Ammonium nitrate: Difference between revisions
No edit summary |
|||
| Line 93: | Line 93: | ||
The industrial production of ammonium nitrate entails the [[acid-base reaction theories|acid-base reaction]] of [[ammonia]] with [[nitric acid]]:<ref>http://www.google.com/patents/pdf/Process_of_producing_concentrated_soluti.pdf?id=XronAAAAEBAJ&output=pdf&sig=ACfU3U0iYFRDUxltKLaVind-3wwP_JYPxg</ref> |
The industrial production of ammonium nitrate entails the [[acid-base reaction theories|acid-base reaction]] of [[ammonia]] with [[nitric acid]]:<ref>http://www.google.com/patents/pdf/Process_of_producing_concentrated_soluti.pdf?id=XronAAAAEBAJ&output=pdf&sig=ACfU3U0iYFRDUxltKLaVind-3wwP_JYPxg</ref> |
||
:HNO<sub>3</sub> + NH<sub>3</sub> → NH<sub>4</sub>NO<sub>3</sub> |
:HNO<sub>3</sub> + NH<sub>3</sub> → NH<sub>4</sub>NO<sub>3</sub> |
||
Ammonia is used in its [[anhydrous]] form gas and the nitric acid is concentrated. This reaction is violent owing to its high exothermic nature. After the solution is formed, typically at about 83% concentration, the excess water is evaporated to an ammonium nitrate (AN) content of 95% to 99.9% concentration (AN melt), depending on grade. The AN melt is then made into "prills" or small beads in a spray tower, or into granules by spraying and tumbling in a rotating drum. The prills or granules may be further dried, cooled, and then coated to prevent caking. These prills or granules are the typical AN products in commerce. |
Ammonia is used in its [[anhydrous]] form (i.e. gas form) and the nitric acid is concentrated. This reaction is violent owing to its high exothermic nature. After the solution is formed, typically at about 83% concentration, the excess water is evaporated to an ammonium nitrate (AN) content of 95% to 99.9% concentration (AN melt), depending on grade. The AN melt is then made into "prills" or small beads in a spray tower, or into granules by spraying and tumbling in a rotating drum. The prills or granules may be further dried, cooled, and then coated to prevent caking. These prills or granules are the typical AN products in commerce. |
||
The ammonia required for this process is obtained by the [[Haber process]] from nitrogen and hydrogen. Ammonia produced by the Haber process is oxidized to nitric acid. Another production method is used in the so-called [[Nitrophosphate process|Odda process]]. |
The ammonia required for this process is obtained by the [[Haber process]] from nitrogen and hydrogen. Ammonia produced by the Haber process is oxidized to nitric acid. Another production method is used in the so-called [[Nitrophosphate process|Odda process]]. |
||
Revision as of 21:48, 11 March 2015
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Ammonium nitrate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.026.680 |
| RTECS number |
|
| UNII | |
| UN number | 0222 – with > 0.2% combustible substances 1942 – with <= 0.2% combustible substances 2067 – fertilizers 2426 – liquid |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| (NH4)(NO3) | |
| Molar mass | 80.052 g/mol |
| Appearance | white/grey solid |
| Density | 1.725 g/cm3 (20 °C) |
| Melting point | 169.6 °C (337.3 °F; 442.8 K) |
| Boiling point | approx. 210 °C;decomposes |
| 118 g/100 ml (0 °C) 150 g/100 ml (20 °C) 297 g/100 ml (40 °C) 410 g/100 ml (60 °C) 576 g/100 ml (80 °C) 1024 g/100 ml (100 °C)[1] | |
| Structure | |
| trigonal | |
| Explosive data | |
| Shock sensitivity | very low |
| Friction sensitivity | very low |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Explosive |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2085–5300 mg/kg (oral in rats, mice)[2] |
| Related compounds | |
Other anions
|
Ammonium nitrite |
Other cations
|
Sodium nitrate Potassium nitrate Hydroxylammonium nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
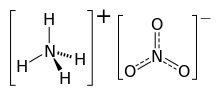
The chemical compound ammonium nitrate, the nitrate salt of ammonium, has the chemical formula NH4NO3, simplified to N2H4O3. It is a white crystalline solid which is highly soluble in water. It is predominantly used in agriculture as a high-nitrogen fertilizer.[3] The compound is used as explosives in mining, and also sometimes in improvised explosive devices. It is the main component of ANFO, a popular explosive, which accounts for 80% explosives used in North America. It is used in instant cold packs, as hydrating the salt is an endothermic process.
Ammonium nitrate is found as a natural mineral (ammonia nitre—the ammonium analogue of saltpetre and other nitre minerals such as sodium nitrate) in the driest regions of the Atacama Desert in Chile, often as a crust on the ground and/or in conjunction with other nitrate, chlorate, iodate, and halide minerals. Ammonium nitrate was mined there in the past, but virtually 100% of the chemical now used is synthetic.
