Menstrual cycle

The menstrual cycle is a series of natural changes in the uterus and ovaries of the female reproductive system that make pregnancy possible. The ovarian cycle controls the production and release of eggs. The uterine cycle governs the preparation and maintenance of the lining of the uterus (womb) to receive a fertilized egg. These cycles are concurrent and coordinated, and last between 25 and 30 days, with a median length of 28 days.[1]
Naturally occurring hormones drive the cycles; the cyclical rise and fall of the hormone estrogen prompts the production and growth of oocytes (immature egg cells). The hormone progesterone stimulates the uterus lining to thicken to accommodate an embryo should fertilization occur.[2] The blood supply of the thickened lining (endometrium) provides nutrients to a successfully implanted embryo. If implantation does not occur, the lining breaks down and blood is released. Triggered by falling progesterone levels, menstruation (a "period", in common parlance) is the cyclical shedding of the lining, and is a sign that pregnancy has not occurred.[3]
Each cycle occurs in phases based on events in the ovary (ovarian cycle) or the uterus (uterine cycle). The ovarian cycle consists of the follicular phase, ovulation, and the luteal phase; the uterine cycle consists of the menstrual, proliferative and secretory phases.[4] Day one of the menstrual cycle is the first day of the period, which lasts for about five days. Around day fourteen, the egg is released from the ovary.[5] Menarche (the onset of the first period) usually occurs around the age of twelve years.[6] Menstrual cycles end at menopause, which is usually between 45 and 55 years of age.[7]
During their menstrual cycle, some women experience problems that disrupt daily life;[8] such problems can include cramps,[9] tender breasts, bloating, tiredness, irritability, mood changes[10] and premenstrual syndrome. More severe problems such as premenstrual dysphoric disorder are experienced by 3 to 8% of women.[11][12] The menstrual cycle can be modified by hormonal birth control.[13]
Cycles and phases
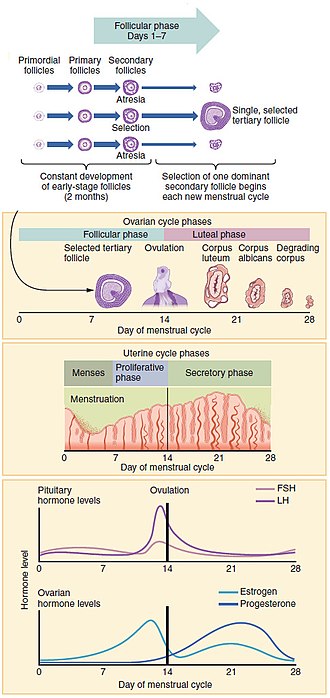
The menstrual cycle encompasses the ovarian and uterine cycles. The ovarian cycle describes changes that occur in the follicles of the ovary,[14] whereas the uterine cycle describes changes in the endometrial lining of the uterus. Both cycles can be divided into phases. The ovarian cycle consists of alternating follicular and luteal phases, and the uterine cycle consists of menstruation, the proliferative phase, and the secretory phase.[4] The menstrual cycle is controlled by the hypothalamus and the pituitary gland in the brain. The hypothalamus releases gonadotropin releasing hormone (GnRH), which causes the nearby anterior pituitary gland to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Before puberty GnRH is released in steady quantities and at a steady rate. After puberty GnRH is released in large pulses and the frequency and magnitude of these determine how much FSH and LH are produced by the pituitary.[2]
Measured from the first day of one menstruation to the first day of the next, the length of a menstrual cycle varies.[1] The cycle is often less regular at the beginning and end of a woman's reproductive life.[1] At puberty a child's body begins to mature into an adult body capable of sexual reproduction; the first period (called menarche) occurs at around 12 years of age.[6][15] The cessation of menstrual cycles at the end of a woman's reproductive period at menopause commonly occurs between the ages of 45 and 55.[7]
Ovarian cycle
Between menarche and menopause the human ovaries regularly alternate between luteal and follicular phases during the monthly menstrual cycle.[16] Stimulated by gradually increasing amounts of estrogen in the follicular phase, discharges of blood flow stop and the uterus lining thickens. Follicles in the ovary begin developing under the influence of a complex interplay of hormones, and after several days one, or occasionally two, become dominant, while non-dominant follicles shrink and die. About mid-cycle, some 34–36 hours after the luteinizing hormone (LH) surges,[1] the dominant follicle releases an oocyte, in an event called ovulation.[17]
