Tracheal intubation
| Tracheal intubation | |
|---|---|
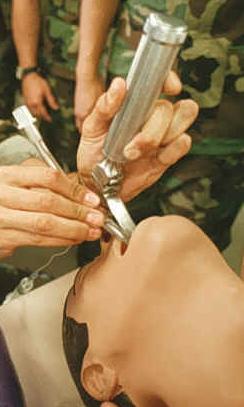 Tracheal intubation being practiced on a mannequin (orotracheal technique using a laryngoscope. | |
| ICD-9-CM | 96.04 |
| MeSH | D007442 |
Tracheal intubation (often simply referred to as intubation) is the placement of a flexible plastic tube into the trachea to protect the airway and provide a means of mechanical ventilation. The most common route for tracheal intubation is orotracheal where, with the assistance of a laryngoscope, an endotracheal tube is passed through the oropharynx, glottis, and larynx into the trachea. A high-volume, low-pressure cuff is then typically inflated near the distal tip of the tube to help secure it in place and protect the airway from blood, gastric contents and other secretions. Another route for tracheal intubation is nasotracheal, where an endotracheal tube is passed through the nasopharynx, glottis, and larynx into the trachea. Other routes for intubation of the trachea include the cricothyrotomy (used almost exclusively in emergency circumstances), and the tracheotomy (used primarily in circumstances where a prolonged need for airway support is anticipated).
After the trachea has been intubated and the tube has been secured to the face or neck, the proximal end of the tube is connected to a T-piece, anesthesia breathing circuit, bag valve mask device, or a mechanical ventilator. Once there is no longer a need for ventilatory assistance and/or protection of the airway, the tracheal tube may be removed; this is referred to as extubation of the trachea (or decannulation, in the case of a surgical airway such as a cricothyrotomy or a tracheotomy).
History
Pre-19th century

Tracheotomy was first depicted on Egyptian artifacts in 3600 BCE.[1] It was described in the Rigveda, a Hindi text, circa 2000 BCE.[1] Homerus of Byzantium is said to have written of Alexander the Great saving a soldier from suffocation in 1000 BCE by making an incision with the tip of his sword in the man's trachea.[1] Hippocrates condemned the practice of tracheotomy. Warning against the unacceptable risk of death from inadvertent laceration of the carotid artery during tracheotomy, he instead advocated the practice of tracheal intubation.[2] Because surgical instruments were not sterilized at that time, infections following surgery also produced numerous complications, including dyspnea, often leading to death.[3]
Despite the concerns of Hippocrates, it is believed that an early tracheotomy was performed by Asclepiades of Bithynia, who lived in Rome around 100 BCE. Galen and Aretaeus, both of whom lived in Rome in the second century AD, credit Asclepiades as being the first physician to perform a non-emergency tracheotomy.[citation needed] Antyllus, another Roman physician of the second century AD, supported tracheotomy when treating oral diseases. He refined the technique to be more similar to that used in modern times, recommending that a transverse incision be made between the third and fourth tracheal rings for the treatment of life-threatening airway obstruction.[2] Antyllus (whose original writings were lost but not before they were preserved by the Greek historian Oribasius) wrote that tracheotomy was not effective however in cases of severe laryngotracheobronchitis because the pathology was distal to the operative site.[3] In AD 131, Galen clarified the anatomy of the trachea and was the first to demonstrate that the larynx generates the voice.[citation needed]
By AD 700, the tracheotomy was well described in Indian and Arabian literature, although it was rarely practiced on humans.[3] Circa AD 1020, Avicenna described tracheal intubation in The Canon of Medicine in order to facilitate breathing.[4] The first correct description of the tracheotomy operation for treatment of asphyxiation was described by Ibn Zuhr in the 12th century,[5]
