Pneumothorax: Difference between revisions
Snowmanradio (talk | contribs) →Classification: ref points |
Snowmanradio (talk | contribs) →Classification: readability |
||
| Line 26: | Line 26: | ||
==Classification== |
==Classification== |
||
Spontaneous pneumothoraces are divided into two types: |
Spontaneous pneumothoraces are divided into two types: primary, which occurs in the absence of apparent lung disease, and secondary, which occurs in the presence of underlying lung disease.<ref name=Rosen2010/> |
||
===Primary spontaneous=== |
===Primary spontaneous=== |
||
Revision as of 18:51, 4 January 2012
| Pneumothorax | |
|---|---|
| Specialty | Emergency medicine, pulmonology, cardiothoracic surgery |
Pneumothorax (plural pneumothoraces) is an abnormal collection of air or gas in the chest between the layers of the pleura, which are normally closely applied. The gas separates the lung from the chest wall and can compromise respiration. A primary pneumothorax is a pneumothorax that occurs spontaneously, without an apparent cause, in the absence of lung disease, while a secondary pneumothorax is a pneumothorax that occurs in the presence of pre-existing lung disease. In addition, pneumothoraces can be caused by physical trauma to the chest, including blast injury and as a complication of medical or surgical intervention.
The symptoms of a pneumothorax are mainly determined by its size and its rate of expansion. Symptoms generally include chest pain and often shortness of breath. Diagnosis by physical examination can be difficult or inconclusive, especially for the smaller pneumothoraces, so the diagnosis is generally confirmed or made with a chest X-ray or sometimes a computed tomography (CT) scan.
Occasionally, the pressure of the air in a pneumothorax increases leading to a tension pneumothorax, which is a medical emergency. When the pressure of the air in the tension pneumothorax increases significantly, the internal mid-line chest structures can be pushed towards the opposite side of the chest compromising the functioning of both lungs. This can lead to oxygen shortage and low blood pressure, progressing to cardiac arrest and death unless treated.
Small spontaneous pneumothoraces typically resolve by themselves and may require monitoring, but no treatment, especially in those with no underlying lung disease. In larger pneumothoraces or when there are marked symptoms, the air may be extracted with a syringe or a one-way chest tube. Occasionally, surgical measures are required, either when tube drainage is unsuccessful or if someone has experienced repeated episodes. The surgical treatments usually involve pleurodesis, where the layers of pleura are stuck together, or pleurectomy, the removal of pleural membranes.
Anatomy and physiology
The thoracic cavity is the hollow space that contains the lungs. The lungs are physically connected at the hila, where the airways and blood vessels enter the lung. They remain inflated inside the thoracic cavity because the pressure inside the pleural space (the potential space between the chest wall and the lung) is almost consistently negative throughout the respiratory cycle, effectively sucking the lung to the chest wall. On each side of the chest a pleural membrane covers the surface of lung (visceral pleura) and also lines the inside of the chest wall (parietal pleura). Normally the two layers are closely applied and only contain a small amount of lubricating serous fluid. Despite the negative pressure, air does not enter the pleural space, because there are no natural connections to an air-containing passage and the pressure of gases in the bloodstream is too low for them to be released into the pleural space.[1]
Classification
Spontaneous pneumothoraces are divided into two types: primary, which occurs in the absence of apparent lung disease, and secondary, which occurs in the presence of underlying lung disease.[2]
Primary spontaneous
The exact cause of primary spontaneous pneumothorax is unknown, but established risk factors include male sex, smoking, and a family history of pneumothorax.[2] The various suspected underlying mechanisms are discussed below.[3][1]
Secondary spontaneous
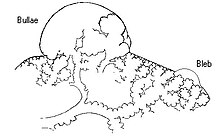
Secondary spontaneous pneumothorax occurs in the setting of a variety of lung diseases. The most common is chronic obstructive pulmonary disease which accounts for approximately 70% of cases.[2] Known lung diseases that may increase the risk for pneumothorax are:[3][1]
