Coeliac disease: Difference between revisions
No edit summary |
No edit summary |
||
| Line 187: | Line 187: | ||
Throughout the 1960s other features of coeliac disease were elucidated. In 1966 dermatitis herpetiformis was linked to gluten sensitivity,<ref name=Marks/> and in 1970 features of [[hyposplenism]] (decreased activity of the spleen) were linked to coeliac disease.<ref name=Ferguson/> The link with tissue transglutaminase was not made until 1997.<ref name=Dieterich/> |
Throughout the 1960s other features of coeliac disease were elucidated. In 1966 dermatitis herpetiformis was linked to gluten sensitivity,<ref name=Marks/> and in 1970 features of [[hyposplenism]] (decreased activity of the spleen) were linked to coeliac disease.<ref name=Ferguson/> The link with tissue transglutaminase was not made until 1997.<ref name=Dieterich/> |
||
'''My Story''' |
|||
I have had coceliac disease since I was 6 and I am now 14. I felt no need to cheat on my diet but I find there is a lot of alternatives out there however I come from quite a well of family so we don't struggle with the cost of the food. the thing that really annoys me is that a gluten free diet only became well known because people feel that it is a great "LOSE WEIGHT" diet. |
I have had coceliac disease since I was 6 and I am now 14. I felt no need to cheat on my diet but I find there is a lot of alternatives out there however I come from quite a well of family so we don't struggle with the cost of the food. the thing that really annoys me is that a gluten free diet only became well known because people feel that it is a great "LOSE WEIGHT" diet. |
||
Revision as of 20:26, 2 October 2006
| Coeliac disease | |
|---|---|
| Specialty | Gastroenterology |
Coeliac disease or celiac disease is an autoimmune disorder of the small bowel that occurs in genetically predisposed individuals in all age groups after early infancy. Symptoms may include diarrhoea, failure to thrive (in children) and fatigue, but these may be absent and associated symptoms in all other organ systems have been described. It affects approximately 1% of Caucasian populations, though it is significantly underdiagnosed. A growing portion of diagnoses are being made in asymptomatic persons as a result of increasing screening.[1]
Coeliac disease is caused by an abnormal reaction to gliadin, a gluten protein found in wheat (and similar proteins of the tribe Triticeae which includes other cultivars such as barley and rye). Upon exposure to gliadin, the body's immune system cross-reacts with the enzyme tissue transglutaminase, causing an inflammatory reaction that leads to flattening of the lining the small intestine, which interferes with the absorption of nutrients. The only effective treatment is a diet, lifelong in principle, from which gluten is absent.
This condition has several other names, including: cœliac disease (with ligature), c(o)eliac sprue, non-tropical sprue, endemic sprue, gluten enteropathy or gluten-sensitive enteropathy, and gluten intolerance. The term coeliac derives from the Greek κοιλια (koilia, abdomen), and was introduced in the 19th century in a translation of what is generally regarded as an ancient Greek description of the disease by Aretaeus of Cappadocia.[2]
Signs and symptoms
Classic symptoms of coeliac disease include diarrhoea, weight loss (or stunted growth in children) and fatigue, but while coeliac disease is a disease of the bowel, it may present with limited bowel symptoms. Some patients are diagnosed with symptoms related to the decreased absorption of nutrients or with symptoms which, although statistically linked, have no clear relationship with the malfunctioning bowel.
Children between 9 and 24 months tend to present with bowel symptoms and growth problems shortly after first exposure to gluten-containing products. Older children may have more malabsorption-related problems and psychosocial problems, while adults generally have malabsorptive problems.[3] Many adults with subtle disease only have fatigue or anaemia.[1]
Gastrointestinal symptoms
The diarrhoea characteristic of coeliac disease is pale, voluminous and malodorous. Abdominal pain and cramping, bloatedness and abdominal distention (thought to be due to fermentative production of bowel gas) and mouth ulcers[4] may be present. As the bowel is more damaged, a degree of lactose intolerance may develop. Constipation is rare, but may be a manifestation of coeliac disease.[3]
Coeliac disease leads to an increased risk of both adenocarcinoma and lymphoma of the small bowel, which returns to baseline with diet. Longstanding disease may lead to other complications, such as ulcerative jejunitis (ulcer formation of the small bowel) and stricturing (narrowing as a result of scarring).[5]
Malabsorption-related symptoms
The changes in the bowel make it less competent in absorbing nutrients, minerals and fat-soluble vitamins.[3]
- The inability to absorb carbohydrates and lipids may cause weight loss (or failure to thrive/stunted growth in children) and fatigue or lack of energy.
