic partial hydrogenation necessarily produces trans-fats, because of the reaction mechanism. In the first reaction step, one hydrogen is added, with the other, coordinatively unsaturated, carbon being attached to the catalyst. The second step is the addition of hydrogen to the remaining carbon, producing a saturated fatty acid. The first step is reversible, such that the hydrogen is readsorbed on the catalyst and the double bond is re-formed. The intermediate with only one hydrogen added contains no double bond and can freely rotate. Thus, the double bond can re-form as either cis or trans, of which trans is favored, regardless the starting material. Complete hydrogenation also hydrogenates any produced trans fats to give saturated fats.
Researchers at the United States Department of Agriculture have investigated whether hydrogenation can be achieved without the side effect of trans fat production. They varied the pressure under which the chemical reaction was conducted — applying 1400 kPa (200 psi) of pressure to soybean oil in a 2 litre vessel while heating it to between 140 °C and 170 °C. The standard 140 kPa (20 psi) process of hydrogenation produces a product of about 40% trans fatty acid by weight, compared to about 17% using the high pressure method. Blended with unhydrogenated liquid soybean oil, the high pressure processed oil produced margarine containing 5 to 6% trans fat. Based on current U.S. labelling requirements (see below) the manufacturer could claim the product was free of trans fat.[28] The level of trans fat may also be altered by modification of the temperature and the length of time during hydrogenation.
Trans fat levels may be measured. Measurement techniques include chromatography (by silver ion chromatography on thin layer chromatography plates, or small high performance liquid chromatography columns of silica gel with bonded phenylsulfonic acid groups whose hydrogen atoms have been exchanged for silver ions). The role of silver lies in its ability to form complexes with unsaturated compounds. Gas chromatography and mid-infrared spectroscopy are other methods in use.
Presence in food

A type of trans fat occurs naturally in the milk and body fat of ruminants (such as cattle and sheep) at a level of 2–5% of total fat.[29] Natural trans fats, which include conjugated linoleic acid (CLA) and vaccenic acid, originate in the rumen of these animals. It should be noted that CLA has two double bonds, one in the cis configuration and one in trans, which makes it simultaneously a cis- and a trans-fatty acid.
Animal-based fats were once the only trans fats consumed, but by far the largest amount of trans fat consumed today is created by the processed food industry as a side-effect of partially hydrogenating unsaturated plant fats (generally vegetable oils). These partially-hydrogenated fats have displaced natural solid fats and liquid oils in many areas, notably in the fast food, snack food, fried food and baked goods industries.
Partially hydrogenated oils have been used in food for many reasons. Partial hydrogenation increases product shelf life and decreases refrigeration requirements. Many baked foods require semi-solid fats to suspend solids at room temperature; partially hydrogenated oils have the right consistency to replace animal fats such as butter and lard at lower cost. They are also an inexpensive alternative to other semi-solid oils such as palm oil.
Partially-hydrogenated plant oils, and also non-hydrogenated plant shortenings made from naturally saturated palm oil, coconut oil and palm kernel oil, can be used to replace animal fats in foodstuffs for adherents to the dietary rules of Kashrut (kosher) and Halal, and for all vegetarians and vegans.
Foods containing artificial trans fats formed by partially hydrogenating plant fats may contain up to 45% trans fat compared to their total fat.[29] Baking shortenings generally contain 30% trans fats compared to their total fats, while animal fats from ruminants such as butter contain up to 4%. Margarines not reformulated to reduce trans fats may contain up to 15% trans fat by weight.[30]
It has been established that trans fats in human milk fluctuate with maternal consumption of trans fat, and that the amount of trans fats in the bloodstream of breastfed infants fluctuates with the amounts found in their milk. Reported percentages of trans fats (compared to total fats) in human milk range from 1% in Spain, 2% in France, 4% in Germany, and 7% in Canada.[31]
Trans fats are used in shortenings for deep frying in restaurants, as they can be used for longer than most conventional oils before becoming rancid. In the early twentyfirst century non-hydrogenated vegetable oils became available that have lifespans exceeding that of the frying shortenings.[32] As fast food chains routinely use different fats in different locations, trans fat levels in fast food can have large variations. For example, an analysis of samples of McDonald's french fries collected in 2004 and 2005 found that fries served in New York City contained twice as much trans fat as in Hungary, and 28 times as much as in Denmark (where trans fats are restricted). At KFC, the pattern was reversed with Hungary's product containing twice the trans fat of the New York product. Even within the US there was variation, with fries in New York containing 30% more trans fat than those from Atlanta.[33]
Nutritional guidelines
Template:Globalize/US The National Academy of Sciences (NAS) advises the United States and Canadian governments on nutritional science for use in Public policy and product labeling programs. Their 2002 Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids[34] contains their findings and recommendations regarding consumption of trans fat (summary).
Their recommendations are based on two key facts. First, "trans fatty acids are not essential and provide no known benefit to human health",[1] whether of animal or plant origin.[35] Second, while both saturated and trans fats increase levels of LDL cholesterol (so-called bad cholesterol), trans fats also lower levels of HDL cholesterol (good cholesterol);[2] thus increasing the risk of coronary heart disease. The NAS is concerned "that dietary trans fatty acids are more deleterious with respect to coronary heart disease than saturated fatty acids".[2] This analysis is supported by a 2006 New England Journal of Medicine (NEJM) scientific review that states "from a nutritional standpoint, the consumption of trans fatty acids results in considerable potential harm but no apparent benefit."[4]
Because of these facts and concerns, the NAS has concluded there is no safe level of trans fat consumption. There is no adequate level, recommended daily amount or tolerable upper limit for trans fats. This is because any incremental increase in trans fat intake increases the risk of coronary heart disease.[2]
Despite this concern, the NAS dietary recommendations have not recommended the elimination of trans fat from the diet. This is because trans fat is naturally present in many animal foods in trace quantities, and therefore its removal from ordinary diets might introduce undesirable side effects and nutritional imbalances if proper nutritional planning is not undertaken. The NAS has therefore "recommended that trans fatty acid consumption be as low as possible while consuming a nutritionally adequate diet".[36] Like the NAS, the World Health Organization has tried to balance public health goals with a practical level of trans fat consumption, recommending in 2003 that trans fats be limited to less than 1% of overall energy intake.[29]
The US National Dairy Council has asserted that the trans fats present in animal foods are of a different type than those in partially hydrogenated oils, and do not appear to exhibit the same negative effects.[37] While a recent scientific review agrees with the conclusion (stating that "the sum of the current evidence suggests that the Public health implications of consuming trans fats from ruminant products are relatively limited") it cautions that this may be due to the low consumption of trans fats from animal sources compared to artificial ones.[4]
Health risks
Partially hydrogenated vegetable oils have been an increasingly significant part of the human diet for about 100 years (particularly since the latter half of the 20th century and in the West where more processed foods are consumed), and some deleterious effects of trans fat consumption are scientifically accepted, forming the basis of the health guidelines discussed above.