- Diseases of the airways: chronic obstructive pulmonary disease (especially when emphysema and lung bullae are present), acute severe asthma, cystic fibrosis
- Lung infections: pneumocystis pneumonia (PCP), tuberculosis, necrotizing pneumonia
- Interstitial lung diseases: sarcoidosis, idiopathic pulmonary fibrosis, histiocytosis X, lymphangioleiomyomatosis (LAM)
- Connective tissue diseases: rheumatoid arthritis, ankylosing spondylitis, polymyositis and dermatomyositis, systemic sclerosis, Marfan's syndrome and Ehlers-Danlos syndrome
- Cancer: lung cancer, sarcomas involving the lung
- Catamenial (occurring in relation to the menstrual cycle): endometriosis in the chest
In children, additional causes include measles, echinococcosis, inhalation of a foreign body, and particular congenital malformations (congenital cystic adenomatoid malformation and congenital lobar emphysema).[4]
A rare genetic disorder, Birt-Hogg-Dubé syndrome, may cause spontaneous pneumothorax in families. It also causes skin lesions (fibrofolliculomas) and lung cysts, and carries an increased risk of kidney cancer. The lung cysts, which probably lead to increased risk of pneumothorax, tend to be in the lower lobes rather than the more common upper lobe cysts encountered in other conditions.[5] Birt-Hogg-Dubé syndrome is caused by mutations in the FLCN gene (chromosome 17p11.2), which encodes a protein named folliculin.[4][5] FLCN mutations and lung lesions have also been identified in familial cases of pneumothorax where other features of Birt-Hogg-Dubé syndrome are absent.[4]
Traumatic

A = air. B = fluid
A traumatic pneumothorax may result from either blunt trauma or penetrating injury to the chest wall.[1] It may be observed in those exposed to an explosive blast, even if no direct injury to the chest has occurred.[6] The most common mechanism is due to sharp bony points at a new rib fracture penetrating pleura and damaging lung tissue.[2]
Medical procedures of the chest (iatrogenic), such as the taking of biopsy samples from lung tissue, inserting a central venous catheter into one of the chest veins, may lead to injury to the lung and resultant pneumothorax. The administration of positive pressure ventilation, either mechanical ventilation or non-invasive ventilation, may result in barotrauma (pressure-related injury) leading to a pneumothorax.[1]
Pathological anatomy and pathophysiology
A pneumothorax can only develop when air can fill pleural cavity, either through damage in the chest wall, damage or disease of lung tissue, or occasionally because of gas producing microorganisms.[1] Physical injury can directly damage the chest wall or lung tissue, as seen in penetrating wounds or fractured ribs.[3]

In secondary spontaneous pneumothorax, vulnerabilities in the lung tissue are caused by a variety of disease processes, such as bullae (large air-containing lesions) in emphysema. Areas of necrosis (tissue death) may precipitate pneumothorax episodes, although the exact mechanism is unclear.[3] Primary spontaneous pneumothorax has for many years been thought to be caused by "blebs", small lesions just under the pleural surface, which were presumed to be more common in those classically at risk of pneumothorax (tall males) due to mechanical factors. Various lines of evidence suggest that this hypothesis may not be correct, such as the fact that pneumothorax may recur even after surgical treatment of blebs, and that blebs occur in 15% of healthy people. It has therefore been suggested that PSP is instead caused by areas of disruption (porosity) in the pleural layer, which are prone to rupture.[3][1] Smoking may lead to inflammation and obstruction of small airways, accounting for the markedly increased risk of PSP in smokers.[7] Once air has stopped entering the pleural cavity, it is gradually resorbed spontaneously. Estimated rates of resorption are between 1.25% and 2.2% the volume of the cavity per day. This would mean that even a completely collapsed lung would spontaneously reinflate over a period of about 6 weeks.[7]
Tension pneumothorax occurs because the opening that allows air to enter the pleural space functions like a valve, and with every breath more air enters and cannot escape. Severe hypoxia follows, with a resultant drop in blood pressure and level of consciousness. A previously uttered theory that the collapsed lung compresses large blood vessels such as the aorta is probably incorrect.[8]
Clinical features
Symptoms