From 1500 to 1833 there are only 28 known reports of successful tracheotomy.[6] Although the Renaissance saw significant advances in science and surgery, and surgeons became increasingly open to experimental surgery on the trachea, nevertheless the mortality rate failed to improve.[6] The next known report on tracheal intubation and subsequent artificial respiration of animals was in 1543, when Andreas Vesalius pointed out that the technique could be life-saving.[citation needed] The next known report of human tracheotomy was in 1546, performed on a patient suffering from complications of a peritonsillar abscess; this patient apparently made a complete recovery.[2] In the late 16th century, anatomist Hieronymus Fabricius described a useful technique in his writings, although he had never actually performed a tracheotomy. He advised using a vertical incision and a straighter, shorter cannula that would prevent the tube from advancing too far into the trachea. He counseled that the operation should be performed only as a last option.[3] The first tracheotomy to be described on a pediatric patient was in 1620, after a boy began to ashyxiate on a bag of gold he had swallowed. The object became lodged in his esophagus, obstructing his trachea. The tracheotomy allowed the surgeon to manipulate the bag so that it passed through his alimentary tract, apparently with no further sequelae.[3]
19th century
In the early 19th century, the tracheotomy finally began to be recognized as a legitimate means of treating severe airway obstruction. In 1832, French physician Pierre Bretonneau employed it as a last resort to treat a case of diphtheria.[7] In 1852, Bretonneau's student Armand Trousseau reported a series of 169 tracheotomies (158 of which were for croup, and 11 for "chronic maladies of the larynx")[8] Trousseau's claimed 73% mortality rate was hailed as "very satisfying".[3] If the procedure is delayed until the patient is close to death, the body will have already incurred major damage due to anoxia. Despite this, surgeons continued to postpone the tracheotomy until it was too late to be effective. In 1869, the German surgeon Friedrich Trendelenburg reported the first successful elective human tracheotomy to be performed for the purpose of administration of general anesthesia.[citation needed] In 1878, the Scottish surgeon William Macewen reported the first orotracheal intubation.[citation needed] At last, in 1880 Morrell Mackenzie's book discussed the symptoms indicating a tracheotomy and when the operation is absolutely necessary.[2]
While all these surgical advances were taking place, many important developments were also taking place in the science of optics. Many new optical instruments with medical applications were invented during the 19th century. In 1805, German physician Philipp von Bozzini used a device he invented and called the lichtleiter (or light-guiding instrument) to examine the human urinary bladder, rectum, nasopharynx and laryngopharynx.[9][10] The practice of gastric endoscopy in humans was pioneered by United States Army surgeon William Beaumont in 1822 with the cooperation of his patient Alexis St. Martin, a victim of an accidental gunshot wound to the stomach.[11] In 1853, Antoine Jean Desormeaux of France examined the human bladder using a device he invented and called the endoscope (this was the first time this term was applied to this practice).[citation needed] In 1854, a singing teacher named Manuel Garcia became the first man to view the functioning glottis in its entirety. Garcia developed a tool that used two mirrors for which the sun served as an external light source.[12] Using this device, he was able to observe the function of his own glottic apparatus and the uppermost portion of his trachea. His findings were presented at the Royal Society of London in 1855.[10] In 1868, Adolph Kussmaul of Germany performed the first esophagogastroduodenoscopy on a living human. The subject was a sword-swallower, who swallowed a metal tube with a length of 47 centimeters and a diameter of 13 millimeters.[citation needed] On 2 October 1877, Berlin physician Maximilian Nitze and instrument maker Josef Leiter introduced the cystourethroscope[13] and in 1881, Polish physician Jan Mikulicz-Radecki created the first rigid gastroscope for practical applications.[14][15][16] All previous observations of the glottis and larynx had been performed under indirect vision (using mirrors) until 23 April 1895, when Alfred Kirstein of Germany first described direct visualization of the vocal cords. Kirstein performed the first direct laryngoscopy in Berlin, using an esophagoscope he had modified for this purpose; he called this device an autoscope.[17] It is believed that the death in 1888 of Kaiser Frederick from laryngeal cancer motivated Kirstein to develop the autoscope. [18]
20th century