- Anaemia may develop in several ways: iron malabsorption may cause iron deficiency anaemia, and folic acid and vitamin B12 malabsorption may give rise to megaloblastic anaemia.
- Calcium and vitamin D (and compensatory secondary hyperparathyroidism) may cause osteopenia (decreased mineral content of the bone) or osteoporosis (bone weakening and risk of fragility fractures). Malabsorption of vitamin D may lead to vitamin D deficiency rickets or even hypocalcemic tetany.
- A small proportion have abnormal coagulation due to deficiency of vitamin K, and are slightly at risk for abnormal bleeding.
- Coeliac disease is also associated with bacterial overgrowth of the small intestine, which can worsen malabsorption, or cause malabsorption after treatment.[6]
Miscellaneous symptoms
Coeliac disease has been linked with a number of conditions. In many cases it is unclear whether the gluten-induced bowel disease is a causative factor or whether these conditions share a common predisposition.
- IgA deficiency is present in 2% of patients with coeliac disease, and in turn this condition features a tenfold increased risk of coeliac disease.[7][8] Other features of this condition are an increased risk of infections and autoimmune disease.
- Dermatitis herpetiformis; this itchy cutaneous condition has been linked to a transglutaminase in the skin, features small bowel changes identical to those in coeliac disease[9] and occurs more often (2%) in patients with coeliac disease.[3]
- Neurological associations: epilepsy, ataxia (coordination problems), myelopathy and peripheral neuropathy have all been linked with coeliac disease, but the strength of these associations and the causality is still subject of debate.[10]
- Growth failure and/or pubertal delay in later childhood can occur even without obvious bowel symptoms or severe malnutrition. Evaluation of growth failure often includes celiac screening.
- Miscarriage and infertility.
- Hyposplenism (a small and underactive spleen) - it is unclear whether this actually increases infection risk in coeliacs.[11]
- Other auto-immune disorders: diabetes mellitus type 1, autoimmune thyroiditis,[12] primary biliary cirrhosis[13] and microscopic colitis.[14]
The role of other grains
Wheat varieties or subspecies such as spelt and Kamut®, and the rye/wheat hybrid triticale, also trigger symptoms.[15]
Barley and rye also induce coeliac disease.[15] A small minority of coeliac patients also react to oats.[16][17] Most probably oats produced symptoms due to cross contamination with other grains in the fields or in the distribution channels. There is at least one oat vendor (McCann's) which, while not claiming to be gluten-free, points out that the risk of contamination is low due to the processes they use.[citation needed] Other cereals, such as maize (corn), sorghum, rice are safe for a patient to consume. Other carbohydrate-rich foods, like potatoes and bananas, do not contain gluten and do not trigger the disease.
Diagnosis
The condition is frequently misdiagnosed or overlooked as it can exhibit multiple symptoms and often the patient or medical staff may not link seemingly unconnected conditions. It is most frequently misdiagnosed when the sufferer complains of diarrhea, persistent indigestion, an itchy rash (dermatitis herpetiformis), or irritable bowel syndrome.
There are several tests that can be used to assist in diagnosis. The level of symptoms may determine the order of the tests, but all tests must be done while the person is on a gluten containing diet. Antibodies are reduced and intestinal damage begins to heal immediately upon removing all gluten from the diet, so the risk of misdiagnosis is increased if the person is not eating gluten. For those who have already commenced themselves on a gluten-free diet, professional guidelines recommend a re-challenge of 2-6 weeks with 10 g of gluten (four slices of bread) before repeating the investigations. Those who experience severe symptoms earlier can be regarded as sufficiently challenged and can be tested earlier.[3]
Blood tests
Serology by blood test is both good at diagnosing coeliac disease (high sensitivity of 98%, i.e. it misses 2 in 100 cases) and excluding it (high specificity of >95%, i.e. a positive test is most likely confirmative of coeliac disease rather than other condition). Because of the major implications of a diagnosis of coeliac disease, many recommend that a positive blood test is still followed by an endoscopy. A negative test may still prompt a biopsy if the suspicion is very high; this would pick up the remaining 2% undiagnosed cases. Due to the few limitations of blood tests, endoscopy and biopsy is still considered the gold standard in the diagnosis of coeliac disease.[3][5]
Due to its high sensitivity, serology has been proposed as a screening measure, because the presence of antibodies would detect previously undiagnosed cases of coeliac disease and prevent its complications in those patients.
Serology may also be used to monitor adherence to diet.[3][5]
Four serological blood tests exist for coeliac disease:
- IgA and IgG anti-tissue transglutaminase antibody (anti-tTG). This test is sometimes used alone. If this test is positive it is highly likely that the patient has coeliac disease. It is not reliable in children before the age of 2.