Primary spontaneous pneumothorax (PSP), which tends to occur in young people without underlying lung problems, usually causes limited symptoms. Chest pain and sometimes mild breathlessness are the predominant symptoms.[3][1] Half of those with primary spontaneous pneumothorax wait several days to seek medical attention.[7] It is rare for PSP to cause tension pneumothorax. The symptoms usually start at rest. Tall males, especially smokers, have a higher risk of PSP.[3] It occurs more commonly during changes in atmospheric pressure and during exposure to loud music, and this explains to an extent why episodes of pneumothorax may happen in clusters.[1]
Secondary spontaneous pneumothorax (SSP) occurs by definition in those with underlying lung diseases. The symptoms tend to be more severe, as the unaffected lung is generally not capable of replacing the loss of function from the affected side. Hypoxemia (decreased blood oxygen levels) is usually present and may be observed as cyanosis (blue discoloration of the lips and skin). Hypercapnia (accumulation of carbon dioxide in the blood) is sometimes encountered; this may cause confusion and coma. Sudden breathlessness in someone with lung problems such as chronic obstructive pulmonary disease or cystic fibrosis may therefore prompt investigations for a possible pneumothorax.[3] The size of the pneumothorax bears limited relationship to the symptoms experienced.[7]
Traumatic pneumothorax occurs either because a hole in the chest wall, such as a stab wound or gunshot wound, allows air to enter the pleural space, or because of injury to the lung. It has been found to occur in half of all cases of injury to the chest, coming second after rib fracture in the complications after chest trauma. The pneumothorax can be small in half of these cases, but they may enlarge if the person requires mechanical ventilation and their presence is therefore still relevant.[1] It is also often encountered in those already receiving mechanical ventilation.[1][8]
Tension pneumothorax is defined differently by different sources,[8] but is generally said to exist when there is severe hypoxia despite administration of oxygen, falling blood pressure or confusion. This is a medical emergency and may require immediate treatment without further investigations (see below).[7][8] Tension pneumothorax may also occur in those receiving mechanical ventilation, in which case it may be difficult to spot as the person is typically sedated; it is often noted because of sudden deterioration.[8]
Physical examination
In pneumothorax, breath sounds, as audible using a stethoscope, may be diminished on the affected side, partly because air in the pleural space dampens sound. Percussion of the chest may sound hyperresonant (like a booming drum), and vocal resonance and tactile fremitus (both a measure of the conduction of vocal vibrations to the surface of the chest) can be decreased. However, there may be no apparent physical signs, especially if the pneumothorax is small.[1][7]
Tension pneumothorax is characterized by rapid breathing, cyanosis, falling blood pressure (hypotension) and confusion. The affected side of the chest may be hyperexpanded and show decreased movement, with increased movement on the other side. In very severe cases, the respiratory rate falls sharply, with shock and coma. Recent studies have shown that the development of tension features may not always be as rapid as previously thought. Particular clinical signs may also be less useful in the recognition of tension pneumothorax, such as the deviation of the trachea (windpipe) to one side and the presence of raised jugular venous pressure (distended neck veins).[8]
Diagnosis
The symptoms of pneumothorax can be vague and inconclusive, especially in those with a small PSP, and confirmation with medical imaging is usually required.[7] In contrast, tension pneumothorax is a medial emergency and may be treated before imaging, especially if there is severe hypoxia, very low blood pressure, or an impaired level of consciousness. In tension pneumothorax, X-rays are sometimes required if there is doubt about the anatomy of the pneumothorax.[8][2]
Chest X-ray

Traditionally a plain radiograph of the chest, ideally with the X-ray beams being projected from the back (posteroanterior or PA), has been the most appropriate first investigation. Usually, these are performed in inspiration (holding one's breath); no added information is gathered by obtaining a chest X-ray in expiration (after exhaling).[1][7] If the PA X-ray does not show a pneumothorax but there is a strong suspicion, lateral X-rays (with beams projecting from the side) may be performed, but this is not routine practice.[7][4] It is not unusual for the mediastinum (the structure between the lungs that contains the heart, great blood vessels and large airways) to be shifted away from the affected lung due to pressure. This is not equivalent to tension pneumothorax, which is determined mainly by symptoms, hypoxia and shock.[1]