The exact biochemical methods by which trans fats produce specific health problems are a topic of continuing research. The most prevalent theory is that the human lipase enzyme is specific to the cis configuration, rendering the human body unable to metabolize or remove trans fat. A lipase is a water-soluble enzyme that catalyzes the hydrolysis of ester bonds in water-insoluble, lipid substrates. Lipases thus comprise a subclass of the esterases. Lipases perform essential roles in the digestion, transport and processing of dietary lipids (e.g. triglycerides, fats, oils) in most – if not all – living organisms. The human lipase enzyme is ineffective with the trans configuration, so trans fat remains in the blood stream for a much longer period of time and is more prone to arterial deposition and subsequent plaque formation. While the mechanisms through which trans fats contribute to coronary heart disease are fairly well understood, the mechanism for trans fat's effect on diabetes is still under investigation.
Coronary heart disease
The primary health risk identified for trans fat consumption is an elevated risk of coronary heart disease (CHD).[38] A comprehensive review of studies of trans fats was published in 2006 in the New England Journal of Medicine reports a strong and reliable connection between trans fat consumption and CHD, concluding that "On a per-calorie basis, trans fats appear to increase the risk of CHD more than any other macronutrient, conferring a substantially increased risk at low levels of consumption (1 to 3% of total energy intake)".[4] This study estimates that between 30,000 and 100,000 cardiac deaths per year in the United States are attributable to the consumption of trans fats.[39]
The major evidence for the effect of trans fat on CHD comes from the Nurses' Health Study — a cohort study that has been following 120,000 female nurses since its inception in 1976. In this study, Hu and colleagues analyzed data from 900 coronary events from the study's population during 14 years of followup. He determined that a nurse's CHD risk roughly doubled (relative risk of 1.94, CI: 1.43 to 2.61) for each 2% increase in trans fat calories consumed (instead of carbohydrate calories). By contrast, it takes more than a 15% increase in saturated fat calories (instead of carbohydrate calories) to produce a similar increase in risk. "The replacement of saturated fat or trans unsaturated fat by cis (unhydrogenated) unsaturated fats was associated with larger reductions in risk than an isocaloric replacement by carbohydrates."[40] Hu also reports on the benefits of reducing trans fat consumption. Replacing 2% of food energy from trans fat with non-trans unsaturated fats more than halves the risk of CHD (53%). By comparison, replacing a larger 5% of food energy from saturated fat with non-trans unsaturated fats reduces the risk of CHD by 43%.[40]
Another study considered deaths due to CHD, with consumption of trans fats being linked to an increase in mortality, and consumption of polyunsaturated fats being linked to a decrease in mortality.[38][41]
There are two accepted tests that measure an individual's risk for coronary heart disease, both blood tests. The first considers ratios of two types of cholesterol, the other the amount of a cell-signalling cytokine called C-reactive protein. The ratio test is more accepted, while the cytokine test may be more powerful but is still being studied.[38] The effect of trans fat consumption has been documented on each as follows:
- Cholesterol ratio: This ratio compares the levels of LDL (so-called "bad" cholesterol) to HDL (so-called "good" cholesterol). Trans fat behaves like saturated fat by raising the level of LDL, but unlike saturated fat it has the additional effect of decreasing levels of HDL. The net increase in LDL/HDL ratio with trans fat is approximately double that due to saturated fat.[42] (Higher ratios are worse.) One randomized crossover study published in 2003 comparing the postprandial effect on blood lipids of (relatively) cis and trans fat rich meals showed that cholesteryl ester transfer (CET) was 28% higher after the trans meal than after the cis meal and that lipoprotein concentrations were enriched in apolipoprotein(a) after the trans meals.[43]
- C-reactive protein (CRP): A study of over 700 nurses showed that those in the highest quartile of trans fat consumption had blood levels of CRP that were 73% higher than those in the lowest quartile.[44]
Other effects
There are suggestions that the negative consequences of trans fat consumption go beyond the cardiovascular risk. In general, there is much less scientific consensus that eating trans fat specifically increases the risk of other chronic health problems:
- Alzheimer's Disease: A study published in Archives of Neurology in February 2003 suggested that the intake of both trans fats and saturated fats promote the development of Alzheimer disease.[45]
- Cancer: There is no scientific consensus that consumption of trans fats significantly increases cancer risks across the board.[38] The American Cancer Society states that a relationship between trans fats and cancer "has not been determined."[46] However, one recent study has found connections between trans fat and prostate cancer.[47] An increased intake of trans-fatty acids may raise the risk of breast cancer by 75%, suggest the results from the French part of the European Prospective Investigation into Cancer and Nutrition.[48][49]
- Diabetes: There is a growing concern that the risk of type 2 diabetes increases with trans fat consumption.[38] However, consensus has not been reached.[4] For example, one study found that risk is higher for those in the highest quartile of trans fat consumption.[50] Another study has found no diabetes risk once other factors such as total fat intake and BMI were accounted for.[51]
- Obesity: Research indicates that trans fat may increase weight gain and abdominal fat, despite a similar caloric intake.[52] A 6-year experiment revealed that monkeys fed a trans-fat diet gained 7.2% of their body weight, as compared to 1.8% for monkeys on a mono-unsaturated fat diet.[53][54] Although obesity is frequently linked to trans fat in the popular media,[55] this is generally in the context of eating too many calories; there is no scientific consensus connecting trans fat and obesity.