- IgA and IgG anti-gliadin antibodies (AGA), IgG and IgA. These tests are often useful when testing young symptomatic children, but they are found in fewer coeliacs than anti-tTG, and their presence does not automatically indicate coeliac disease because they are found in some other disorders.
- IgA anti-endomysial antibodies (EMA). This test is being replaced by the anti-tTG test because both tests measure the autoantibodies that cause the tissue damage associated with coeliac disease. Many physicians still order this test. This test as tTG test is also not reliable in children before the age of 2.
- An older test, the IgA anti-reticulin antibodies (ARA). IgA anti-ARA is not ordered as frequently as it once was, because it is less sensitive and less specific than the other tests. It is found in about 60% of people with coeliac disease and 25% of those with dermatitis herpetiformis.
For those based on IgA, a total IgA level is checked in parallel, as patients with IgA deficiency may have a normal result while still having coeliac disease ("false negative"). In those patients, IgG antibodies may be diagnostic.[18]
Many doctors consider coeliac disease to be diagnosed where the patient has positive blood tests and shows improved symptoms after the adoption of a gluten-free diet, while others require at least one upper endoscopy with biopsy. The problem with not doing a biopsy at all is that patients later commonly want to know if they really have coeliac disease and need to remain gluten restricted. A diagnosis with biopsy confirmation at the time of initial diagnosis eliminates this common clinical problem.
Endoscopy
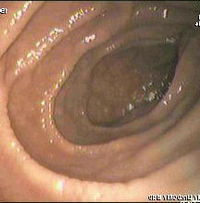
An upper endoscopy with biopsy of the duodenum (beyond the duodenal bulb) or jejunum is performed. It is important for the physician to obtain multiple samples (three or more) from various places throughout the intestine. Not all areas may be equally affected, which is why even upper endoscopy carries a small risk of false negative results.[5] Most patients with coeliac disease have a normal appearing small bowel on endoscopy; however, five endoscopic findings have been associated with a high specificity for coeliac disease when all are found: scalloping of the small bowel folds (pictured), paucity in the folds, a mosaic pattern to the mucosa (described as a cracked-mud appearance), prominence of the submucosal blood vessels and a nodular pattern to the mucosa. [19]
The classic pathology changes of coeliac disease in the small bowel are categorized by the "Marsh classification":[20]
- Marsh stage 0: normal mucosa
- Marsh stage 1: increased number of intra-epithelial lymphocytes, usually exceeding 20 per 100 enterocytes
- Marsh stage 2: proliferation of the crypts of Lieberkuhn
- Marsh stage 3: partial or complete villous atrophy
- Marsh stage 4: hypoplasia of the small bowel architecture
The changes classically improve or reverse after gluten is removed from the diet, so many official guidelines recommend a repeat biopsy several months after commencement of gluten exclusion.
In some cases a deliberate gluten challenge, followed by biopsy, may be conducted to confirm or refute the diagnosis. A normal biopsy and normal serology after challenge indicates the diagnosis may have been incorrect.[3] Patients are warned that one does not "outgrow" coeliac disease in the same way as childhood food intolerances.
Other diagnostic tests
Other tests that may assist in the diagnosis are blood tests for a full blood count, electrolytes, calcium, renal function and liver enzymes. Coagulation testing may be useful to identify deficiency of vitamin K, which predisposes patients to hemorrhage. These tests should be repeated on follow-up, as well as anti-tTG titres.[3]
Some professional guidelines[3] recommend screening of all patients for osteoporosis by DXA/DEXA scanning.