The 20th century saw the transformation of the practices of endoscopy and tracheal intubation from rarely employed procedures to essential components of the practices of anesthesia, critical care medicine, emergency medicine, gastroenterology, pulmonology, and surgery. Until 1913, surgery involving the mouth and nose was performed by mask inhalation anesthesia, topical application of local anesthetics to the mucosa, rectal anesthesia, or intravenous anesthesia. While otherwise effective, these techniques did not protect the airway from obstruction and also exposed patients to the risk of aspiration of blood and mucus into the tracheobronchial tree. In 1913, Chevalier Jackson, a professor of laryngology at Jefferson Medical College in Philadelphia, Pennsylvania, was the first to report a high rate of success for the use of direct laryngoscopy in orotracheal intubation.[19] Jackson introduced a new laryngoscope blade that had a light source at the distal tip, rather than the proximal light source used by Kirstein.[20] This new blade incorporated a component that the operator could slide out to allow room for passage of an endotracheal tube or bronchoscope.[9][20]
That same year, Henry Janeway published results he had achieved using another new laryngoscope he had recently developed.[21] An American anesthesiologist practicing at Bellevue Hospital in New York City, Janeway believed that direct intratracheal insufflation of volatile anesthetics would provide improved conditions for surgery of the nose, mouth and throat. With this in mind, he developed a laryngoscope designed for the sole purpose of tracheal intubation. Similar to Jackson's device, Janeway's instrument incorporated a distal light source. Unique however was the inclusion of batteries within the handle of the laryngoscope. Additional features included a central notch for maintaining the tracheal tube in the midline of the oropharynx during intubation, and a slight curve to the distal tip of the blade to help guide the tube through the glottis. The success of this design led to its subsequent use in other types of surgery in addition to surgery of the nose, mouth and throat. Janeway was instrumental in popularizing the widespread use of direct laryngoscopy and tracheal intubation in the practice of anesthesiology.
After World War I, further advances were made in the field of intratracheal anesthesia. Perhaps most notable among these were those made by Sir Ivan Whiteside Magill (1888–1986). Working at the Queen's Hospital for Facial and Jaw Injuries in Sidcup with Sir Harold Gillies (a surgeon) and E. Stanley Rowbotham (an anesthetist), Magill developed the technique of awake blind nasotracheal intubation.[22][23][24][25][26][27] Magill devised a new type of angulated forceps (the Magill forceps) that are still used today to facilitate nasotracheal intubation in a manner that is little changed from Magill's original technique.[28] Other devices invented by Magill include the Magill laryngoscope blade,[29] as well as several apparati for the administration of volatile anesthetic agents.[30][31][32] The Magill curve of an endotracheal tube is also named for Magill.
Robert Macintosh also achieved significant advances in techniques for tracheal intubation when he introduced his new curved laryngoscope blade in 1943.[33] The most widely used curved laryngoscope blade is named after Macintosh.[34] In 1932, Rudolph Schindler of Germany introduced the first semi-flexible gastroscope.[35] This device had numerous lenses positioned throughout the tube and a miniature light bulb at the distal tip. The tube of this device was 75 centimeters in length and 11 millimeters in diameter, and the distal portion was capable of a certain degree of flexion. Between 1945 and 1952, optical engineers (notably Karl Storz of Germany, Harold Hopkins of England, and Mutsuo Sugiura of the Japanese Olympus Corporation) built upon this early work, leading to the development of the first gastrocamera.[36] In 1964, Fernando Alves Martins of Portugal applied optical fiber technology to one of these early gastrocameras to produce the first gastrocamera with a flexible fiberscope.[37][38] Initially used in esophagogastroduodenoscopy, newer devices were developed in the late 1960s for use in bronchoscopy, rhinoscopy, and laryngoscopy. The concept of using a fiberoptic endoscope for tracheal intubation was introduced by Peter Murphy in 1967.[39] By the mid-1980s, the flexible fiberoptic bronchoscope had become an indispensable instrument within the pulmonology and anesthesia communities.
