Addition reaction
- العربية
- Bosanski
- Català
- Čeština
- Dansk
- Deutsch
- Eesti
- Ελληνικά
- Español
- Euskara
- فارسی
- Français
- Gaeilge
- 한국어
- हिन्दी
- Hrvatski
- Bahasa Indonesia
- Italiano
- עברית
- Magyar
- മലയാളം
- Bahasa Melayu
- Nederlands
- 日本語
- Norsk bokmål
- Norsk nynorsk
- Oʻzbekcha / ўзбекча
- پښتو
- Polski
- Português
- Română
- Русский
- Simple English
- Slovenčina
- Slovenščina
- کوردی
- Српски / srpski
- Srpskohrvatski / српскохрватски
- Suomi
- Svenska
- தமிழ்
- Türkçe
- Українська
- Tiếng Việt
- 中文
This is an old revision of this page, as edited by Nickpayifson (talk | contribs) at 20:05, 24 May 2021 (some of it was ambiguous so I clarified the definition with my own brain. My own creative work). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
An addition reaction, in organic chemistry, is a reaction driven by electrophilic and nucleophilic mechanism. Where the double or triple bond attacks and electrophile producing a carbocation intermediate or a haloni ion intermediat. In most cases, trans- isomers are observed due to a halonium ion intermediate rather than a carbocation intermediate. The product results in the addition of new atoms and no by products.[1]
Addition reactions are limited to chemical compounds that have multiple bonds, such as molecules with carbon–carbon double bonds (alkenes), or with triple bonds (alkynes), and compounds that have rings, which are also considered points of unsaturation. Molecules containing carbon—hetero double bonds like carbonyl (C=O) groups, or imine (C=N) groups, can undergo addition, as they too have double-bond character.
An addition reaction is the reverse of an elimination reaction. For instance, the hydration of an alkene to an alcohol is reversed by dehydration.
There are two main types of polar addition reactions: electrophilic addition and nucleophilic addition. Two non-polar addition reactions exist as well, called free-radical addition and cycloadditions. Addition reactions are also encountered in polymerizations and called addition polymerization.
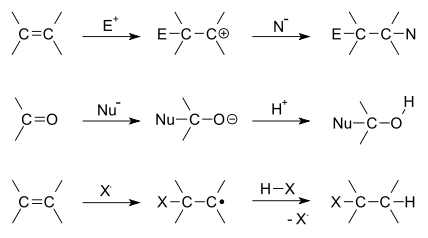
Depending on the product structure, it could promptly react further to eject a leaving group to give the addition–elimination reaction sequence.
References
- ^ Armstrong, Susan (2002-12). [http://dx.doi.org/10.1007/s00897020632a "The Molecular World: Alkenes and Aromatics. Edited by Peter Taylor and Michael Gagan. The Royal Society ofChemistry and The Open University: Cambridge, U.K., July2002. 184 pp, paperback, 263 � 210 mm. �17.50. ISBN0854046801"]. The Chemical Educator. 7 (6): 388–389. doi:10.1007/s00897020632a. ISSN 1430-4171.
{{cite journal}}: Check date values in:|date=(help); replacement character in|title=at position 192 (help)
External links
Basic reaction mechanisms | |
|---|---|
| Nucleophilic substitutions | |
| Electrophilic substitutions | |
| Elimination reactions | |
| Addition reactions | |
| Unimolecular reactions | |
| Electron/Proton transfer reactions | |
| Medium effects | |
| Related topics | |
| Chemical kinetics | |