- Liver Dysfunction: Trans fats are metabolized differently by the liver than other fats and interfere with delta 6 desaturase. Delta 6 desaturase is an enzyme involved in converting essential fatty acids to arachidonic acid and prostaglandins, both of which are important to the functioning of cells.[56]
- Infertility in women: One 2007 study found, "Each 2% increase in the intake of energy from trans unsaturated fats, as opposed to that from carbohydrates, was associated with a 73% greater risk of ovulatory infertility...".[57]
Public response and regulation
International
The international trade in food is standardized in the Codex Alimentarius. Hydrogenated oils and fats come under the scope of Codex Stan 19.[58] Non-dairy fat spreads are covered by Codex Stan 256-2007.[59] In the Codex Alimentarius, trans fat to be labelled as such is defined as the geometrical isomers of monounsaturated and polyunsaturated fatty acids having non-conjugated [interrupted by at least one methylene group (-CH2-CH2-)] carbon-carbon double bonds in the trans configuration. This definition excludes specifically the healthy 'trans fats' (vaccenic acid and conjugated linoleic acid) which are present especially in human milk, dairy products, and beef.
Australia
The Australian federal government has indicated that it wants to pursue actively a policy of reducing trans fats from fast foods. The former federal assistant health minister, Christopher Pyne, asked fast food outlets to reduce their trans fat usage. A draft plan was proposed, with a September 2007 timetable, in order to reduce reliance on trans fats and saturated fats.[60] Currently, Australia's food labeling laws do not require trans fats to be shown separately from the total fat content. However, margarine in Australia has been free of trans fat since 1996.[61] In spite of the efforts mentioned above, Australia has chosen to define trans fats strictly as any fat containing a trans bond. In this sense Australia is diverting from codex (although having agreed on codex definition for trans fats), and also from the regulatory definitions implemented in the US, and EU member states regulations. Considering this, the present Australian/New Zealand food act is positioning human milk as rich (3-6%) in trans fat, and as such unacceptable for human use. Both scientifically and politically seen, this is an isolated position implicitly considering human milk as unhealthy. How the act positions beef meat and dairy products is another story altogether.
Canada
In November 2004, an opposition day motion seeking a ban similar to Denmark's was introduced by Jack Layton of the New Democratic Party, and passed through the House of Commons by an overwhelming 193-73 vote.[62] Like all Commons motions, it served as an expression of the views of the House but was not binding on the government and has no force under the law.
Since December 2005, Health Canada has required that food labels list the amount of trans fat in the nutrition facts section for most foods. Products with less than 0.2 grams of trans fat per serving may be labeled as free of trans fats.[63] These labelling allowances are not widely known, but as an awareness of them develops, controversy over truthful labelling is growing. In Canada, trans fat quantities on labels include naturally occurring trans fats from animal sources.[64]
In June 2006, a task force co-chaired by Health Canada and the Heart and Stroke Foundation of Canada recommended a limit of 5% trans fat (of total fat) in all products sold to consumers in Canada (2% for tub margarines and spreads).[29] The amount was selected such that "most of the industrially produced trans fats would be removed from the Canadian diet, and about half of the remaining trans fat intake would be of naturally occurring trans fats". This recommendation has been endorsed by the Canadian Restaurant and Foodservices Association[65] and Food & Consumer Products of Canada has congratulated the task force on the report,[66] although it did not recommend delaying implementation to 2010 as they had previously advocated.[67]
Ten months after submitting their report the Heart and Stroke Foundation of Canada and Toronto Public Health issued a plea to the government of Canada: "to act immediately on the task force's recommendations and to eliminate harmful trans fat from Canada's food supply."[68]
On June 20, 2007, the federal government announced its intention to regulate trans fats to the June 2006 standard unless the food industry voluntarily complied with these limits within two years.[69][70]
On January 1, 2008, Calgary became the first city in Canada to ban trans fats from restaurants and fast food chains. Trans fats present in cooking oils may not exceed 2% of the total fat content.[71] However, the replacement of local health regions with the Alberta Health Services Board in 2009 has temporarily eliminated all enforcement of the ban.[72]
Effective September 30, 2009, British Columbia became the first province in Canada to mandate the June 2006 recommendation in provincially regulated food services establishments.[73][74]
Denmark
Denmark became the first country to introduce laws strictly regulating the sale of many foods containing trans fats in March 2003, a move which effectively bans partially hydrogenated oils. The limit is 2% of fats and oils destined for human consumption. It should be noted that this restriction is on the ingredients rather than the final products. This regulatory approach has made Denmark the only country in which it is possible to eat "far less" than 1 g of industrially produced trans fats on a daily basis, even with a diet including prepared foods.[75] It is hypothesized that the Danish government's efforts to decrease trans fat intake from 6g to 1g per day over 20 years is related to a 50% decrease in deaths from ischemic heart disease.[76]
Switzerland
Switzerland followed Denmark's trans fats ban, and implemented its own beginning in April 2008.[77]
European Union
On request the European Food Safety Authority produced a scientific opinion on trans fatty acids.[78]
United Kingdom
In October 2005, the Food Standards Agency (FSA) asked for better labelling in the UK.[79] In the July 29, 2006 edition of the British Medical Journal, an editorial also called for better labelling.[80] In January 2007, the British Retail Consortium announced that major UK retailers, including Asda, Boots, Co-op, Iceland, Marks and Spencer, Sainsbury's, Tesco and Waitrose intend to cease adding trans fatty acids to their own products by the end of 2007.[81]
Sainsbury's became the first UK major retailer to ban all trans fat from all their own brand foods.
On 13 December 2007, the Food Standards Agency issued news releases stating that voluntary measures to reduce trans fats in food had already resulted in safe levels of consumer intake.[82][83]
On 15 April 2010, a BMJ (British Medical Journal) editorial called for trans fats to be "virtually eliminated in the United Kingdom by next year".[84]
United States
Before 2006, consumers in the United States could not directly determine the presence (or quantity) of trans fats in food products. This information could only be inferred from the ingredient list, notably from the partially hydrogenated ingredients. According to the FDA, the average American consumes 5.8 grams of trans fat per day (2.6% of calories.)[85]
On July 11, 2003, the Food and Drug Administration (FDA) issued a regulation requiring manufacturers to list trans fat on the Nutrition Facts panel of foods and some dietary supplements.[16][17] The new labeling rule became mandatory across the board, even for companies that petitioned for extensions, on January 1, 2008. However, unlike in many other countries, trans fat levels of less than 0.5 grams per serving can be listed as 0 grams trans fat on the food label.[86] According to a study published in the Journal of Public Policy & Marketing, without an interpretive footnote or further information on recommended daily value, many consumers do not know how to interpret the meaning of trans-fat content on the Nutrition Facts panel. In fact, without specific prior knowledge about trans fat and its negative health effects, consumers, including those at risk for heart disease, may misinterpret nutrient information provided on the panel.[18] The FDA did not approve nutrient content claims such as "trans fat free" or "low trans fat", as they could not determine a "recommended daily value". Nevertheless, the agency is planning a consumer study to evaluate the consumer understanding of such claims and perhaps consider a regulation allowing their use on packaged foods.[87] However, there is no requirement to list trans fats on institutional food packaging; thus bulk purchasers such as schools, hospitals, and cafeterias are unable to evaluate the trans fat content of commercial food items.[88] The FDA defines trans fats as containing one or more trans linkage that are not in a conjugated system. This is an important distinction, as it distinguishes non-conjugated synthetic trans fats from naturally occurring fatty acids with conjugated trans double bonds, such as conjugated linoleic acid.