The size of the pneumothorax, i.e. the volume of air in the pleural space, can be determined with a reasonable degree of a accuracy by measuring the distance between the chest wall and the lung. This is relevant as smaller pneumothoraces may be treated differently. An air rim of 2 cm means that the pneumothorax occupies about 50% of the hemithorax.[7] British professional guidelines have traditionally stated that the measurement should be performed at the level of the hilum (where blood vessels and airways enter the lung) with 2 cm as the cutoff,[7] while American guidelines state that the measurement should happen at the apex (top) of the lung with 3 cm differentiating between a small and a large pneumothorax.[9] The latter method may overestimate the size of a pneumothorax if it is located mainly at the apex, which is a common occurrence.[7] The various methods correlate poorly, but are the best easily available ways of estimating pneumothorax size.[7][4] CT scanning (see below) would provide a more accurate determination of the size of the pneumothorax, but its routine use in this setting is not recommended.[9]
Not all pneumothoraces are uniform; some only form a pocket of air in a particular place in the chest.[7] Small amounts of fluid (which may be blood—hemopneumothorax) may be noted on the chest X-ray.[1] In some cases, the only significant abnormality may be the "deep sulcus sign", in which the usually small space between the chest wall and the diaphragm appears enlarged due to the presence of air.[8]
Computed tomography
Computed tomography (CT or CAT scan) can be useful in particular situations. In some lung diseases, especially emphysema, it is possible for abnormal lung areas such as bullae (large air-filled sacs) to have the same appearance as a pneumothorax on chest X-ray, and it may not be safe to apply any treatment before the distinction is made and before the exact location and size of the pneumothorax is determined.[7] In trauma, where it may not be possible to perform an upright film, chest radiography may miss up to a third of pneumothoraces, while CT remains very sensitive.[2]
A further use of CT is in the identification of underlying lung lesions. In presumed primary pneumothorax, it may help to identify blebs or cystic lesions (in anticipation of treatment, see below), and in secondary pneumothorax it can help to identify most of the causes listed above.[7][4]
Ultrasound
Ultrasound is commonly used in the evaluation of people who have sustained physical trauma, for example with the FAST protocol.[10] Ultrasound may be more sensitive than chest X-rays in the identification of pneumothorax after blunt trauma to the chest.[11] Ultrasound may also provide a rapid diagnosis in other emergency situations, and allow quantification of the size of the pneumothorax. Four particular features on ultrasonography of the chest can be used to confirm or exclude the diagnosis.[12]
Treatment
The treatment of pneumothorax depends on a number of factors, and may vary from discharge with early follow-up to immediate needle decompression or insertion of a chest tube. Treatment is determined by the severity of symptoms and indicators of acute illness, the presence of underlying lung disease, the estimated size of the pneumothorax on X-ray, and in some instances also on the personal preference of the person involved. In spontaneous pneumothorax, air travel is discouraged until it has completely resolved.[7]
In traumatic pneumothorax, chest tubes are usually inserted (unless iatrogenic, see below). It is not yet clear if there is a subgroup of people with small pneumothoraces who do not require tube treatment and could be managed conservatively. If mechanical ventilation is required, the insertion of a chest tube is mandatory as it would increase the risk of tension pneumothorax.[1][13]