After ovulation, the oocyte lives for 24 hours or less without fertilization,[18] while the remains of the dominant follicle in the ovary become a corpus luteum—a body with the primary function of producing large amounts of the hormone progesterone.[19] Under the influence of progesterone, the uterine lining changes to prepare for potential implantation of an embryo to establish a pregnancy. The thickness of the endometrium continues to increase in response to mounting levels of estrogen, which is released by the antral follicle (a mature ovarian follicle) into the blood circulation. Peak levels of estrogen are reached at around day thirteen of the cycle and coincide with ovulation. If implantation does not occur within about two weeks, the corpus luteum degenerates into the corpus albicans, which does not produce hormones, causing a sharp drop in levels of both progesterone and estrogen. This hormone drop causes the uterus to loose its lining in menstruation; it is around this time that the lowest levels of estrogen are reached.[20]
On average the ovarian cycle lasts about 28 days;[1][16] for most women, cycle lengths are between 25 and 30 days.[1] Although the average length of the human menstrual cycle is similar to that of the lunar cycle, there is no causal relation between the two.[21]
Follicular phase
The ovaries contain a finite number of egg stem cells, granulosa cells and theca cells, which together form primordial follicles.[19] Beginning at puberty, these mature to primary follicles independently of the menstrual cycle.[22] The development of the egg is called oogenesis and only one cell survives the divisions to await fertilization. The other cells are discarded as polar bodies, which cannot be fertilized.[23] The follicular phase is the first part of the ovarian cycle and it ends with the completion of the antral follicles.[16] Meiosis (cell division) remains incomplete in the egg cells until the antral follicle is formed. During this phase usually only one ovarian follicle fully matures and gets ready to release an egg.[24] The follicular phase shortens significantly with age, lasting around 14 days in women aged 18–24 compared with 10 days in women aged 40–44).[20]
Through the influence of a rise in follicle stimulating hormone (FSH) during the first days of the cycle, a few ovarian follicles are stimulated. These follicles, which have been developing for the better part of a year in a process known as folliculogenesis, compete with each other for dominance. Under the influence of several hormones, all but one of these follicles will stop growing, while one dominant follicle in the ovary will continue to maturity. The remaining follicles die in a process called follicular atresia.[25] Luteinising hormone (LH) stimulates further development of the ovarian follicle. The follicle that reaches maturity is called an antral follicle, and it contains the ovum (egg cell).[2]
The theca cells develop receptors that bind LH, and in response secrete large amounts of androstenedione. At the same time the granulosa cells surrounding the maturing follicle develop receptors that bind FSH, and in response start secreting androstenedione, which is converted to estrogen by the enzyme aromatase. The estrogen inhibits further production of FSH and LH by the pituitary gland. This negative feedback regulates levels of FSH and LH. The dominant follicle continues to secrete estrogen, and the rising estrogen levels make the pituitary more responsive to GnRH from the hypothalamus. As estrogen increases this becomes a positive feedback signal, which makes the pituitary secrete more FSH and LH. This surge of FSH and LH usually occurs one to two days before ovulation, and is responsible for stimulating the rupture of the antral follicle and release of the oocyte.[22][26]
Ovulation

At around 20 weeks gestation some 7 million immature eggs are already formed in an ovary. This decreases to around 2 million by the time a girl is born, and 300,000 by the time she has her first period. On average one egg matures and is released during ovulation each month after menarche.[27]
Ovulation occurs when a mature egg is released from the ovarian follicles into the fallopian tube about 10 to 12 hours after the peak in LH levels.[1] Most times only one of the 15–20 follicles that have been stimulated reaches full maturity, and just one egg is released.[28] Ovulation only occurs in around 10% of cycles during the first two years following menarche, and by the age of 40–50 the number of ovarian follicles is depleted.[29] Luteinising hormone initiates ovulation at around day 14 and stimulates the formation of the corpus luteum.[4] In turn, and following further stimulation by luteinising hormone, the corpus luteum produces and releases estrogen, progesterone, relaxin (which relaxes the uterus by inhibiting contractions of the myometrium), and inhibin (which inhibits secretion of more luteinising hormone).[2]