Screening and case finding
The is significant debate as to the benefits of screening. Some studies suggest that early detection would decrease the risk of osteoporosis and anaemia. In contrast, a cohort studied in Cambridge suggested that people with undetected coeliac disease had a beneficial risk profile for cardiovascular disease (less overweight, lower cholesterol levels).[1]
Clinical scenarios in which screening may be justified include type 1 diabetes, unexplained iron-deficiency anemia, Down's syndrome, Turner's syndrome, irritable bowel syndrome, lupus, and autoimmune thyroid disease.[citation needed]
Pathophysiology
Genetics
Coeliac disease is linked to the CELIAC1 locus (HLA DQ) and occurs almost exclusively (over 95%) in patients with human leukocyte antigen types DQ2 (DQA1*0501:DQB1*0201) and DQ8 (DQA1*0301:DQB1*0302), which is inherited in families. Over 95% of coeliac patients carry one or two of the DQ2 or DQ8 genes. DQ2 and DQ8 are serotypes defined by immunological reactivity where as DQA1:DQB1 are two locus haplotypes that are commonly found that result in the serotypes, and any given person can produce 4 DQ molecules, 2 in the cis-haplotype dimer pairing and 2 in the transhaplotype dimer pairing. DQA1*0501:DQB1*0201 and DQ8 produce susceptibility in the cis-haplotype pairing configuration. There is a third pairing of haplotypes, DQA1*0201:DQB1*0202 / DQA1*0505:DQB1*0301 (DQ7) that when both are found in a single patient can produce susceptibility to coeliac disease via a trans-pairing of the DQA1*0505-DQB1*0202 gene products. With this exception DQB1*0202 is atypically assocaited with coeliac disease.[citation needed]
It appears that HLA-DQ2-(DQA1*05) is more avid in presenting gliadin peptides to T lymphocytes, which then initiate the autoimmune process.[1]
Every person carries two HLA-DQ_ genes, one from their mother and one from their father. About 20% of normal people carry HLA-DQ2, which raises the question of what other factors cause a subgroup of about 5% of those people to develop coeliac disease.[1]. The frequency of these genes vary greatly, DQ2(DQA1*05) is at high frequencies in the Sardinians, Basque, British Ilses, Scandinavia. DQ8 is at high frequencies in the South and Central America (up to 90% phenotype frequency), Mexico, and Sweden. HLA-DQ2(DQA1*05)/DQ8 heterozygotes are at slightly increased risk for coeliac disease versus homozygotes of either and may have more severe disease.[citation needed]
In addition to the HLA locus on chromosome 6q21.3, several other regions have been linked (especially 19p13.3 and 4p14).[21] Mendelian Inheritance in Man designates five regions as CELIAC1-5 (6q21.3, 5q31-q33, 2q33, 19p13.1, 15q11-q13). For CELIAC3, the CTLA4 gene was found to be linked, and CELIAC4 the gene coding for myosin IXB. CELIAC2 and CELIAC5 have no suspected gene association.
Prolamins
The proteins responsible for the immunological reaction in coeliac disease are the prolamins, storage proteins rich in proline (prol-) and glutamine- (-amin) that dissolve in alcohols and are resistant to pepsin and chymotrypsin, two main digestive proteases. Apart from gliadin (from wheat) the main putative prolamins are hordein (from barley), and secalin (from rye). Recent research has identified a 33 amino acid-long homologous polypeptide in these species that may be responsible for its effects in coeliac disease.[1] There is ongoing controversy on the ability of avenin (from oats) to induce the coeliac response.
Tissue transglutaminase
Antibodies to the enzyme tissue transglutaminase (tTG) are found in an overwhelming majority of cases, and cross-react to gluten.[22] Tissue transglutaminase participates in the modification of gluten peptides, the product of which may be more potent in stimulating the immune system.[1]
Stored biopsies from suspected celiac patients has revealed that autoantibody deposits in the subclinical celiacs are detected prior to clinical disease. These deposits are found in patients who present with other autoimmune diseases, anemia or maladsoption phenomena at a much increased rate over the normal population.[23]. Endomysial component of antibodies (EMA) to tTG are believed to be directed toward cell surface transglutaminase, and these antibodies are still used in confirming a celiac disease diagnosis. However, a recent study has shown that EMA negative celiac patients tend to be older, more typically male with more severe abdominal symptoms and a lower frequency of "atypical" symptoms including autoimmune disease.[24] In this study the anti-tTG antibody deposits did not correlate with the severity of villous destruction. These findings, coupled with recent work showing that gliadin has an innate response component,[25] suggests that gliadin may be more responsible for the primary manifestations of celiac disease whereas as tTG is a bigger factor in secondary effects such as allegic responses and secondary autoimmune diseases.
Villous atrophy and malabsorption
The inflammatory process, mediated by T cells, leads to disruption of the structure and function of the small bowel's mucosa, and causes malabsorption (it impairs the body's ability to absorb nutrients, minerals and fat-soluble vitamins A, D, E and K from food). Lactose intolerance may be present due to the decreased bowel surface and reduced production of lactase, but typically resolves once the condition is treated.
Some research suggests an alternative route mediated by interleukin 15 in which the innate immune system is induced by a shorter gluten peptide (p31-43/49) that causes killing of enterocytes by lymphocytes in the epithelium.[1]
The villous atrophy seen on biopsy may also be due to unrelated causes, such as tropical sprue, giardiasis, radiation enteritis and several rarer causes. While positive serology and typical biopsy are highly suggestive of coeliac disease, lack of response to diet may require these conditions to be considered.[5]
Triggers
There are various theories as to what determines whether a genetically susceptible individual will go on to develop coeliac disease. Major theories include:
- An environmental agent, such as infection or a chemical substance
- Stress
- Timing of the exposure to gluten (before the gut barrier has developed fully): those exposed to wheat, barley, or rye at any time in the first three months had five times the risk of developing coeliac over those exposed at 4 to 6 months. Those exposed later had a slightly increased risk relative to those exposed at 4-6 months.[26]
Some research has suggested that smoking is protective against coeliac disease.[27] Results on this topic are however inconsistent, and smoking cannot be recommended as a means to avoid developing coeliac disease.