In the early 20th century, physicians began to use the tracheotomy in the treatment of patients afflicted with paralytic poliomyelitis who required mechanical ventilation. The currently used surgical tracheotomy technique was described in 1909 by Chevalier Jackson.[40] However, surgeons continued to debate various aspects of the tracheotomy well into the 20th century. Many techniques were described and employed, along with many different surgical instruments and tracheal tubes. Surgeons could not seem to reach a consensus on where or how the tracheal incision should be made, arguing whether the "high tracheotomy" or the "low tracheotomy" was more beneficial. Ironically, the newly developed inhalational anesthetic agents and techniques of general anesthesia actually seemed to increase the risks, with many people suffering fatal postoperative complications. Jackson emphasised the importance of postoperative care, which dramatically reduced the death rate. By 1965, the surgical anatomy was thoroughly and widely understood, antibiotics were widely available and useful for treating postoperative infections, and other major complications of tracheotomy had also become more manageable. The current perioperative mortality rate for tracheotomy is less than 1%.[41][42]
21st century
The "digital revolution" has brought newer technology to the art and science of tracheal intubation. Several manufacturers have developed video laryngoscopes which employ digital technology such as the CMOS active pixel sensor (CMOS APS) to generate a view of the glottis so that the trachea may be intubated. The Glidescope video laryngoscope is one example of such a device.[43][44]
Indications
Tracheal intubation (orotracheal, nasotracheal, cricothyrotomy, or tracheotomy) is indicated under any of the following circumstances:[45]
- Comatose or intoxicated patients with a depressed level of consciousness who are unable to protect their airways. This is commonly defined as those subjects with a Glasgow Coma Scale ≤ 8. In such cases, the throat muscles may lose their tone so that the hypopharynx becomes obstructed, impeding the free flow of air into the lungs. Furthermore, protective airway reflexes such as coughing and swallowing, which serve to protect the airways against aspiration of secretions and foreign bodies, may be absent. With tracheal intubation, airway patency is restored and the lower airways can be protected from aspiration.
- Requirement for mechanical ventilation, including cardiopulmonary resuscitation and general anesthesia. In such situations, spontaneous ventilation may be decreased or absent due to the effect of injury, disease, anesthetic agents, opioids, or neuromuscular-blocking drugs. To enable mechanical ventilation, a tracheal tube is often used, although there are alternative devices such as the laryngeal mask airway[46] or the CPAP mask.
- Apnea or hypoventilation (e.g., closed head injury, intoxication or poisoning, cervical spine injury, flail chest)
- Persistent or recurrent airway obstruction
- Impending or potential compromise of the airway (e.g., sustained seizure activity, facial fractures, expanding neck hematoma, laryngeal or tracheobronchial injury, airway burns, inhalation injury)
- Inability to maintain oxygenation using face mask oxygen supplementation (severe pneumonia, acute respiratory distress syndrome (ARDS), near-drowning, etc.)
- Diagnostic or therapeutic manipulation of the airway (such as bronchoscopy, laser therapy or stenting of the bronchi).
Predicting difficulty of tracheal intubation
All persons performing tracheal intubation must be familiar with alternative techniques of securing the airway. Because the life of a patient can depend on the success of tracheal intubation, it is important to assess possible obstacles beforehand. The history is helpful in this respect. The diagnosis and/or proposed surgical procedure may offer clues to a potentially difficult airway. The subject should be questioned about any significant signs or symptoms, such as dysphonia or dyspnea. Such findings may suggest obstructing lesions in various locations within the pharynx, larynx, or tracheobronchial tree. It is also important to elicit any history of previous surgery (e.g., previous cervical fusion), trauma, radiation therapy, or tumors involving the head, neck, and mediastinum, as well as any prior experiences with tracheal intubation (especially prior tracheotomy).
A detailed physical examination is also critical to evaluation of the airway. Specifically, one should evaluate:
- the range of motion of the cervical spine: the subject should be able to tilt the head back and then forward so that the mentum touches the chest.
- the range of motion of the temporomandibular joint: three of the subject's fingers should be able to fit between the upper and lower incisors.
- the size and shape of the maxilla and mandible, looking especially for problems such as maxillary hypoplasia, prominent maxillary incisors or retrognathia.
- the thyromental distance: three of the subject's fingers should be able to fit between the thyroid cartilage and the mentum.