Critics of the plan, including FDA advisor Dr. Carlos Camargo, have expressed concern that the 0.5 gram per serving threshold is too high to refer to a food as free of trans fat. This is because a person eating many servings of a product, or eating multiple products over the course of the day may still consume a significant amount of trans fat.[19] Despite this, the FDA estimates that by 2009, trans fat labeling will have prevented from 600 to 1,200 cases of coronary heart disease and 250 to 500 deaths each year. This benefit is expected to result from consumers choosing alternative foods lower in trans fats as well as manufacturers reducing the amount of trans fats in their products.
The American Medical Association supports any state and federal efforts to ban the use of artificial trans fats in U.S. restaurants and bakeries.[89]
The American Public Health Association adopted a new policy statement regarding trans fats in 2007. These new guidelines, entitled Restricting Trans Fatty Acids in the Food Supply, recommend that the government require nutrition facts labeling of trans fats on all commercial food products. They also urge federal, state, and local governments to ban and monitor use of trans fats in restaurants. Furthermore, the APHA recommends barring the sales and availability of foods containing significant amounts of trans fat in public facilities including universities, prisons, and day care facilities etc.[88]
Local regulation in the United States
Some US cities are acting to reduce consumption of trans fats. In May 2005, Tiburon, California, became the first American city wherein all restaurants voluntarily cook with trans fat-free oils.[90] Montgomery County, Maryland approved a ban on partially hydrogenated oils, becoming the first county in the nation to restrict trans fats.[91]
New York City embarked on a campaign in 2005 to reduce consumption of trans fats, noting that heart disease is the primary cause of resident deaths. This has included a Public education campaign (see trans fat pamphlet) and a request to restaurant owners to eliminate trans fat from their offerings voluntarily.[92] Finding that the voluntary program was not successful, New York City's Board of Health in 2006 solicited public comments on a proposal to ban artificial trans fats in restaurants.[93] The board voted to ban trans fat in restaurant food on December 5, 2006. New York was the first large US city to strictly limit trans fats in restaurants. Restaurants were barred from using most frying and spreading fats containing artificial trans fats above 0.5 g per serving on July 1, 2007, and were supposed to have met the same target in all of their foods by July 1, 2008.[94]
The Philadelphia City Council voted unanimously to pass a ban on February 8, 2007, which was signed into law on February 15, 2007, by Mayor John F. Street.[95][96] By September 1, 2007, eateries must cease frying food in trans fats. A year later, trans fat must not be used as an ingredient in commercial kitchens. The law does not apply to prepackaged foods sold in the city. On October 10, 2007, the Philadelphia City Council approved the use of trans-fats by small bakeries throughout the city.[97]
Albany County of New York passed a ban on trans fats. The ban was adopted after a unanimous vote by the county legislature on May 14, 2007. The decision was made after New York City's decision, but no plan has been put into place. Legislators received a letter from Rick J. Sampson, president and CEO of the New York State Restaurant Association, calling on them to "delay any action on this issue until the full impact of the New York City ban is known."
San Francisco officially asked its restaurants to stop using trans fat in January 2008. The voluntary program will grant a city decal to restaurants that comply and apply for the decal.[98] Legislators say the next step will be a mandatory ban.
Chicago also considered a ban on oils containing trans fats for large chain restaurants, and finally settled on a partial ban on oils and posting requirements for fast food restaurants.[99][100]
On December 19, 2006, Massachusetts state representative Peter Koutoujian filed the first state level legislation that would ban restaurants from preparing foods with trans fats.[101] The statewide legislation has not yet passed. However, the city of Boston did ban the sale of foods containing artificial trans fats at more than 0.5 grams per serving, which is similar to the New York City regulation; there are some exceptions for clearly labeled packaged foods and charitable bake sales.[102]
Maryland and Vermont were considering statewide bans of trans fats as of March 2007.[103][104]
King County of Washington passed a ban on artificial trans fats effective February 1, 2009.[105]
On July 25, 2008, California became the first state to ban trans fats in restaurants effective January 1, 2010.[106] California restaurants are prohibited from using oil, shortening, and margarine containing artificial trans fats in spreads or for frying, with the exception of deep frying donuts.[106][107][108] Donuts and other baked goods will be prohibited from containing artificial trans fats as of January 1, 2011.[106][107][108] Packaged foods are not covered by the ban and can legally contain trans fats.[109]
Food industry response
Manufacturer response
Palm oil, a natural oil extracted from the fruit of oil palm trees that is semi-solid at room temperature (15–25 degrees Celsius), is increasingly being used as an alternative to partially hydrogenated fats in baking and processed food applications.[110][111]
The J.M. Smucker Company, American manufacturer of Crisco (the original partially hydrogenated vegetable shortening), in 2004 released a new formulation made from solid saturated palm oil cut with soybean oil and sunflower oil. This blend yielded an equivalent shortening much like the previous partially hydrogenated Crisco, and was labelled zero grams of trans fat per 1 tablespoon serving (as compared with 1.5 grams per tablespoon of original Crisco).[112] As of January 24, 2007, Smucker claims that all Crisco shortening products in the US have been reformulated to contain less than one gram of trans fat per serving while keeping saturated fat content less than butter.[113] The separately marketed trans-fat free version introduced in 2004 was discontinued.