Fertilizer
Ammonium nitrate is an important fertilizer with the NPK rating 34-0-0 (34% nitrogen).[4] It is less concentrated than urea (46-0-0), giving ammonium nitrate a slight transportation disadvantage. Ammonium nitrate's advantage over urea is that it is more stable and does not lose nitrogen to the atmosphere. During warm weather it is best to apply urea soon before rain is expected to minimize nitrogen loss.[5][6]
Safety, handling, and storage
Health and safety data are shown on the material safety data sheets available from suppliers and found on the internet.[7] In response to several explosions resulting in the deaths of numerous people, U.S. agencies of Environmental Protection (EPA), Occupational Health and Safety (OSHA) and the Bureau of Alcohol, Tobacco and Firearms jointly issued safety guidelines.[8]
Heating or any ignition source may cause violent combustion or explosion.[9] Ammonium nitrate reacts with combustible and reducing materials as it is a strong oxidant. Although it is mainly used for fertilizer, it can be used for explosives. It was sometimes used to blast away earth to make farm ponds.[10][11] Ammonium nitrate is also used to modify the detonation rate of other explosives, such as ammonia-based dynamites, for example nitroglycerin[citation needed] and amatol.
Numerous safety guidelines are available for storing and handling ammonium nitrate.[12] It should not be stored near combustible substances.
Ammonium nitrate has a critical relative humidity of 59.4%, above which it will absorb moisture from the atmosphere. Therefore, it is important to store ammonium nitrate in a tightly sealed container. Otherwise, it can coalesce into a large, solid mass. Ammonium nitrate can absorb enough moisture to liquefy. Blending ammonium nitrate with certain other fertilizers can lower the critical relative humidity.[13]
The potential for use of the material as an explosive has prompted regulatory measures. For example in Australia, the Dangerous Goods Regulations came into effect in August 2005 to enforce licensing in dealing with such substances.[14] Licenses are granted only to applicants (industry) with appropriate security measures in place to prevent any misuse.[15] Additional uses such as education and research purposes may also be considered, but individual use will not. Employees of those with licenses to deal with the substance are still required to be supervised by authorized personnel and are required to pass a security and national police check before a license may be granted.
Production
The industrial production of ammonium nitrate entails the acid-base reaction of ammonia with nitric acid:[16]
- HNO3 + NH3 → NH4NO3
Ammonia is used in its anhydrous form (i.e. gas form) and the nitric acid is concentrated. This reaction is violent owing to its high exothermic nature. After the solution is formed, typically at about 83% concentration, the excess water is evaporated to an ammonium nitrate (AN) content of 95% to 99.9% concentration (AN melt), depending on grade. The AN melt is then made into "prills" or small beads in a spray tower, or into granules by spraying and tumbling in a rotating drum. The prills or granules may be further dried, cooled, and then coated to prevent caking. These prills or granules are the typical AN products in commerce.
The ammonia required for this process is obtained by the Haber process from nitrogen and hydrogen. Ammonia produced by the Haber process is oxidized to nitric acid. Another production method is used in the so-called Odda process.
Ammonium nitrate can also be made via metathesis reactions:
- (NH4)2SO4 + 2 NaNO3 → 2 NH4NO3 + Na2SO4
- (NH4)2SO4 + Ca(NO3)2 → 2 NH4NO3 + CaSO4
Sodium sulfate is removed by lowering the temperature of the mixture. Since sodium sulfate is much less water-soluble than ammonium nitrate, it precipitates, and may be filtered off. For the reaction with calcium nitrate, the calcium sulfate generated is quite insoluble, even at room temperature.