The release of LH matures the egg and weakens the wall of the follicle in the ovary, causing the fully developed follicle to release its oocyte.[30] If it is fertilized by a sperm, the oocyte promptly matures into an ootid which blocks the other sperm cells and becomes a mature egg. If it is not fertilized by a sperm, the oocyte will degenerate. The mature egg has a diameter of about 0.1 mm (0.0039 in),[31] and is the largest human cell.[32]
Which of the two ovaries—left or right—ovulates appears random;[33] no left and right coordinating process is known.[34] Occasionally both ovaries will release an egg; if both eggs are fertilized, the result is fraternal twins.[35] After release from the ovary, the egg is swept into the fallopian tube by the fimbria—a fringe of tissue at the end of each fallopian tube. After about a day, an unfertilized egg will disintegrate or dissolve in the fallopian tube, and a fertilized egg will reach the uterus in three to five days.[36]
Fertilization usually takes place in the ampulla, the widest section of the fallopian tubes. A fertilized egg immediately starts the process of embryogenesis (development). The developing embryo takes about three days to reach the uterus, and another three days to implant into the endometrium. It has usually reached the blastocyst stage at the time of implantation: this is when pregnancy begins.[37] The loss of the corpus luteum is prevented by fertilization of the egg. The syncytiotrophoblast (the outer layer of the resulting embryo-containing blastocyst that later becomes the outer layer of the placenta), produces human chorionic gonadotropin (hCG), which is very similar to LH and which preserves the corpus luteum. The corpus luteum continues to secrete progestoerone and estrogens at levels slightly above those at ovulation during the first few months of pregnancy. After this and for the rest of the pregnancy, the placenta secretes high levels of these hormones along with human chorionic gonadotropin (hCG), which stimulates the corpus luteum to secrete more progesterone and estrogens, blocking the menstrual cycle.[38] These hormones also prepare the mammary glands for milk[a] production.[38]
Luteal phase
Lasting about 14 days,[1] the luteal phase is the final phase of the ovarian cycle and it corresponds to the secretory phase of the uterine cycle. During the luteal phase, the pituitary hormones FSH and LH cause the remaining parts of the dominant follicle to transform into the corpus luteum, which produces progesterone.[40] The increased progesterone in the adrenal cortex starts to induce the production of estrogen. The hormones produced by the corpus luteum also suppress production of the FSH and LH that the corpus luteum needs to maintain itself. The level of FSH and LH fall quickly, and the corpus luteum atrophies.[41] Falling levels of progesterone trigger menstruation and the beginning of the next cycle. From the time of ovulation until progesterone withdrawal has caused menstruation to begin, the process typically takes about two weeks. For an individual woman, the follicular phase often varies in length from cycle to cycle; by contrast, the length of her luteal phase will be fairly consistent from cycle to cycle.[20] The luteal phase is about the same length in most individuals (average 14 days) whereas the follicular phase tends to show much more variability lasting from 10 to 16 days.[20]
Uterine cycle

The uterine cycle has three phases: menses, proliferative and secretory.[42]
Menstruation
Menstruation (also called menstrual bleeding, menses or a period) is the first and most evident phase of the uterine cycle and begins at puberty. Called menarche, the first period occurs at the age of around twelve or thirteen years.[15] The average age is generally later in the developing world and earlier in developed world.[43] In precocious puberty, it can occur as early as age eight years,[44] and this can still be normal.[45][46]
Menstruation is initiated each month by falling levels of estrogen and progesterone and the release of prostaglandins,[24] which constrict the spiral arteries. This causes them to spasm, contract and break up.[47] The blood supply to the endometrium is cut off and the cells of the top layer of the endometrium (the stratum functionalis) become deprived of oxygen and die. Later the whole layer is lost and only the bottom layer, the stratum basalis, is left in place.[24] An enzyme called plasmin breaks up the blood clots in the menstrual fluid, which eases the flow of blood and broken down lining from the uterus.[48]
The flow of blood normally serves as a sign that a woman has not become pregnant, but this cannot be taken as certainty, as several factors can cause bleeding during pregnancy.[49] Menstruation occurs on average once a month from menarche to menopause, which corresponds with a woman's fertile years. The average age of menopause in women is 52 years, and it typically occurs between 45 and 55 years of age.[50] Menopause is preceded by a stage of hormonal changes and irregular cycles called perimenopause.[7]