Treatment
Diet
The only treatment is a life-long gluten-free diet.[28] At this time no medication will prevent damage, nor prevent the body from attacking the gut when gluten is present. The disease is controlled by strict adherence to a gluten-free diet, which allows the intestines to heal and resolves all symptoms in the vast majority of cases and, depending on how soon the diet is begun, can also eliminate the heightened risk of osteoporosis and intestinal cancer.[29] Dietician input is generally requested to ensure the patient is aware which food contain gluten, which foods are safe, and how to have a balanced diet despite the limitations. In many countries gluten-free products are available on prescription and may be reimbursed by health insurance plans. More manufacturers are producing gluten-free products, some of which are almost indistinguishable from their gluten-containing counterparts.
The diet can be cumbersome; while young children can be kept compliant by their parents, teenagers may wish to hide their problem or rebel against the dietary restrictions, risking relapse. Many food products contain traces of gluten even if apparently wheat-free. Gluten-free products are usually more expensive and harder to find than common wheat-containing foods.
Even while on a diet, health-realted quality of life (HRQOL) is decreased in people with coeliac disease. Some have persisting digestive symptoms or dermatitis herpetiformis, mouth ulcers, osteoporosis and fractures. Symptoms suggestive of irritable bowel syndrome may be present, and there is an increased rate of anxiety, fatigue, dyspeptic and musculoskeletal pain.[30]
Refractory disease
A tiny minority of patients suffer from refractory disease, which means they do not improve on a gluten-free diet. This may be because the disease has been present for so long that the intestines are no longer able to heal, or because the patient is not adhering to the diet. If alternative causes have been eliminated, steroids or immunomodulators (such as azathioprine) may be considered in this scenario.[5]
Experimental treatments
Various other approaches are being studied that would reduce the need of dieting. All are still under development, and are not expected to be available to the general public for a while:[1]
- Genetically engineered wheat species, or wheat species that have been selectively bred to be minimally immunogenic. This, however, could interfere with the effects that gliadin has on the quality of dough.
- A combination of enzymes (prolyl endopeptidase and a barley glutamine-specific cysteine endopeptidase (EP-B2)) that degrade the putative 33-mer peptide in the duodenum. This combination would enable coeliac disease patients to consume gluten-containing products.[31]
- Inhibition of zonulin, a substance linked to increased permeability of the bowel wall and hence increased presentation of gliadin to the immune system.[32]
- Other treatments aimed at other well-understood steps in the pathogenesis of coeliac disease, such as the action of HLA-DQ2 or tissue transglutaminase and the MICA/NKG2D interaction that may be involved in the killing of enterocytes (bowel lining cells).
Epidemiology
The prevalence of clinically diagnosed disease (symptoms prompting diagnostic testing) is 0.05-0.27% in various studies. However, population studies from Europe, South America, Australasia and the USA (using serology and biopsy) indicate that the prevalence may be between 0.33 and 1.06% in children (5.66% in one study of Saharawi children[33][34]) and 0.18-1.2% in adults.[1] It is commoner in people of British or Italian ancestry, owing to both the genetic component of the disease and the tendency to feed infants gluten products very early in life in these populations.[citation needed] People of African, Japanese, and Chinese descent are rarely diagnosed.[citation needed] Risk is elevated in those with other autoimmune diseases.[citation needed] A large multicentre study in the US found a prevalence of 0.75% in not-at-risk groups, rising to 1.8% in symptomatic patients, 2.6% in second-degree relatives of a patient with coeliac disease and 4.5% in first-degree relatives. This profile is similar to the prevalence in Europe.[35]
From the fact that prevalence seems to remain the same between childhood and adulthood one may deduce that the principal trigger occurs early in life.[1]
Social and religious issues
Roman Catholic position
Roman Catholic doctrine states that for a valid Eucharist the bread must be made from wheat. The Catholic Church has approved the use of low-gluten hosts, but even these are not gluten-free. Some Catholic coeliac sufferers have requested permission to use rice wafers; such petitions have always been denied.[36]