On May 22, 2004, Unilever, the corporate descendant of Joseph Crosfield & Sons (the original producer of Wilhelm Normann's hydrogenation hardened oils) announced that they have eliminated transfats from all their margarine products in Canada, including their flagship Becel brand.[114]
Agribusiness giant Bunge Limited, through their Bunge Oils division, are now producing and marketing an NT product line of non-hydrogenated oils, margarines and shortenings, made from corn, canola, and soy oils.[115]
Since 2003,[116] Loders Croklaan, a wholly-owned subsidiary of Malaysia's IOI Group has been providing trans fat free bakery and confectionery fats, made from palm oil, for giant food companies in the United States to make more heart healthy margarine.[117]
Major users' response
Some major food chains have chosen to remove or reduce trans fats in their products. In some cases these changes have been voluntary. In other cases, however, food vendors have been targeted by legal action that has generated a lot of media attention. In May 2003, BanTransFats.com Inc., a U.S. non-profit corporation, filed a lawsuit against the food manufacturer Kraft Foods in an attempt to force Kraft to remove trans fats from the Oreo cookie. The lawsuit was withdrawn when Kraft agreed to work on ways to find a substitute for the trans fat in the Oreo. In November 2006, Arby's announced[118] that by May 2007, it would be eliminating trans fat from its french fries and reducing it in other products.
Similarly, in 2006, the Center for Science in the Public Interest sued KFC over its use of trans fats in fried foods.[119] concerning their class action complaint.[120] KFC reviewed alternative oil options, saying "there are a number of factors to consider including maintaining KFC's unique taste and flavor of Colonel Sanders' Original Recipe".[121] On October 30, 2006, KFC announced that it will replace the partially hydrogenated soybean oil it currently uses with a zero-trans-fat low linoleic soybean oil in all restaurants in the US by April 2007, although its biscuits will still contain trans-fats.[122] Despite the US-specific nature of the lawsuit, KFC is making changes outside of the US as well; in Canada, KFC's brand owner is switching to trans-fat free Canadian canola oil by early 2007.[123] Wendy's announced in June 2006 plans to eliminate trans-fats from 6,300 restaurants in the United States and Canada, starting in August 2006.[124] In November 2006, Taco Bell made a similar announcement, pledging to remove Trans Fat from many of their menu items by switching to canola oil. By April 2007, 15 Taco Bell menu items were completely free of Trans Fat. In January 2007, McDonald's announced they will start phasing out the trans fat in their fries after years of testing and several delays.[125] This can be partially attributed to New York's recent ban, with the company stating they would not be selling a unique oil just for New York customers but would implement a nationwide change. Chick-fil-A's menu is Trans Fat free as of October 9, 2007.[126] Raising Canes fast food chicken restaurant recently tested a trans-fat free chicken strip, but there is no plan to reduce their current menu due to the new strip being considered tasting "unsatisfactory."
In response to a May 2007 law suit from the Center for Science in the Public Interest, Burger King announced that its 7,100 US restaurants will begin the switch to zero trans-fat oil by the end of 2007.[127]
The Walt Disney Company announced that they will begin getting rid of trans fats in meals at US theme parks by the end of 2007, and will stop the inclusion of trans fats in licensed or promotional products by 2008.[128]
The Girl Scouts of America announced in November 2006 that all of their cookies will contain less than 0.5g trans fats per serving, thus meeting or exceeding the FDA guidelines for the "zero trans fat" designation.[129]
Health Canada's monitoring program, which tracks the changing amounts of TFA and SFA in fast and prepared foods shows considerable progress in TFA reduction by some industrial users while others lag behind. In many cases, SFAs are being substituted for the TFAs.[130][131]
See also
References
- ^ a b Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 423.
- ^ a b c d Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 504.
- ^ "Trans fat: Avoid this cholesterol double whammy". Mayo Foundation for Medical Education and Research (MFMER). Retrieved 2007-12-10.
- ^ a b c d e Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC (April 13, 2006). "Trans Fatty Acids and Cardiovascular Disease". New England Journal of Medicine. 354 (15): 1601–1613. doi:10.1056/NEJMra054035. PMID 16611951.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nobel Lectures, Chemistry, 1901–1921. Elsevier. 1966. Reprinted online: "Paul Sabatier, The Nobel Prize in Chemistry 1912". Nobel Foundation. Retrieved 2007-01-07.
- ^ de 141029 Process for converting unsaturated fatty acids or their glycerides into saturated compounds
- ^ gb 190301515 Process for converting unsaturated fatty acids or their glycerides into saturated compounds
- ^ Patterson, HBW (1998). "Hydrogenation" (PDF). Sci Lecture Papers Series. Retrieved 2007-01-07.
- ^ "Normann bio (in German)".
- ^
Shurtleff, William. "History of Soybeans and Soyfoods: 1100 B.C. to the 1980s". Archived from the original on 2005-10-18.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Wilhelm Normann — Erfinder der Fetthärtung(in German)".
- ^ Fred A. Kummerow (2008). Cholesterol Won't Kill You — But Trans Fat Could. Trafford Publishing. ISBN 142513808X.
- ^ a b
Ascherio A, Stampfer MJ, Willett WC. "Trans fatty acids and coronary heart disease". Retrieved 2006-09-14.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^
Booyens J, Louwrens CC, Katzeff IE (1988). "The role of unnatural dietary trans and cis unsaturated fatty acids in the epidemiology of coronary artery disease". Medical Hypotheses. 25 (3): 175–182. doi:10.1016/0306-9877(88)90055-2. PMID 3367809.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Willett WC, Ascherio A (1995). "Trans fatty acids: are the effects only marginal?". American Journal of Public Health. 85 (3): 411–412. PMC 1615057. PMID 8179036.
- ^ a b Regulation: 21 CFR 101.9 (c)(2)(ii). Food and Drug Administration (2003-07-11). "21 CFR Part 101. Food labeling; trans fatty acids in nutrition labeling; consumer research to consider nutrient content and health claims and possible footnote or disclosure statements; final rule and proposed rule" (PDF). National Archives and Records Administration. Retrieved 2007-01-18.
- ^ a b "FDA acts to provide better information to consumers on trans fats". Food and Drug Administration. Retrieved 2005-07-26.
- ^ a b "Newswise: most consumers misinterpret meaning of trans-fat information on Nutrition Facts panel". Retrieved 2008-06-19.
- ^ a b Shockman, Luke (2005-12-05). "Trans fat: 'Zero' foods add up". Toledo Blade. Retrieved 2007-01-18.
- ^ "CBC Trans Fats Headed for the Exit".
- ^ "Trans fat free future".
- ^
Alonso L, Fontecha J, Lozada L, Fraga MJ, Juárez M (1999). "Fatty acid composition of caprine milk: major, branched-chain, and trans fatty acids". Journal of Dairy Science. 82 (5): 878–84. PMID 10342226.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Alfred Thomas (2002). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_173.