Eumenorrhea denotes normal, regular menstruation that lasts for a few days (usually 3 to 5 days, but anywhere from 2 to 7 days is considered normal).[51] The average blood loss during menstruation is 30 milliliters (mL), and more than 80 mL is considered abnormal.[1] Women who experience menorrhagia (heavy menstrual bleeding) are more susceptible to iron deficiency than the average person.[52]
Proliferative phase
The proliferative phase is the second phase of the uterine cycle when estrogen causes the lining of the uterus to grow and proliferate.[41] The latter part of the follicular phase overlaps with the proliferative phase of the uterine cycle.[33] As they mature, the ovarian follicles secrete increasing amounts of estradiol, an estrogen. The estrogens initiate the formation of a new layer of endometrium in the uterus with the spiral arterioles.[4]
As estrogen levels increase, cells in the cervix produce a type of cervical mucus[53] that has a higher pH and is less viscous than usual, rendering it more friendly to sperm.[54] This increases the chances of fertilization, which occurs around day 11 to day 14.[18] This cervical mucus can be detected as a vaginal discharge that is copious and resembles raw egg whites.[55] For women who are practicing fertility awareness, it is a sign that ovulation is about to take place.[55]
Secretory phase
The secretory phase is the final phase of the uterine cycle and it corresponds to the luteal phase of the ovarian cycle. During the secretory phase, the corpus luteum produces progesterone, which plays a vital role in making the endometrium receptive to the implantation of a blastocyst (a fertilized egg, which has begun to grow).[56] Glycogen, lipids, and proteins are secreted into the uterus[57] and the cervical mucus thickens.[58] In early pregnancy progesterone also increases blood flow and reduces the contractility of the smooth muscle in the uterus[2] and raises the woman's basal body temperature.[59]
If pregnancy does not occur the ovarian and uterine cycles start over again.[48]
Menstrual health

Some women experience problems sufficient to disrupt their lives as a result their menstrual cycle.[8] These include acne, tender breasts, bloating, feeling tired, irritability mood changes,[10] and premenstrual syndrome (PMS). More severe problems such as premenstrual dysphoric disorder are experienced by 3 to 8% of women.[1][11] Dysmenorrhea is frequently a source of pelvic pain,[60] causing cramps in the abdomen, back, or upper thighs that occur during the first few days of menstruation.[61] These issues can significantly affect a woman's health and quality of life and timely interventions can improve the lives of these women.[62]
There is little evidence to support the common belief that the menstrual cycle affects a woman's moods, but this belief can cause women to blame their normal mood variations on their menstrual cycle. The belief that the premenstrual phase causes depression or that menstruation is painful, shameful, or "unclean" could be a self-fulfilling prophecy. Non-emotional changes are also minor and rare; athletic and general intellectual achievement—including academic performance, problem-solving, memory, and creativity—do not vary during the menstrual cycle. Any improvements in spatial reasoning ability are probably caused by the lower levels of estrogen and progesterone during the menstruation phase of the cycle.[63]
When menstruation begins, symptoms of PMS such as breast tenderness and irritability generally decrease.[51] In some women, ovulation features a characteristic pain[b] called mittelschmerz (a German term meaning middle pain).[51] This occurs when an egg does not enter the fallopian tube but the pelvic cavity where it breaks up. The cause of the pain is the small amount of blood loss associated with the ruptured follicle.[24]
Even when normal, the changes in hormone levels during the menstrual cycle can increase the incidence of disorders such as autoimmune diseases,[67] which might be caused by estrogen enhancement of the immune system.[1]
Hormonal contraception
Hormonal contraceptives prevent pregnancy by inhibiting the secretion of the hormones, FSH, LH and GnRH. Containing estrogen, combined oral contraceptive pills (COCs, often referred to as birth control pills) stop the development of the dominant follicle and the mid-cycle LH surge and thus ovulation.[68] Another hormone in COCs called progestin stops the cervical mucus from becoming sperm-friendly but does not always prevent ovulation when used alone in progestin-only pills. COCs regulate the length of the menstrual cycle and reduce the amount of blood lost. Hormonal contraception is available in a variety of forms such as pills, patches, skin implants and hormonal intrauterine devices (IUDs).[69]