The issue is more complex for priests. Although a Catholic (lay or ordained) receiving under either form is considered to have received Christ "whole and entire", the priest, who is acting in persona Christi, is required to receive under both species when offering Mass — not for the validity of his Communion, but for the fullness of the sacrifice of the Mass. On August 22, 1994, the Congregation for the Doctrine of the Faith apparently barred coeliacs from ordination, stating, "Given the centrality of the celebration of the Eucharist in the life of the priest, candidates for the priesthood who are affected by coeliac disease or suffer from alcoholism or similar conditions may not be admitted to holy orders." After considerable debate, the congregation softened the ruling on July 24, 2003 to "Given the centrality of the celebration of the Eucharist in the life of a priest, one must proceed with great caution before admitting to Holy Orders those candidates unable to ingest gluten or alcohol without serious harm."[citation needed]
Eastern Orthodox Position
The Orthodox Church also requires that the bread used at the Eucharist be made with wheat flour; here the bread is leavened with yeast. Orthodox Christians receive Communion under the species of bread and wine; both are given together from the chalice with a spoon. Some Orthodox coeliac sufferers have been able to receive communion simply by having the priest take only the consecrated wine in the spoon; others, more sensitive to wheat, have had to use a special chalice containing only the consecrated wine. This latter case is extremely unusual, and is strictly speaking only permissible with the permission of the diocesan bishop. While Orthodox Christians do not have such an explicit rationale as the Roman Catholic Church, their general understanding is that, in the case of exceptions made for the sake of Economy, the Holy Spirit makes up whatever is lacking.[citation needed]
The Church of Jesus Christ of Latter-day Saints
The Sacrament of the Lord's Supper as observed by members of The Church of Jesus Christ of Latter-day Saints allows for some flexibility in adapting to the needs of coeliac congregants. Section 27 of the church's canonical Doctrine and Covenants reads:
- "it mattereth not what ye shall eat or what ye shall drink when ye partake of the sacrament, if it so be that ye do it with an eye single to my glory—remembering unto the Father my body which was laid down for you, and my blood which was shed for the remission of your sins."
As awareness of coeliac disease has increased, many congregations have made allowance for Coeliac congregants where necessary by permitting gluten-free breadstuff to be used, sometimes alongside regular breads (where possible).
Coeliacs and Passover
The Jewish festival of Pesach (Passover) may present problems with its obligation to eat matzo. Matzo is normally made from wheat or other gluten-containing grains, so oat matzo is used. Many products prepared for Passover are free of wheat, barley, spelt, oats, and rye, as many Orthodox (especially Hasidic) Jews avoid wheat products altogether (gebroks) apart from matzo. Potato starch is the primary starch used to replace the grains. [37]
History
Aretaeus of Cappadocia, living in the second century, described a malabsorptive syndrome now widely perceived as an early description of coeliac disease. It gained the attention of Western medicine when Francis Adams presented a translation of Aretaeus' work at the Sydenham Society in 1856. Adams introduced the term "coeliac".[2] In 1888 Dr Samuel Gee, a paediatrician at Great Ormond Street Hospital in London gave the first modern-day description of the condition, as well as improvement on a diet of mussels.[38] Dr Sydney V. Haas, an American paediatrician, reported positive effects of a diet of bananas in 1924.[39] This diet remained in vogue until the actual cause of coeliac disease was determined.
While a role for carbohydrates had been suspected, the link with wheat was not made until 1950 by the Dutch paediatrician Dr Willem Dicke.[40] It is likely that clinical improvement of his patients during the Dutch famine of 1944 (during which flour was sparse) may have contributed to his discovery.[41] The link with the gluten component of wheat was made in 1952 by a team from Birmingham, England.[42] Villous atrophy was described by British physician Paulley in 1954.[43] Dr Margo Shiner, working on Prof Sheila Sherlock's team at the Postgraduate Medical School in London, described the principles of small bowel biopsy in 1956.[44]
Throughout the 1960s other features of coeliac disease were elucidated. In 1966 dermatitis herpetiformis was linked to gluten sensitivity,[9] and in 1970 features of hyposplenism (decreased activity of the spleen) were linked to coeliac disease.[11] The link with tissue transglutaminase was not made until 1997.[22] My Story
I have had coceliac disease since I was 6 and I am now 14. I felt no need to cheat on my diet but I find there is a lot of alternatives out there however I come from quite a well of family so we don't struggle with the cost of the food. the thing that really annoys me is that a gluten free diet only became well known because people feel that it is a great "LOSE WEIGHT" diet.
References
- ^ a b c d e f g h i j k van Heel D, West J (2006). "Recent advances in coeliac disease". Gut. 55 (7): 1037–46. PMID 16766754..
- ^ a b Adams F, translator (1856). "On The Cœliac Affection". The extant works of Aretaeus, The Cappadocian. London: Sydenham Society. Retrieved 2006-09-04.