- ^
Food lipids: chemistry, nutrition, and biotechnology. New York: M. Dekker. 2002. pp. 1–2. ISBN 0824707494.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^
IUPAC Gold book. International Union of Pure and Applied Chemistry.
{{cite book}}: Unknown parameter|section_title=ignored (help) - ^ Hill John W, Kolb Doris K (2007). Chemistry for changing times. Pearson / Prentice Hall.
- ^ a b
"Section 7: Biochemistry". Handbook of chemistry and physics. 2007-2008 (88th ed.). Taylor and Francis. 2007. Retrieved 2007-11-19.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Eller FJ; List, GR; Teel, JA; Steidley, KR; Adlof, RO (2005). "Preparation of spread oils meeting U.S. Food and Drug Administration labeling requirements for trans fatty acids via pressure-controlled hydrogenation". Journal of Agricultural and Food Chemistry. 53 (15): 5982–5984. doi:10.1021/jf047849. PMID 16028984.
- ^ a b c d Trans Fat Task Force (2006). "TRANSforming the Food Supply". Retrieved 2007-01-07.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|month=ignored (help) - ^ Hunter, JE (2005). "Dietary levels of trans fatty acids" basis for health concerns and industry efforts to limit use". Nutrition Research. 25: 499–513. doi:10.1016/j.nutres.2005.04.002.
- ^ Innis, Sheila M and King, D Janette (September 1, 1999). "trans fatty acids in human milk are inversely associated with concentrations of essential all-cis n-6 and n-3 fatty acids and determine trans, but not n-6 and n-3, fatty acids in plasma lipids of breast-fed infants". American Journal of Clinical Nutrition. 70 (3): 383–390. PMID 10479201.
{{cite journal}}: CS1 maint: multiple names: authors list (link) PMID 10479201 - ^ NYC Board of Health. "Board of Health Approves Regulation to Phase Out Artificial Trans Fat: FAQ". Retrieved 2007-01-07.
- ^ "What's in that french fry? Fat varies by city". MSNBC. 2006-04-12. Retrieved 2007-01-07. AP story concerning PMID 16611965
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for [[Energy]], [[Carbohydrate]], [[Fiber]], [[Fat]], [[Fatty Acid]]s, [[Cholesterol]], [[Protein]], and [[Amino Acid]]s (Macronutrients). National Academies Press. pp. i.
{{cite book}}: URL–wikilink conflict (help) - ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 447.
- ^ Food and nutrition board, institute of medicine of the national academies (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press. p. 424.
- ^ National Dairy Council (2004-06-18). "comments on 'Docket No. 2003N-0076 Food Labeling: Trans Fatty Acids in Nutrition Labeling'" (PDF). Retrieved 2007-01-07.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c d e Trans Fat Task Force (2006). "TRANSforming the Food Supply (Appendix 9iii)". Retrieved 2007-01-09.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|month=ignored (help) (Consultation on the health implications of alternatives to trans fatty acids: Summary of Responses from Experts) - ^ Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC (2006). "Trans fatty acids and cardiovascular disease". N. Engl. J. Med. 354 (15): 1601–13. doi:10.1056/NEJMra054035. PMID 16611951.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Hu, FB (1997). "Dietary fat intake and the risk of coronary heart disease in women" (PDF). New England Journal of Medicine. 337 (21): 1491–1499. doi:10.1056/NEJM199711203372102. PMID 9366580. Retrieved 2009-06-22.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) PMID 9366580. - ^ Oh, K (2005). "Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses' health study". American Journal of Epidemiology. 161 (7): 672–679. doi:10.1093/aje/kwi085. PMID 15781956.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) PMID 15781956 - ^ A Ascherio; Katan, MB; Zock, PL; Stampfer, MJ; Willett, WC (1999). "Trans fatty acids and coronary heart disease". New England Journal of Medicine. 340 (25): 1994–1998. doi:10.1056/NEJM199906243402511. PMID 10379026.
- ^
Gatto, Lissa M (2003). "Postprandial effects of dietary trans fatty acids on apolipoprotein(a) and cholesteryl ester transfer" (PDF). Am J Clin Nutr. 77: 1119–1124.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lopez-Garcia, Esther; S; M; M; R; S; W; H (March 1, 2005). "Consumption of Trans Fatty Acids Is Related to Plasma Biomarkers of Inflammation and Endothelial Dysfunction". The Journal of Nutrition. 135 (3): 562–566. PMID 15735094.
- ^
Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Schneider J, Wilson RS (2003). "Dietary fats and the risk of incident Alzheimer disease". Arch Neurol. 60 (2): 194–200. doi:10.1001/archneur.60.2.194. PMID 12580703.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ American Cancer Society. "Common questions about diet and cancer". Retrieved 2007-01-09.
- ^ Jorge, Chavarro (April 1, 2006). "A prospective study of blood trans fatty acid levels and risk of [[prostate cancer]]". Proc. Amer. Assoc. Cancer Res. 47 (1). American Association for Cancer Research: 943. Retrieved 2007-01-09.
{{cite journal}}: URL–wikilink conflict (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Breast cancer: a role for trans fatty acids?" (Press release).
- ^ Chajès V, A. Thiébaut CM, Rotival M, Gauthier E, Maillard V; Boutron-Ruault MC, Joulin V, Lenoir GM, Clavel-Chapelon F (2008). "Serum trans-monounsaturated fatty acids are associated with an increased risk of breast cancer in the E3N-EPIC Study". Am. J. Epidemiol. 167 (11): 1312. doi:10.1093/aje/kwn069. PMC 2679982. PMID 18390841.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hu FB, van Dam RM, Liu S (2001). "Diet and risk of Type II diabetes: the role of types of fat and carbohydrate". Diabetologia. 44 (7): 805–817. doi:10.1007/s001250100547. PMID 11508264.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ van Dam RM, Stampfer M, Willett WC, Hu FB, Rimm EB (2002). "Dietary fat and meat intake in relation to risk of type 2 diabetes in men". Diabetes care. 25 (3): 417–424. doi:10.2337/diacare.25.3.417. PMID 11874924.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gosline, Anna (2006-06-12). "Why fast foods are bad, even in moderation". New Scientist. Retrieved 2007-01-09.
- ^ "Six years of fast-food fats supersizes monkeys". New Scientist (2556): 21. 2006-06-17.
- ^ Kavanagh, K; Jones, KL; Sawyer, J; Kelley, K; Carr, JJ; Wagner, JD; Rudel, LL (2007-07-15). "Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys". Obesity (Silver Spring). 15 (7): 1675–84. doi:10.1038/oby.2007.200. PMID 17636085.