- the size and shape of the tongue and palate relative to the size of the oral cavity:
- the teeth, especially noting the presence of any loose or damaged teeth or crowns.
Besides the physical examination, many classification systems have been developed in an effort to predict difficulty of tracheal intubation, including the Cormack-Lehane grading system,[47][48] the Intubation Difficulty Scale (IDS),[49] and the Mallampati score.[50] The Mallampati score is determined by looking at the anatomy of the mouth and based on the visibility of the base of uvula, faucial pillars and the soft palate.
Such medical scoring systems correlate to some extent with the degree of difficulty of laryngoscopy and tracheal intubation, and may aid in the evaluation of factors linked to difficult tracheal intubation. It should however be noted that no single score or combination of scores can be trusted to detect all patients who are difficult to intubate. No system has yet been devised that can claim 100% positive predictive value, or 100% sensitivity and specificity. Furthermore, one recent study has demonstrated that even among experienced anesthesiologists, only 25% could correctly define all four grades of the widely used Cormack–Lehane classification system, and intra-observer reliability (reproducibility of results) was poor.[51] While perfection may be an unrealistic expectation from a statistical standpoint, the grave consequences of failed tracheal intubation require the highest possible degree of certainty in predicting the difficulty of intubation.
Equipment
Laryngoscopes


The vast majority of tracheal intubations involve the use of a "scope" of one type or another. Since its introduction by Kirstein in 1895, the most common device used for this purpose has been the conventional laryngoscope. Today, the typical conventional laryngoscope consists of a handle, usually containing batteries, and a set of interchangeable blades. Two basic styles of laryngoscope blade are commercially available: the straight blade and the curved blade. The Macintosh blade is the most widely used of the curved laryngoscope blades,[34] while the Miller blade[52] is the most popular style of straight blade.[53] There are many other styles of straight and curved blades (e.g., Phillips, Robertshaw, Sykes, Wisconsin, Wis-Hipple, etc.) with accessories such as mirrors for enlarging the field of view and even ports for the administration of oxygen. These specialty blades are primarily designed for use by anesthetists, most commonly in the operating room.
Besides the conventional laryngoscopes, many other devices have been developed as alternatives to direct laryngoscopy. These include a number of indirect fiberoptic viewing laryngoscopes such as the flexible fiberoptic bronchoscope, Bullard scope,[54] UpsherScope,[55][56] and the WuScope.[57] These devices are widely employed for tracheal intubation, especially in the setting of the difficult intubation (see below). Several types of video laryngoscopes are also currently available, such as the Glidescope,[43][44] McGrath laryngoscope,[58] Daiken Medical Coopdech C-scope VLP-100,[59] the Storz C-Mac,[60] Pentax AWS,[61][62][63][64] Video Macintosh Intubating Laryngoscope System (VMS)[65] and the Berci DCI[66]. Other "noninvasive" devices which are commonly employed for tracheal intubation are the laryngeal mask airway[46] (used as a guide for endotracheal tube placement), the lighted stylet,[67][68] and the AirTraq.[69] Due to the widespread availability of such devices, the technique of blind digital intubation[70] of the trachea is rarely practiced today, though it may still be useful in emergency situations under austere conditions such as natural or man-made disasters.
Stylets
An intubating stylet is a malleable metal wire which can be inserted into the endotracheal tube to make the tube conform better to the laryngopharyngeal anatomy of the specific individual, thus facilitating its insertion. It is commonly employed under circumstances of difficult laryngoscopy. Just as with laryngoscope blades, there are also several types of available stylets. The Verathon Stylet is a rigid stylet that is curved to follow the 60° angulation of the blade of the GlideScope® video laryngoscope.[43]
The Eschmann tracheal tube introducer (often referred to as a gum elastic bougie) is another specialized type of stylet, which can also be used to facilitate difficult intubation.[71][72] This flexible device is 60 cm in length, 15 French (5 mm diameter) with a small "hockey-stick" angle at its distal tip. Unlike the stylet, the Eschmann tracheal tube introducer is typically inserted directly into the trachea and then used as a guideover which the endotracheal tube can be passed (in a manner analogous to the Seldinger technique). As the Eschmann tracheal tube introducer is considerably less rigid than a conventional stylet, this technique is considered to be a relatively atraumatic means of tracheal intubation.