{{cite book}}:|last=has generic name (help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ a b c d e f g h i j Ciclitira P. Interim Guidelines for the Management of Patients with Coeliac Disease. British Society of Gastroenterology, 2002. MS Word document.
- ^ Ferguson R, Basu M, Asquith P, Cooke W (1976). "Jejunal mucosal abnormalities in patients with recurrent aphthous ulceration". Br Med J. 1 (6000): 11–13. PMID 1247715.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ a b c d e f "American Gastroenterological Association medical position statement: Celiac Sprue". Gastroenterology. 120 (6): 1522–5. 2001. PMID 11313323..
- ^ Tursi A, Brandimarte G, Giorgetti G (2003). "High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal". Am J Gastroenterol. 98 (4): 839–43. PMID 12738465.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Crabbé P, Heremans J (1967). "Selective IgA deficiency with steatorrhea. A new syndrome". Am J Med. 42 (2): 319–26. PMID 4959869..
- ^ Collin P, Mäki M, Keyriläinen O, Hällström O, Reunala T, Pasternack A (1992). "Selective IgA deficiency and coeliac disease". Scand J Gastroenterol. 27 (5): 367–71. PMID 1529270.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ a b Marks J, Shuster S, Watson A (1966). "Small-bowel changes in dermatitis herpetiformis". Lancet. 2 (7476): 1280–2. PMID 4163419.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Pengiran Tengah D, Wills A, Holmes G (2002). "Neurological complications of coeliac disease". Postgrad Med J. 78 (921): 393–8. PMID 12151653.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ a b Ferguson A, Hutton M, Maxwell J, Murray D (1970). "Adult coeliac disease in hyposplenic patients". Lancet. 1 (7639): 163–4. PMID 4189238.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Collin P, Kaukinen K, Välimäki M, Salmi J (2002). "Endocrinological disorders and celiac disease". Endocr Rev. 23 (4): 464–83. PMID 12202461.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Kingham J, Parker D (1998). "The association between primary biliary cirrhosis and coeliac disease: a study of relative prevalences". Gut. 42 (1): 120–2. PMID 9518232.
- ^ Matteoni C, Goldblum J, Wang N, Brzezinski A, Achkar E, Soffer E (2001). "Celiac disease is highly prevalent in lymphocytic colitis". J Clin Gastroenterol. 32 (3): 225–7. PMID 11246349.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Grain toxicity" (RTF). The CELIAC list. Retrieved 2006-08-27.
- ^ Lundin K, Nilsen E, Scott H, Løberg E, Gjøen A, Bratlie J, Skar V, Mendez E, Løvik A, Kett K (2003). "Oats induced villous atrophy in coeliac disease". Gut. 52 (11): 1649–52. PMID 14570737.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Størsrud S, Olsson M, Arvidsson Lenner R, Nilsson L, Nilsson O, Kilander A (2003). "Adult coeliac patients do tolerate large amounts of oats". Eur J Clin Nutr. 57 (1): 163–9. PMID 12548312.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Korponay-Szabó I, Dahlbom I, Laurila K, Koskinen S, Woolley N, Partanen J, Kovács J, Mäki M, Hansson T (2003). "Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency". Gut. 52 (11): 1567–71. PMID 14570724.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Niveloni S, Fiorini A, Dezi R, Pedreira S, Smecuol E, Vazquez H, Cabanne A, Boerr LA, Valero J, Kogan Z, Maurino E, Bai JC. (1998). "Usefulness of videoduodenoscopy and vital dye staining as indicators of mucosal atrophy of celiac disease: assessment of interobserver agreement". Gastrointestinal Endoscopy. 47 (3): 223–229. PMID 9580349.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marsh M (1992). "Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue')". Gastroenterology. 102 (1): 330–54. PMID 1727768..