Reactions
Ammonium nitrate reacts with metal hydroxides, releasing ammonia and forming alkali metal nitrate:
- NH4NO3 + MOH → NH3 + H2O + MNO3 (M = Na, K)
Ammonium nitrate gives ammonium chloride and nitric acid upon reaction with hydrochloric acid:
- NH4NO3 + HCl → NH4Cl + HNO3
Ammonium nitrate leaves no residue when heated:
- NH4NO3 → N2O + 2H2O
Ammonium nitrate is also formed in the atmosphere from emissions of NO, SO2, and NH3, and is a secondary component of PM10.[17]
Crystalline phases
Transformations of the crystal states due to changing conditions (temperature, pressure) affect the physical properties of ammonium nitrate. These crystalline states have been identified:
| System | Temperature (°C) | State | Volume change (%) |
|---|---|---|---|
| > 169.6 | liquid | ||
| I | 169.6 to 125.2 | cubic | +2.1 |
| II | 125.2 to 84.2 | tetragonal | −1.3 |
| III | 84.2 to 32.3 | α-rhombic | +3.6 |
| IV | 32.3 to −16.8 | β-rhombic | −2.9 |
| V | −16.8 | tetragonal |
The type V crystal is a quasicubic form which is related to caesium chloride, the nitrogen atoms of the nitrate anions and the ammonium cations are at the sites in a cubic array where Cs and Cl would be in the CsCl lattice.[18]
Health hazards
Health and safety data are shown on the material safety data sheets which are available from suppliers and can be found on the internet.[19]
Ammonium nitrate is not very hazardous to health and is usually used in fertilizer products.[19][20][21] The chances of direct personal exposure to the chemical are very low, because the fertilization of the soil by use of ammonium nitrate is done at early stages of plant growth and usually does not remain detectable on the harvested plants or when the plants reach the consumer.[citation needed]
Ammonium nitrate has an LD50 of 2217 mg/kg,[19] which for comparison is about two-thirds that of table salt.
Acute health effects
Short-term exposure to ammonium nitrate can cause symptoms ranging from minor irritation to nausea, vomiting, gastric irritation, headaches, dizziness, and hypertension.[22]
| Area of exposure | Hazard level |
|---|---|
| Ingestion | Moderately hazardous |
| Skin contact | Moderately hazardous (irritant) |
| Eye contact | Moderately hazardous |
| Inhalation | Moderately hazardous |
Long-term health effects
The toxicity of nitrates when ingested is due to in vivo conversion to nitrites. The material safety data sheet considers chronic ingestion of more than 5 mg/kg/day unacceptable. The primary overdose effects of chronic exposure are orthostatic hypotension and methemoglobinemia. Other common effects include: faintness, fatigue, weakness, depression, mental impairment, dizziness, shortness of breath, and reflex tachycardia; headache, nausea, vomiting, and nephritis may also occur.[19]
| Types of effect | Effect level |
|---|---|
| Carcinogenic effects | Though no ammonium nitrate-specific studies are available, nitrates can be reduced to nitrites in the body, and the formed nitrites can subsequently react with amines to form suspect carcinogens N-nitrosamine.[19] |
| Mutagenic effects | In general, nitrates and nitrites are genotoxic.[19] |
| Teratogenic effects | None |
| Developmental toxicity | Though not specific to ammonium nitrate, some studies have shown a link between birth defects (particularly neural tube defects) and nitrate-contaminated well water. |
| Prolonged exposure | Causes damage to lungs and mucous membranes and may also cause damage to blood and gastrointestinal tract. Chronic ingestion may also cause nephritis.[19] |
Disasters
Ammonium nitrate decomposes into the gases nitrous oxide and water vapor when heated (not an explosive reaction); however, it can be induced to decompose explosively by detonation. Large stockpiles of the material can be a major fire risk due to their supporting oxidation, and may also detonate, as happened in the Texas City disaster of 1947, which led to major changes in the regulations for storage and handling.
The two major classes of incidents resulting in explosions are:
- The explosion happens by the mechanism of shock-to-detonation transition. The initiation happens by an explosive charge going off in the mass, by the detonation of a shell thrown into the mass, or by detonation of an explosive mixture in contact with the mass. The examples are Kriewald, Morgan (present-day Sayreville, New Jersey), Oppau, and Tessenderlo.
- The explosion results from a fire that spreads into the ammonium nitrate itself (Texas City, Brest, Oakdale[disambiguation needed]), or from a mixture of ammonium nitrate with a combustible material during the fire (Repauno, Cherokee, Nadadores). The fire must be confined at least to a degree for successful transition from a fire to an explosion (a phenomenon known as "deflagration-to-detonation transition"). Pure, compact AN is stable and very difficult to ignite, and numerous cases exist when even impure AN did not explode in a fire.
Ammonium nitrate-based explosives were used in the Oklahoma City and 2011 Delhi bombings, the 2013 Hyderabad blasts, and the bombing in Oslo 2011.
Ammonium nitrate decomposes in temperatures normally well above 200°C. However, the presence of impurities (organic and/or inorganic) often reduce the temperature point when heat is being generated. Once the AN has started to decompose, then a runaway reaction will normally occur as the heat of decomposition is very large. AN evolves so much heat that this runaway reaction is normally impossible to stop. This is a well-known hazard with some types of N-P-K Fertilizers, and it is responsible for the loss of several cargo ships.