Evolution and other species
Most female mammals have an estrous cycle, but only ten primate species, four bat species, the elephant shrew and the spiny mouse have a menstrual cycle.[70] The cycles are the same as in humans apart from the length, which ranges from 21 to 37 days.[71] The lack of immediate relationship between these groups suggests that four distinct evolutionary events have caused menstruation to arise.[72] In species that have a menstrual cycle, ovulation is not obvious to potential mates and there is no mating season.[73][74] There are four theories on the evolutionary significance of menstruation:[72]
- Control of sperm-borne pathogens.[75][76][77] This hypothesis held that menstruation protected the uterus against pathogens introduced by sperm. Hypothesis 1 does not take into account that copulation can take place weeks before menstruation and that potentially infectious semen is not controlled by menstruation in other species.[72]
- Energy conservation.[76][78] This hypothesis claimed that it took less energy to rebuild a uterine lining than to maintain it if pregnancy did not occur. Hypothesis 2 does not explain other species that also do not maintain a uterine lining but do not menstruate.[72]
- A theory based on spontaneous decidualization (a process that results in significant changes to cells of the endometrium in preparation for, and during, pregnancy, in which the endometrium changes into the decidua). Decidualization leads to the development of the endothelium, which involves cells of the immune system,[71] the formation of a new blood supply, hormones and tissue differentiation. In non-menstruating mammals, decidualization is driven by the embryo, not the mother.[76] It evolved in some placental mammals because it confers advantages in that it allows females to prepare for pregnancy without needing a signal from the fetus.[72] Hypothesis 3 defers to an explanation of the evolutionary origin of spontaneous decidualization and does not explain the evolution of menstruation alone.[72]
- Uterine pre-conditioning.[79] This hypothesis claims that a monthly pre-conditioning of the uterus is needed in species, such as humans, that have deeply invasive (deep-rooted) placentas. In the process leading to the formation of a placenta, maternal tissues are invaded. This hypothesis holds that menstruation was not evolutionary, rather the result of a coincidental pre-conditioning of the uterus to protect uterine tissue from the deeply rooting placenta, in which a thicker endometrium develops.[79] Hypothesis 4 does not explain menstruation in non-primates.[72]
Notes
- ^ Breastfeeding women can experience complete suppression of follicular development, follicular development but no ovulation, or resumption of normal menstrual cycles.[39]
- ^ Uncharacteristic mid-cycle pain may be caused by medical conditions such as ectopic pregnancy or ruptured ovarian cyst[64][65] or may be confused with appendicitis.[66]
References
- ^ a b c d e f g h i j k Reed BF, Carr BR, Feingold KR, et al. (2018). "The Normal Menstrual Cycle and the Control of Ovulation". Endotext (Review). PMID 25905282.
- ^ a b c d e Tortora 2017, p. 942.
- ^ Johnson 2007, p. 99.
- ^ a b c d Tortora 2017, p. 944.
- ^ Tortora 2017, p. 943.
- ^ a b Lacroix AE, Gondal H, Langaker MD (2020). "Physiology, Menarche". StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (Review). PMID 29261991.
- ^ a b c Rodriguez-Landa 2017, p. 8.
- ^ a b Gudipally PR, Sharma GK (2020). Premenstrual syndrome (Review). StatPearls [Internet]. PMID 32809533.
- ^ Else-Quest & Hyde 2021, pp. 258.
- ^ a b "Premenstrual syndrome (PMS) fact sheet". Office of Women's Health. United States Department of Health and Human Services . 23 December 2014. Archived from the original on 28 June 2015. Retrieved 23 June 2015.
- ^ a b Appleton SM (March 2018). "Premenstrual syndrome: evidence-based evaluation and treatment". Clinical Obstetrics and Gynecology (Review). 61 (1): 52–61. doi:10.1097/GRF.0000000000000339. PMID 29298169. S2CID 28184066.
- ^ Biggs WS, Demuth RH (October 2011). "Premenstrual syndrome and premenstrual dysphoric disorder". American Family Physician (Review). 84 (8): 918–24. PMID 22010771.
- ^ Johnson 2007, p. 285.
- ^ Richards JS (2018). "The ovarian cycle". Vitamins and Hormones (Review). 107: 1–25. doi:10.1016/bs.vh.2018.01.009. ISBN 978-0-128-14359-9. PMID 29544627.