Tension pneumothorax is usually treated with urgent needle decompression. This may be required before transport to hospital, and can be performed by an emergency medical technician or other trained professional.[8][14] If tension pneumothorax leads to cardiac arrest, needle compression is performed as part of resuscitation as it may restore cardiac output.[15] The needle or cannula is left in place until a chest tube can be inserted.[8][14] Any open chest wound is covered, as it carries a high risk of leading to tension pneumothorax, ideally with a dressing called the Asherman seal, which appears to be more effective than standard "three-sided" dressing. The Asherman seal is a specially designed device that adheres to the chest wall and allows air to escape but not to enter the chest through a valve-like mechanism.[14]
Conservative
Small spontaneous pneumothoraces do not always require treatment, as they are unlikely to proceed to respiratory failure or tension pneumothorax and generally resolve spontaneously. This approach is most appropriate if the estimated size of the pneumothorax is small (e.g. <50%), there is no breathlessness, and there is no underlying lung disease.[4][9] It may be appropriate to treat a larger PSP conservatively if the symptoms are limited.[7] Admission to hospital is often not required, as long as clear instructions are given to return to hospital if there are worsening symptoms. Further investigations may be performed as an outpatient, at which time X-rays are repeated to confirm improvement, and advice may be given with regards to preventing recurrence such as surgery (see below).[7] Secondary pneumothoraces are only treated conservatively if the size is very small (1 cm or less air rim) and there are limited symptoms. Admission to hospital is usually recommended. Oxygen given at a high flow rate may accelerate resorption.[7]
Aspiration
In a large primary spontaneous pneumothorax (>50%) or PSP associated with breathlessness, some professional guidelines recommend that reducing the size by aspiration is equally effective as insertion of a chest tube. This involves the administration of local anesthetic and inserting a needle connected to a three-way tap; up to 2.5 liters of air (in adults) are removed. If there has been significant reduction in the size of the pneumothorax on a further X-ray, the remainder of the treatment can be conservative. This approach is effective in over 50% of cases.[3][7][4] First-line aspiration in PSP reduces the number of people requiring admission to hospital significantly as opposed to tube drainage, without increasing the risk of complications.[16]
Aspiration may also be considered in secondary pneumothorax of moderate size (air rim 1–2 cm) without breathlessness, with the difference that ongoing observation in hospital is required even after a successful procedure.[7] American professional guidelines state that all large pneumothoraces, even those due to PSP, should be treated with a chest tube.[9] Moderately-sized traumatic pneumothorax due to medical procedures (iatrogenic) may initially be treated with aspiration.[1]
Chest tube
A chest tube (or intercostal drain) is the most definitive initial treatment of a pneumothorax. This is typically inserted in an area under the axilla (armpit) called the "safe triangle", where damage to internal organs can be avoided; this is delineated by a horizontal line at the level of the nipple and two muscles of the chest wall (latissimus dorsi and pectoralis major). Local anesthetic is applied. Two types of tubes may be used. In spontaneous pneumothorax, small-bore (smaller than 14 FG, 4.7 mm diameter) tubes may be inserted by the Seldinger technique, and larger tubes do not have an advantage.[7] In traumatic pneumothorax, larger tubes (28 FG, 9.3 mm) are used.[14]
Chest tubes are required in PSP that has not responded to needle aspiration, in any SSP that is large (>50%), and in cases of tension pneumothorax. They are connected to a one-way valve system that allows air to escape but not to reenter the chest. This may include a bottle with water that functions like a water seal, or a Heimlich valve. They are not normally connected to a negative pressure circuit, as this would result in rapid reexpansion of the lung and a risk of pulmonary edema ("reexpansion pulmonary edema"). The tube is left in place until no air is seen to escape from it for a period of time, and X-rays confirm reexpansion of the lung.[7][4][9]
If after 2–4 days there is still evidence of air leak, various options are available. Negative pressure suction (at low pressures of –10 to –20 cmH2O) at a high flow rate may be attempted, especially in PSP; it is thought that this may accelerate the healing of the leak. In SSP, assistance from a thoracic surgeon may be required earlier.[7] Surgical options are similar to those used to prevent further episodes, and are discussed below (thoracotomy and VATS).