Under normal handling conditions, ammonium nitrate is not harmful. However, inhalation of high concentrations of its dust can cause respiratory tract irritation. Symptoms may include: coughing, sore throat, shortness of breath, or even suffocation. When swallowed in high concentrations, ammonium nitrate may cause headache, dizziness, abdominal pain, vomiting, bloody diarrhea, weakness, a tingling sensation, heart and circulation irregularities, convulsions, collapse, and suffocation. It forms a mild acid when mixed with water. This acid can cause irritation to the eyes, nose, and skin.[23]
In November 2009, a ban on ammonium sulfate, ammonium nitrate, and calcium ammonium nitrate fertilizers was imposed in the former Malakand Division—comprising the Upper Dir, Lower Dir, Swat, Chitral, and Malakand districts of the North West Frontier Province (NWFP) of Pakistan—by the NWFP government, following reports that those chemicals were used by militants to make explosives. In January 2010, these substances were also banned in Afghanistan for the same reason. After several cases, AN has now been legalised due to the Pakistani forces of NWFP.
Ammonium nitrate was suspected as the explosive responsible for the fertilizer plant explosion in West, Texas on April 17, 2013. Investigators said they believe it exploded following a fire that began in the plant's office.[24]
Mixture with fuel oil
ANFO is a mixture of 94% ammonium nitrate ("AN") and 6% fuel oil ("FO") widely used as a bulk industrial explosive.[25]: 1 It is used in coal mining, quarrying, metal mining, and civil construction in undemanding applications where the advantages of ANFO's low cost and ease of use matter more than the benefits offered by conventional industrial explosives, such as water resistance, oxygen balance, high detonation velocity, and performance in small diameters.[25]: 2
References
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ Martel, B.; Cassidy, K. (2004). Chemical Risk Analysis: A Practical Handbook. Butterworth–Heinemann. p. 362. ISBN 1-903996-65-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Karl-Heinz Zapp "Ammonium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.a02_243
- ^ Nutrient Content of Fertilizer Materials
- ^ [1]
- ^ [2]
- ^ Ammonium nitrate MSDS
- ^ Chemical Advisory: Safe Storage, Handling, and Management of Ammonium Nitrate United States Environmental Protection Agency
- ^ Pradyot Patnaik (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0-07-049439-8.
- ^ Pothole pond
- ^ Progressive Farmer Magazine
- ^ Storing and handling ammonium nitrate
- ^ Fertilizers Europe (2006). "Guidance for Compatibility of Fertilizer Blending Materials" (PDF).
- ^ Dangerous Goods (HCDG) Regulations
- ^ Ammonium Nitrate-Regulating its use, Balancing Access & Protection from "Worksafe Victoria".
- ^ http://www.google.com/patents/pdf/Process_of_producing_concentrated_soluti.pdf?id=XronAAAAEBAJ&output=pdf&sig=ACfU3U0iYFRDUxltKLaVind-3wwP_JYPxg
- ^ Int Panis, LLR (2008). "The Effect of Changing Background Emissions on External Cost Estimates for Secondary Particulates" (PDF). Open Environmental Sciences. 2: 47–53. doi:10.2174/1876325100802010047.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Choi, C. S.; Prask, H. J. (1983). "The structure of ND4NO3 phase V by neutron powder diffraction". Acta Crystallographica B. 39 (4): 414–420. doi:10.1107/S0108768183002669.
- ^ a b c d e f g CF Industries. "Ammonium nitrate MSDS" (PDF). Cite error: The named reference "MSDS" was defined multiple times with different content (see the help page).
- ^ "Chemicalland21 – Ammonium Nitrate".
- ^ "Ammonium Nitrate". Paton Fertilizers Pty Ltd. 2005.
{{cite web}}: Missing or empty|url=(help) - ^ Martel, B.; Cassidy, K. (2004). Chemical Risk Analysis: A Practical Handbook. Butterworth–Heinemann. ISBN 1-903996-65-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ The Hazards and Dangers of Ammonium Nitrate [Ammonium Nitrate Dangers | http://www.nortechlabs.com/hazards-ammonium-nitrate.html]
- ^ http://www.dallasnews.com/news/west-explosion/headlines/20130506-investigators-blame-ammonium-nitrate-in-massive-west-explosion.ece Investigators blame ammonium nitrate in massive West explosion
- ^ a b Cook, Melvin A. (1974). The Science of Industrial Explosives. IRECO Chemicals. p. 1. ASIN B0000EGDJT.
- Properties: UNIDO and International Fertilizer Development Center (1998), Fertilizer Manual, Kluwer Academic Publishers, ISBN 0-7923-5032-4.
External links
- International Chemical Safety Card 0216
- "Storing and Handling Ammonium Nitrate", United Kingdom Health and Safety Executive publication INDG230 (1986)
- Chemical Advisory: Safe Storage, Handling, and Management of Ammonium Nitrate United States Environmental Protection Agency
- Calculators: surface tensions, and densities, molarities and molalities of aqueous ammonium nitrate