- ^ a b Papadimitriou A (December 2016). "The evolution of the age at menarche from prehistorical to modern times". Journal of Pediatric and Adolescent Gynecology (Review). 29 (6): 527–30. doi:10.1016/j.jpag.2015.12.002. PMID 26703478.
- ^ a b c Sherwood 2016, p. 741.
- ^ Sherwood 2016, p. 747.
- ^ a b Tortora 2017, p. 957.
- ^ a b Tortora 2017, p. 929.
- ^ a b c d Tortora 2017, pp. 942–46.
- ^ Norris & Carr 2013, p. 361.
- ^ a b Watchman 2020, p. 8.
- ^ Schmerler S, Wessel GM (January 2011). "Polar bodies – more a lack of understanding than a lack of respect". Molecular Reproduction and Development (Review). 78 (1): 3–8. doi:10.1002/mrd.21266. PMC 3164815. PMID 21268179.
- ^ a b c d Tortora 2017, p. 945.
- ^ Johnson 2007, p. 86.
- ^ Sherwood 2016, p. 745.
- ^ Ugwumadu 2014, p. 115.
- ^ Sadler 2019, p. 48.
- ^ Tortora 2017, p. 953.
- ^ Sherwood 2016, p. 746.
- ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Eggs". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 0-8153-3218-1.
- ^ Iussig B, Maggiulli R, Fabozzi G, Bertelle S, Vaiarelli A, Cimadomo D, Ubaldi FM, Rienzi L (May 2019). "A brief history of oocyte cryopreservation: Arguments and facts". Acta Obstetricia et Gynecologica Scandinavica (Review). 98 (5): 550–58. doi:10.1111/aogs.13569. PMID 30739329.
- ^ a b Parker 2019, p. 283.
- ^ Johnson 2007, p. 192–93.
- ^ Johnson 2007, p. 192.
- ^ Sadler 2019, p. 36.
- ^ Tortora 2017, p. 959.
- ^ a b Tortora 2017, p. 976.
- ^ Carr SL, Gaffield ME, Dragoman MV, Phillips S (September 2016). "Safety of the progesterone-releasing vaginal ring (PVR) among lactating women: A systematic review". Contraception (Review). 94 (3): 253–61. doi:10.1016/j.contraception.2015.04.001. PMID 25869631.
- ^ Johnson 2007, p. 91.
- ^ a b Ugwumadu 2014, p. 117.
- ^ Salamonsen LA (December 2019). "Women in reproductive science: Understanding human endometrial function". Reproduction (Cambridge, England) (Review). 158 (6): F55–F67. doi:10.1530/REP-18-0518. PMID 30521482.
- ^ Alvergne A, Högqvist Tabor V (June 2018). "Is female health cyclical? Evolutionary perspectives on menstruation". Trends in Ecology & Evolution (Review). 33 (6): 399–414. arXiv:1704.08590. doi:10.1016/j.tree.2018.03.006. PMID 29778270. S2CID 4581833.
- ^ Ibitoye M, Choi C, Tai H, Lee G, Sommer M (2017). "Early menarche: A systematic review of its effect on sexual and reproductive health in low- and middle-income countries". PLOS ONE (Review). 12 (6): e0178884. Bibcode:2017PLoSO..1278884I. doi:10.1371/journal.pone.0178884. PMC 5462398. PMID 28591132.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Menstruation and the menstrual cycle fact sheet". Office of Women's Health. US Department of Health and Human Services. 23 December 2014. Archived from the original on 26 June 2015. Retrieved 25 June 2015.
- ^ Sultan C, Gaspari L, Maimoun L, Kalfa N, Paris F (April 2018). "Disorders of puberty" (PDF). Best Practice & Research. Clinical Obstetrics & Gynaecology (Review). 48: 62–89. doi:10.1016/j.bpobgyn.2017.11.004. PMID 29422239.
- ^ Johnson 2007, pp. 152.
- ^ a b Tortora 2017, p. 600.
- ^ Breeze C (May 2016). "Early pregnancy bleeding". Australian Family Physician (Review). 45 (5): 283–86. PMID 27166462.
- ^ Towner MC, Nenko I, Walton SE (April 2016). "Why do women stop reproducing before menopause? A life-history approach to age at last birth". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences (Review). 371 (1692): 20150147. doi:10.1098/rstb.2015.0147. PMC 4822427. PMID 27022074.