- ^ e.g. Thompson Tommy G. "Trans Fat Press Conference"., US Secretary of health and human services
- ^ Mahfouz M (1981). "Effect of dietary trans fatty acids on the delta 5, delta 6 and delta 9 desaturases of rat liver microsomes in vivo". Acta biologica et medica germanica. 40 (12): 1699–1705. PMID 7345825.
- ^ Chavarro Jorge E, Rich-Edwards Janet W, Rosner Bernard A and Willett Walter C (2007-01). "Dietary fatty acid intakes and the risk of ovulatory infertility". American Journal of Clinical Nutrition. 85 (1): 231–237. PMID 17209201.
{{cite journal}}: Check date values in:|date=(help); More than one of|number=and|issue=specified (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "CODEX STAN 19-1999" (PDF).
- ^ CODEX STAN 256 – 2007 "Standard for Fat Spreads and Blended Spreads" (PDF file)
- ^ "Fast food outlets asked to cut down trans fat usage". ABC. March 12, 2007. Retrieved 2007-03-12.
- ^ Peter M. Clifton, Jennifer B. Keogh, and Manny Noakes (April 1, 2004). "Trans fatty acids in adipose tissue and the food supply are associated with myocardial infarction". The Journal of Nutrition. 134 (4). Amer Inst Nutrition: 874–879. ISSN 0022-3166. PMID 15051840. Retrieved 2007-12-26.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "The motion and the vote in the Canadian House of Commons on November 23, 2004". Retrieved 2007-06-07.
- ^ "Canadian Regulations" (PDF).
- ^ Canadian Food Inspection Agency. "Information letter: Labelling of trans fatty acids". Retrieved 2007-01-18.
- ^ Canadian Restaurant and foodservices association. "Restaurant industry commits to Trans Fat Task Force recommendations". Retrieved 2007-01-18.
- ^ Food & Consumer Products of Canada (2006-06-08). "Food industry congratulates trans fat task force on report" (PDF) (Press release). Retrieved 2007-01-18.
- ^ "Cut trans fats from food supply, health groups tell Ottawa". CBC News. Retrieved 2007-06-21.
- ^ "In Depth — Trans fats". CBC News. Retrieved 2007-06-07.
- ^ Health Canada. "Canada's New Government Calls on Industry to Adopt Limits for Trans Fat". Retrieved 2007-06-20.
- ^ "Health Canada delays trans fat regulations". CBC News. Retrieved 2007-06-21.
- ^ "Calgary moves against trans fats". CBC News. 2007-12-29. Retrieved 2008-01-13.
- ^ "Calgary's trans fat ban fizzles". CBC News. 12 March 2009.
- ^ "Province restricts trans fat in B.C." (Press release). British Columbia Ministry of Healthy Living and Sport. March 7, 2009.
- ^ "B.C. tackles trans fat in food service establishments" (Press release). British Columbia Ministry of Healthy Living and Sport. September 30, 2009.
- ^ Stender, Steen (2006). "A trans world journey". Atherosclerosis Supplements. 7 (2). Elsevier: 47–52. doi:10.1016/j.atherosclerosissup.2006.04.011. PMID 16713385.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Stender S, Dyerberg J (2004). "Influence of trans fatty acids on health". Ann. Nutr. Metab. 48 (2): 61–6. doi:10.1159/000075591. PMID 14679314.
- ^ "Deadly fats: why are we still eating them?". London: The Independent. 2008-06-10. Retrieved 2008-06-16.
- ^
"Opinion of the Scientific Panel on Dietetic products, nutrition and allergies [[NDA]] related to the presence of trans fatty acids in foods and the effect on human health of the consumption of trans fatty acids. Question number: EFSA-Q-2003-022". 2004. Retrieved 20 February 2009.
{{cite web}}: URL–wikilink conflict (help) - ^ Gray, Richard (February 5, 2006). "Forced to own up to the killer fat in our food". Scotsman.com. Retrieved 2007-01-18.
- ^ "Call to label hidden fats in food". BBC. 2006-07-20. Retrieved 2007-01-18. reporting on Clarke, Robert (2006-07-29). "Trans fatty acids and coronary heart disease". British Medical Journal. 333 (7561): 214. doi:10.1136/bmj.333.7561.214. PMC 1523500. PMID 16873835. Retrieved 2007-01-18.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Retailers to stop trans-fat use". BBC. January 31, 2007. Retrieved 2007-01-31.
- ^ Food Standards Agency Board recommends voluntary approach for trans fats 13 December 2007
- ^ Food Standards Agency FSA Board to advise the Department of Health to maintain successful voluntary approach for trans fats in food 13 December 2007
- ^ Dariush Mozaffarian, Meir J Stampfer Removing industrial trans fat from foods: A simple policy that will save lives BMJ 2010;340:c1826
- ^ "Revealing 'trans' fats" US Food and Drug Administration
- ^ "FDA requires trans fatty acid labeling for foods and dietary supplements" article by George Misko from Food & Drug Packaging on AllBusiness.com, October 1 2003, retrieved June 2, 2010
- ^ Food and Drug Administration (2003-07-11). "FDA food labeling: trans fatty acids in nutrition labeling; consumer research to consider nutrient content and health claims and possible footnote or disclosure statements". p. 41059. Retrieved 2007-01-18.
- ^ a b American Public Health Association. "Restricting trans fatty acids in the food supply". Retrieved 2008-02-28.
- ^ American Medical Association. "American Medical Association position on trans fats". Retrieved 2008-11-10.
- ^ BanTransFats.com. "Project Tiburon: America's first trans fat-free city!!!". Retrieved 2007-01-18.
- ^ Spivack, Miranda S. (2007-05-16). "Montgomery bans trans fats in restaurants, markets". The Washington Post. Retrieved 2007-06-28.
- ^ "Health department asks restaurateurs and food suppliers to voluntarily make an oil change and eliminate artificial trans fat" (Press release). City of New York. 2005-08-10. Retrieved 2007-01-18.
- ^ "Health department proposes two changes to city's health code for public comment" (Press release). City of New York. 2006-09-26. Retrieved 2007-01-18.
- ^ "Board of health votes to phase out artificial trans fat from New York City's restaurants" (Press release). City of New York. 2006-12-05. Retrieved 2007-01-18.
- ^ Kerkstra, Patrick; Stoiber, Julie (2007-02-09). "Ban gives Phila. a healthy lead in trans-fat fight". Philadelphia Inquirer. Retrieved 2007-02-23.