- ^ Popat S, Bevan S, Braegger C, Busch A, O'Donoghue D, Falth-Magnusson K, Godkin A, Hogberg L, Holmes G, Hosie K, Howdle P, Jenkins H, Jewell D, Johnston S, Kennedy N, Kumar P, Logan R, Love A, Marsh M, Mulder C, Sjoberg K, Stenhammar L, Walker-Smith J, Houlston R (2002). "Genome screening of coeliac disease". J Med Genet. 39 (5): 328–31. PMID 12011149.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ a b Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken E, Schuppan D (1997). "Identification of tissue transglutaminase as the autoantigen of celiac disease". Nat Med. 3 (7): 797–801. PMID 9212111.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Kaukinen K, Peraaho M, Collin P, Partanen J, Woolley N, Kaartinen T, Nuuntinen T, Halttunen T, Maki M, Korponay-Szabo I (2005). "Small-bowel mucosal tranglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: A Prospective and radmonized clinical study". Scand J Gastroenterology. 40: 564–572. PMID 16036509.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Salmi TT, Collin P, Korponay-Szabo IR, Laurila K, Partanen J, Huhtala H, Kiraly R, Lorand L, Reunala T, Maki M, and Kaukinen K. (2006). "Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits". Gut 2006 Mar 29; [Epub ahead of print]. PMID 16571636.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Londei M, Ciacci C, Ricciardelli I, Vacca L, Quaratino S, and Maiuri L. (2005). "Gliadin as a stimulator of innate responses in celiac disease". Mol Immunol. 42 (8): 913–918. PMID 15829281.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, Emery LM, Sokol RJ, Erlich HA, Eisenbarth GS, Rewers M. (2005). "Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease". JAMA. 293 (19): 2343–2351. PMID 15900004.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Suman S, Williams E, Thomas P, Surgenor S, Snook J (2003). "Is the risk of adult coeliac disease causally related to cigarette exposure?". Eur J Gastroenterol Hepatol. 15 (9): 995–1000. PMID 12923372.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Kupper C (2005). "Dietary guidelines and implementation for celiac disease". Gastroenterology. 128 (4 Suppl 1): S121-7. PMID 15825119.
- ^ Treem W (2004). "Emerging concepts in celiac disease". Curr Opin Pediatr. 16 (5): 552–9. PMID 15367850.
- ^ Häuser W, Gold J, Stein J, Caspary W, Stallmach A (2006). "Health-related quality of life in adult coeliac disease in Germany: results of a national survey". Eur J Gastroenterol Hepatol. 18 (7): 747–54. PMID 16772832.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Siegel M, Bethune M, Gass J, Ehren J, Xia J, Johannsen A, Stuge T, Gray G, Lee P, Khosla C (2006). "Rational design of combination enzyme therapy for celiac sprue". Chem Biol. 13 (6): 649–58. PMID 16793522.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum S (2000). "Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease". Lancet. 355 (9214): 1518–9. PMID 10801176.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Catassi C, Rätsch I, Gandolfi L, Pratesi R, Fabiani E, El Asmar R, Frijia M, Bearzi I, Vizzoni L (1999). "Why is coeliac disease endemic in the people of the Sahara?". Lancet. 354 (9179): 647–8. PMID 10466670.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Carlo Catassi (2002). "Coeliac disease, an emerging problem in developing countries". Celiachia News Inglese 2002. Associazione Italiana Celiachia. Retrieved 2006-09-04.
- ^ Fasano A, Berti I, Gerarduzzi T, Not T, Colletti R, Drago S, Elitsur Y, Green P, Guandalini S, Hill I, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman S, Murray J, Horvath K (2003). "Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study". Archives of Internal Medicine. 163 (3): 286–92. PMID 12578508.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Associated Press (December 8, 2004). "Girl with digestive disease denied Communion". MSNBC. Microsoft. Retrieved 2006-05-30..
- ^ Rabbi Avraham Juravel. "Gluten Intolerance, Celiac, Allergies And Pesach". Orthodox Union. Retrieved 2006-09-03.
- ^ Gee SJ (1888). On the coeliac affection. St Bartholomew's Hospital Report 24 : 17-20.
- ^ Haas SV (1924). The value of the banana in the treatment of coeliac disease. Am J Dis Child 24: 421-37.
- ^ van Berge-Henegouwen G, Mulder C (1993). "Pioneer in the gluten free diet: Willem-Karel Dicke 1905-1962, over 50 years of gluten free diet". Gut. 34 (11): 1473–5. PMID 8244125..
- ^ Dicke WK. Coeliakie: een onderzoek naar de nadelige invloed van sommige graansoorten op de lijder aan coeliakie [PhD thesis]. Utrecht, the Netherlands: University of Utrecht, 1950.
- ^ Anderson C, French J, Sammons H, Frazer A, Gerrard J, Smellie J (1952). "Coeliac disease; gastrointestinal studies and the effect of dietary wheat flour". Lancet. 1 (17): 836–42. PMID 14918439.
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Paulley J. "Observation on the aetiology of idiopathic steatorrhoea; jejunal and lymph-node biopsies". Br Med J. 4900: 1318–21. PMID 13209109..
- ^ Shiner M (1956). "Duodenal biopsy". Lancet. 270 (6906): 17–9. PMID 13279152..
External links
- Coeliac UK (charity)
- The Celiac Disease Foundation (U.S.)
- National Digestive Diseases Clearinghouse - page on coeliac disease
- National Foundation for Celiac Awareness (U.S.)
- University of Maryland Center for Celiac Research