- ^ a b c Goldenring JM (1 February 2019). "All About Menstruation". WebMD. Retrieved 26 February 2021.
- ^ Harvey LJ, Armah CN, Dainty JR, Foxall RJ, John Lewis D, Langford NJ, Fairweather-Tait SJ (October 2005). "Impact of menstrual blood loss and diet on iron deficiency among women in the UK". The British Journal of Nutrition (Comparative study). 94 (4): 557–64. doi:10.1079/BJN20051493. PMID 16197581.
- ^ Simmons RG, Jennings V (July 2020). "Fertility awareness-based methods of family planning". Best Practice & Research. Clinical Obstetrics & Gynaecology (Review). 66: 68–82. doi:10.1016/j.bpobgyn.2019.12.003. PMID 32169418.
- ^ Tortora 2017, pp. 936–37.
- ^ a b Su HW, Yi YC, Wei TY, Chang TC, Cheng CM (September 2017). "Detection of ovulation, a review of currently available methods". Bioeng Transl Med (Review). 2 (3): 238–46. doi:10.1002/btm2.10058. PMC 5689497. PMID 29313033.
- ^ Lessey BA, Young SL (April 2019). "What exactly is endometrial receptivity?". Fertility and Sterility (Review). 111 (4): 611–17. doi:10.1016/j.fertnstert.2019.02.009. PMID 30929718.
- ^ Salamonsen LA, Evans J, Nguyen HP, Edgell TA (March 2016). "The microenvironment of human implantation: determinant of reproductive success". American Journal of Reproductive Immunology (New York, N.Y. : 1989) (Review). 75 (3): 218–25. doi:10.1111/aji.12450. PMID 26661899.
- ^ Han L, Taub R, Jensen JT (November 2017). "Cervical mucus and contraception: what we know and what we don't". Contraception (Review). 96 (5): 310–321. doi:10.1016/j.contraception.2017.07.168. PMID 28801053.
- ^ Charkoudian N, Hart EC, Barnes JN, Joyner MJ (June 2017). "Autonomic control of body temperature and blood pressure: influences of female sex hormones" (PDF). Clinical Autonomic Research (Review). 27 (3): 149–55. doi:10.1007/s10286-017-0420-z. PMID 28488202. S2CID 3773043.
- ^ Nagy H, Khan MA (2020). "Dysmenorrhea". StatPearls (Review). PMID 32809669.
- ^ Baker FC, Lee KA (September 2018). "Menstrual cycle effects on sleep". Sleep Medicine Clinics (Review). 13 (3): 283–94. doi:10.1016/j.jsmc.2018.04.002. PMID 30098748.
- ^ Matteson KA, Zaluski KM (September 2019). "Menstrual health as a part of preventive health care". Obstetrics and Gynecology Clinics of North America (Review). 46 (3): 441–53. doi:10.1016/j.ogc.2019.04.004. PMID 31378287.
- ^ Else-Quest & Hyde 2021, pp. 258–61.
- ^ Kruszka PS, Kruszka SJ (July 2010). "Evaluation of acute pelvic pain in women". Am Fam Physician (Review). 82 (2): 141–47. PMID 20642266.
- ^ Cleary M, Flanagan KW (2019). Acute and Emergency Care in Athletic Training. Human Kinetics. p. 340.
- ^ Brott NR, Le JK (2020). "Mittelschmerz". Stat Pearls [Internet] (Review). PMID 31747229.
- ^ Talsania M, Scofield RH (May 2017). "Menopause and rheumatic disease". Rheumatic Diseases Clinics of North America (Review). 43 (2): 287–302. doi:10.1016/j.rdc.2016.12.011. PMC 5385852. PMID 28390570.
- ^ Tortora 2017, p. 948.
- ^ Tortora 2017, pp. 948–49.
- ^ Bellofiore N, Ellery SJ, Mamrot J, Walker DW, Temple-Smith P, Dickinson H (January 2017). "First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus)". American Journal of Obstetrics and Gynecology (Journal article). 216 (1): 40.e1–40.e11. doi:10.1016/j.ajog.2016.07.041. PMID 27503621. S2CID 88779.
- ^ a b Catalini L, Fedder J (May 2020). "Characteristics of the endometrium in menstruating species: lessons learned from the animal kingdom†". Biology of Reproduction (Journal article). 102 (6): 1160–1169. doi:10.1093/biolre/ioaa029. PMC 7253787. PMID 32129461.