- ^ McCaffrey, Jim (2007-02-16). "Street signs trans-fat ban bill". The Evening Bulletin. Retrieved 2007-02-23.
- ^ "Amending Section 6-307 of The Philadelphia Code, entitled 'Foods containing artificial trans fats,' by exempting certain bakeries from the provisions prohibiting the use of artificial trans fats, under certain terms and conditions" (Press release). Philadelphia City Council. 2007-10-10. Retrieved 2007-10-11.
- ^ "San Francisco restaurants asked to ban trans fats". The Union Tribune. 2008-02-02. Retrieved 2008-02-02.
- ^ "No more trans fat". Chicago Sun-Times. 2007-07-02. Retrieved 2008-02-02.
- ^ Davey, Monica (2006-07-18). "Chicago weighs new prohibition: bad-for-You fats". New York Times. Retrieved 2007-01-18.
- ^ "Lawmaker wants to ban trans fats from Mass. restaurants". Boston Globe. 2006-12-19. Retrieved 2007-03-20.
- ^ "Trans Fats Facts and Information". Boston Public Health Commission. Retrieved 2008-12-08.
- ^ "Trans Fat Ban Considered in Maryland". Associated Press. 2007-03-08. Retrieved 2007-03-20.
- ^ "Trans fat ban bill proposed in Senate". Daniel Barlow Vermont Press Bureau. 2007-03-10. Retrieved 2007-03-20.
- ^
Black, Cherie. "King County restaurants told to phase out trans fats". Seattle P-I date = 2007-06-19. Retrieved 2007-07-16.
{{cite web}}: Missing pipe in:|work=(help) - ^ a b c McGreevy, Patrick (2008-07-25). "Gov. Schwarzenegger signs law banning trans fats in restaurants". Los Angeles Times. Retrieved 2008-07-25.
- ^ a b Sondag, Samantha (2008-07-25). "Gov. signs nation's first statewide ban on trans fats in restaurants". San Francisco Chronicle. Retrieved 2008-07-25.
- ^ a b "Assembly Bill No. 97" (PDF). California State Assembly. 2008-07-25. Retrieved 2008-07-25.
- ^ Sanders, Jim (2008-07-25). "Schwarzenegger wages war on trans fats". McClatchy Newspapers. The Atlanta Journal-Constitution. Retrieved 2008-07-25.
- ^ Palm oil 'reasonable' replacement for trans fats, say experts Food Navigator, 16 Dec 2005
- ^ Palm oil blend could offer trans-fat free shortenings for bakery: Study Food Navigator, 17 Feb 2009
- ^ Crisco. "Crisco 0 Grams Trans Fat Per Serving All-Vegetable Shortening". Retrieved 2007-01-18.
- ^ "Crisco Frequently Asked Questions." Crisco. Retrieved on September 13, 2007.
- ^ "List of Canadian industry actions to reduce transfats". Food & Consumer Products of Canada (FCPC). Retrieved on September 13, 2007.
- ^ "Bunge Oils".
- ^ Loders builds on trans fat alternatives Food Navigator, 5 Feb 2004
- ^ Loders Croklaan targets trans fat free demand By Anthony Fletcher, Food Navigator, 10 Nov 2005
- ^ Turner, Dorie (2006-11-29). "Arby's Announces Trans Fat Reduction". USA Today. Associated Press. Retrieved 2007-12-13.
- ^ "KFC Sued for Fouling Chicken with Partially Hydrogenated Oil: Lawsuit Aimed at Eliminating, or Disclosing Use of Artery-Clogging Frying Oil" (Press release). Center for Science in the Public Interest. 2006-06-12. Retrieved 2007-01-18.
- ^ "Class action complaint" (PDF). 2006-06-12. Retrieved 2007-01-18.
- ^ Burros, Marian (2006-06-14). "KFC Is Sued Over the Use of Trans Fats in Its Cooking". New York Times. Retrieved 2007-01-18.
- ^ "KFC announces switch to zero trans fat cooking oil following two-year test for same great taste" (Press release). KFC. 2006-10-30. Retrieved 2007-01-18.
- ^ "KFC Canada phasing in zero grams trans fat menu in all 786 restaurants nationally early in the new year" (Press release). KFC Canada. 2006-10-30. Retrieved 2007-01-18.
- ^ "Wendy's Significantly Cuts Trans Fats — Switch to New Cooking Oil Under Way" (Press release). Wendy's. 2006-06-08. Retrieved 2007-01-18.
- ^ McDonald’s finally picks trans-fat-free oil. [[MSNBC. January 30, 2007. Retrieved on September 13, 2007]
- ^ "Bot generated title ->". MarketWatch.com<!. Retrieved 2010-01-03.
- ^ Adrian Sainz (2006-07-06). "Burger King to Use Trans-Fat-Free Oil" (Press release). AP. Retrieved 2007-07-06.
- ^ "The Walt Disney Company Introduces New Food Guidelines To Promote Healthier Kids' Diets" (Press release). Walt Disney Company. 2006-10-16. Retrieved 2007-09-12.
- ^ "Statement from GSUSA CEO Kathy Cloninger: Girl Scout Cookies Now Have Zero Trans Fats" (Press release). 2006-11-13. Retrieved 2008-02-26.
- ^ "Health Canada. Trans Fat Monitoring Program".
- ^ "CBC News Trans-fat levels dropping, though Burger King in the hot seat 2007-12-20 accessed 2007-12-21".
Further reading
- Dijkstra Albert, Hamilton Richard J., Hamm Wolf (eds.) (2008). Trans Fatty Acids. Blackwell. ISBN 9781405156912.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - Jang ES, Jung MY, Min DB (2005). "Hydrogenation for Low Trans and High Conjugated Fatty Acids" (PDF). Comprehensive Reviews in Food Science and Food Safety. 1.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links and further reading
- Trans-fat and the Link to Lipoproteins & Exercise
- Ban Trans Fat
- "Ban the Trans: These Sorry Lipids Should Go Away"
- Center for Science in the Public Interest Trans Fat Page
- Chemical Structure of Fats and Fatty Acids
- No Trans Club Malaysia
- Federal Register - 68 FR 41433 July 11, 2003: Food Labeling; Trans Fatty Acids in Nutrition Labeling; Consumer Research to Consider Nutrient Content and Health Claims and Possible Footnote or Disclosure Statements; Final Rule and Proposed Rule