Prevention
Both medical and surgical treatments exist to reduce the risk of recurrence of a pneumothorax.[17] The main aim is to achieve pleurodesis, the adherence between the lung and the chest wall. The evidence on the most effective treatment is still conflicting in some areas, and there is variation between treatments available in Europe and the USA.[3] Not all episodes of pneumothorax require such interventions; the decision depends largely on the suspected risk of recurrence. They however are often recommended after a second pneumothorax.[17] An exception applies in those who engage in diving; diving is considered unsafe unless permanent treatment has been applied; professional guidelines suggest that pleurectomy is performed on both lungs (see below) and that lung function tests and CT scan must be normal before diving is resumed.[7][9]
The best results, with a recurrence rate less than 1%, are achieved with a thoracotomy (surgical opening of the chest) with identification of any clear air leak and stapling of blebs, followed by pleurectomy (stripping of the pleural lining) of the outer pleural layer and pleural abrasion (scraping of the pleura) of the inner layer. During the healing process, the lung adheres to the chest wall, effectively obliterating the pleural space. Thoracotomy is always performed under a general anesthetic.[3][7]
A less invasive approach is thoracoscopy, usually in the form of a procedure called video-assisted thoracoscopic surgery. This also involves a general anesthetic but the lung is approached through a number of small incisions between the ribs. The results from VATS-based pleural abrasion are slightly worse than those achieved by thoracotomy, but achieved with smaller scars in the skin. VATS may be also be used to achieve chemical pleurodesis; this involves insufflation of talc, which activates a inflammatory reaction that may also stick the lung to the chest wall.[3][7]
Not everyone may be prepared to undergo surgery. If a chest tube is already in place, various agents may be instilled through the tube to achieve pleurodesis, specifically talc and the antibiotic tetracycline. The results from this tend to be worse than from surgical approaches.[3][7] Talc pleurodesis has few long term consequences in young people.[3]
Epidemiology
Spontaneous pneumothorax is more common in males than in females. The annual incidence of PSP is 18–28 per 100,000 in males and 1.2–6.0 in females. Secondary spontaneous pneumothorax is less common, with 6.3 for males and 2.0 for females. Risk of recurrence depends on underlying lung disease. Once a second episode has occurred, there is a high likelihood of subsequent further episodes.[3] Smokers have an increased risk of contracting a first spontaneous pneumothorax of approximately ninefold among women and 22-fold among men compared to non-smokers.[18] The incidence in children has not been well studied, but it is probably less than that of adults and often reflects underlying lung disease.[4]
Death from pneumothorax is very uncommon (except for tension pneumothorax). British statistics have revealed an annual mortality of 1.26 per million per year in men and 0.62 in women.[7] Mortality is higher in older people and those with secondary pneumothorax.[3]
History
Jean Marc Gaspard Itard, a student of René Laennec, first recognised pneumothorax in 1803, and Laennec himself described the full clinical picture in 1819.[19] While Itard and Laennec recognized that some cases were not due to tuberculosis (then the most common cause), the concept of primary spontaneous pneumothorax was reintroduced by the Danish physician Hans Kjærgaard in 1932.[7][20]
Prior to the advent of anti-tuberculous medications, iatrogenic pneumothoraces were intentionally given to people with tuberculosis in an effort to collapse a lobe, or entire lung around a cavitating lesion. This was known as "resting the lung". It was introduced by the Italian surgeon Carlo Forlanini in 1888 and publicized by the American surgeon John Benjamin Murphy in the early 20th century after discovering the same procedure independently. Murphy used the then recently discovered X-ray technology to create pneumothoraces of the correct size.[21]
References
- ^ a b c d e f g h i j k l m n o p q Noppen M, De Keukeleire T (2008). "Pneumothorax". Respiration. 76 (2): 121–7. doi:10.1159/000135932. PMID 18708734.
- ^ a b c d e f Marx J (2010). Rosen's emergency medicine: concepts and clinical practice 7th edition. Philadelphia, PA: Mosby/Elsevier. pp. 393–396. ISBN 9780323054720.