- ^ a b c d e f g Emera D, Romero R, Wagner G (January 2012). "The evolution of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness". BioEssays : News and Reviews in Molecular, Cellular and Developmental Biology (Journal article). 34 (1): 26–35. doi:10.1002/bies.201100099. PMC 3528014. PMID 22057551.
{{cite journal}}: Unknown parameter|lay-date=ignored (help); Unknown parameter|lay-source=ignored (help); Unknown parameter|lay-url=ignored (help) - ^ Schjenken JE, Robertson SA (July 2020). "The female response to seminal fluid". Physiological Reviews (Review). 100 (3): 1077–117. doi:10.1152/physrev.00013.2018. PMID 31999507.
- ^ Muller MN (May 2017). "Testosterone and reproductive effort in male primates". Hormones and Behavior (Review). 91: 36–51. doi:10.1016/j.yhbeh.2016.09.001. PMC 5342957. PMID 27616559.
- ^ Martin RD (2007). "The evolution of human reproduction: a primatological perspective". American Journal of Physical Anthropology (Review). Suppl 45: 59–84. doi:10.1002/ajpa.20734. PMID 18046752.
- ^ a b c Finn CA (June 1998). "Menstruation: a nonadaptive consequence of uterine evolution". The Quarterly Review of Biology (Review). 73 (2): 163–73. doi:10.1086/420183. PMID 9618925. S2CID 25135630.
- ^ Profet M (September 1993). "Menstruation as a defense against pathogens transported by sperm". The Quarterly Review of Biology (Review). 68 (3): 335–86. doi:10.1086/418170. PMID 8210311. S2CID 23738569.
- ^ Strassmann BI (June 1996). "The evolution of endometrial cycles and menstruation". The Quarterly Review of Biology (Review). 71 (2): 181–220. doi:10.1086/419369. PMID 8693059. S2CID 6207295.
- ^ a b Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, Brosens IA (June 2009). "A role for menstruation in preconditioning the uterus for successful pregnancy". American Journal of Obstetrics and Gynecology (Journal article). 200 (6): 615.e1–6. doi:10.1016/j.ajog.2008.11.037. PMID 19136085.
Book sources
- Else-Quest N, Hyde JS (2021). "Psychology, gender, and health: psychological aspects of the menstrual cycle". The psychology of women and gender: half the human experience + (10th ed.). Los Angeles: SAGE publications. ISBN 978-1-544-39360-5.
- Johnson MH (2007). Essential Reproduction. Malden, Massachusetts: Blackwell Publishing. ISBN 978-1-4051-1866-8. OCLC 76074156.
- Norris DA, Carr JA (2013). Vertebrate Endocrinology (5th ed.). Academic Press. ISBN 978-0-123-96465-6.
- Parker S (2019). The Concise Human Body Book: An Illustrated Guide to its Structures, Function and Disorders. London: Dorling Kindersley. ISBN 978-0-241-39552-3. OCLC 1091644711.
- Rodriguez-Landa J (2017). A Multidisciplinary Look at Menopause. Rijeka, Croatia: IntechOpen. ISBN 978-953-51-3405-3. OCLC 1193045564.
- Sadler TW (2019). Langman's Medical Embryology. Philadelphia: Wolters Kluwer. ISBN 978-1-4963-8390-7. OCLC 1042400100.
- Sherwood L (2016). Human Physiology: From Cells to Systems. Boston, MA, USA: Cengage Learning. ISBN 978-1-285-86693-2. OCLC 905848832.
- Tortora G (2017). Tortora's Principles of Anatomy & Physiology. Hoboken, NJ: John Wiley & Sons, Inc. ISBN 978-1-119-38292-8. OCLC 990424568.
- Ugwumadu A (2014). Basic Sciences for Obstetrics and Gynaecology: Core Material for MRCOG. Oxford, England: Oxford University Press. ISBN 978-0-19-953508-8. OCLC 889303297.
- Watchman T (2020). Zero to Finals : Obstetrics and Gynaecology. Manchester: Zero to Finals. ISBN 979-8-6037-9726-7. OCLC 1233034578.
External links
![]() Media related to Menstrual cycle at Wikimedia Commons
Media related to Menstrual cycle at Wikimedia Commons