- ^ a b c d e f g h i j k l m n o p Tschopp JM, Rami-Porta R, Noppen M, Astoul P (2006). "Management of spontaneous pneumothorax: state of the art". Eur. Respir. J. 28 (3): 637–50. doi:10.1183/09031936.06.00014206. PMID 16946095.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j Robinson PD, Cooper P, Ranganathan SC (2009). "Evidence-based management of paediatric primary spontaneous pneumothorax". Paediatr. Respir. Rev. 10 (3): 110–7. doi:10.1016/j.prrv.2008.12.003. PMID 19651381.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Menko FH, van Steensel MA, Giraud S; et al. (2009). "Birt-Hogg-Dubé syndrome: diagnosis and management". Lancet Oncol. 10 (12): 1199–206. doi:10.1016/S1470-2045(09)70188-3. PMID 19959076.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wolf SJ, Bebarta VS, Bonnett CJ, Pons PT, Cantrill SV (2009). "Blast injuries". Lancet. 374 (9687): 405–15. doi:10.1016/S0140-6736(09)60257-9. PMID 19631372.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae MacDuff A, Arnold A, Harvey J, BTS Pleural Disease Guideline Group (2010). "Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010". Thorax. 65 (8): ii18–ii31. doi:10.1136/thx.2010.136986. PMID 20696690.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j Leigh-Smith S, Harris T (2005). "Tension pneumothorax—time for a re-think?". Emerg. Med. J. 22 (1): 8–16. doi:10.1136/emj.2003.010421. PMC 1726546. PMID 15611534.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e f Baumann MH, Strange C, Heffner JE; et al. (2001). "Management of spontaneous pneumothorax: an [[American College of Chest Physicians]] Delphi consensus statement". Chest. 119 (2): 590–602. doi:10.1378/chest.119.2.590. PMID 11171742.
{{cite journal}}: Explicit use of et al. in:|author=(help); URL–wikilink conflict (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Scalea T, Rodriguez A, Chiu W, Brenneman F, Fallon W, Kato K, McKenney M, Nerlich M, Ochsner M, Yoshii H (1999). "Focused Assessment with Sonography for Trauma (FAST): results from an international consensus conference". Journal of Trauma. 46 (3): 466–72. doi:10.1097/00005373-199903000-00022. PMID 10088853.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wilkerson RG, Stone MB (2010). "Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma". Acad. Emerg. Med. 17 (1): 11–17. doi:10.1111/j.1553-2712.2009.00628.x. PMID 20078434.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Volpicelli G (2011). "Sonographic diagnosis of pneumothorax". Intensive Care Med. 37 (2): 224–32. doi:10.1007/s00134-010-2079-y. PMID 21103861.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Keel M, Meier C (2007). "Chest injuries - what is new?". Curr. Opin. Crit. Care. 13 (6): 674–9. doi:10.1097/MCC.0b013e3282f1fe71. PMID 17975389.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d Lee C, Revell M, Porter K, Steyn R (2007). "The prehospital management of chest injuries: a consensus statement. Faculty of Pre‐hospital Care, Royal College of Surgeons of Edinburgh". Emerg. Med. J. 24 (3): 220–4. doi:10.1136/emj.2006.043687. PMC 2660039. PMID 17351237.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Neumar RW, Otto CW, Link MS; et al. (2010). "Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 122 (18 Suppl 3): S729–67. doi:10.1161/CIRCULATIONAHA.110.970988. PMID 20956224.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wakai A, O'Sullivan RG, McCabe G (2007). "Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults". Cochrane Database Syst Rev (1): CD004479. doi:10.1002/14651858.CD004479.pub2. PMID 17253510.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Baumann MH, Noppen M (2004). "Pneumothorax". Respirology. 9 (2): 157–64. doi:10.1111/j.1440-1843.2004.00577.x. PMID 15182264.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bense L, Eklund G, Wiman LG (1987). "Smoking and the increased risk of contracting spontaneous pneumothorax". Chest. 92 (6): 1009–12. doi:10.1378/chest.92.6.1009. PMID 3677805.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Laennec RTH (1819). Traité de l'auscultation médiate et des maladies des poumons et du coeur - part II (in French). Paris.
- ^ Kjaergard H (1932). "Spontaneous pneumothorax in the apparently healthy". Acta Med. Scand. 43 Suppl: 1–159. doi:10.1111/j.0954-6820.1932.tb05982.x.
- ^ Herzog H (1998). "History of tuberculosis" (PDF). Respiration. 65 (1): 5–15. doi:10.1159/000029220. PMID 9523361.