The concept of using a stylet for replacing or exchanging orotracheal tubes was introduced by Finucane and Kupshik in 1978, using a central venous catheter.[73] The modern tracheal tube exchanger is a hollow catheter, 56-81 cm in length, that can be used for removal and replacement of tracheal tubes without the need for laryngoscopy.[74] The Cook Airway Exchange Catheter (CAEC) is another example of this type of catheter.[75]
Tracheal tubes
Tracheal tubes are commonly used for airway management in the settings of general anesthesia, critical care, mechanical ventilation, and emergency medicine. They may also be used as an alternative route for many medications, in the event an intravenous infusion cannot be established. The tube is inserted into the trachea in order to ensure that the airway is not closed off and that air is able to reach the lungs. The tracheal tube is regarded as the most reliable available method for protecting a patient's airway.
Sir Ivan Whiteside Magill (1888–1986) was an Irish born anesthetist who is famous for his involvement in much of the innovation and development in modern anesthesia. Originally a general practitioner, he accepted a post at the Queen's Hospital, Sidcup in 1919 as an anesthetist. The hospital had been established for the treatment of facial injuries sustained in the World War I. Working with plastic surgeon Harold Gillies, he was responsible for the development of numerous items of anesthetic equipment but most particularly the single-tube technique of endotracheal anesthesia. This was driven by the immense difficulties of administering "standard" anesthetics such as chloroform and ether to men with severe facial injury using masks; they would cover the operative field. Following the closure of the hospital, and the diminishing numbers of patients seen from the war era, he continued to work with Gillies in private practice but was also appointed to the Westminster Hospital and Brompton Hospital in London. He was Knighted by Queen Elizabeth II in 1960.
The original tubes were cut from a roll of rubber industrial tubing by his assistant, hence the natural curve of the tube. A curved metal adaptor was designed (Magill oral & nasal connectors) and a 4" black rubber connecting hose to fit to the anesthetic circuit was adapted from an MG brake hose and named the 'catheter mount' by Magill's theatre technician at Westminster Hospital. Originally, there was no inflatable cuff, the tube was packed either side of the sub-glottis by two green anesthetic swabs, with ribbon gauze sewn on by hand to aid extraction at extubation of the trachea. Anesthetic gel or ointment was used to lubricate the tube and provide some relief for the patient's throat soreness after the procedure.

Portex Medical (England and France) produced the first cuffless plastic 'Ivory' tracheal tubes, in conjunction with Magill's design later adding a cuff as manufacturing techniques became more viable, these were glued on by hand to make the famous Blue-line tube copied by many other manufacturers. Mallinckrodt GmBH developed the disposable endotracheal tube and produced a plethora of design variations, adding the 'Murphy Eye' to their tubes in case of 'accidental' placement of the tube to avoid right bronchial occlusion. David S. Sheridan was one of the manufacturers of the American markets "disposable" plastic endotracheal tube now used routinely in surgery. Previously, red rubber (Rusch-Germany) tubes were used, then sterilized for re-use.
Tracheal intubation usually requires general anesthesia and muscle relaxation but can be achieved in the awake patient with local anesthesia or in an emergency without any anesthesia, although this is extremely uncomfortable. It is usually performed by visualising the glottis by means of a hand-held laryngoscope that has a variety of curved and straight blades, with a light source. Intubation can also be performed using a flexible fiberoptic bronchoscope, video laryngoscope, or simply with the use of the attendant's fingers (this technique is referred to as blind digital intubation). The goal is to position the end of the tracheal tube two centimeters above the bifurcation of the lungs or the carina. If inserted too far into the trachea it often goes into the right main bronchus (because the right main brochus is less angled than the left.
Most tracheal tubes today are constructed of polyvinyl chloride, but specialty tubes constructed of silicone rubber, latex rubber, or stainless steel are also widely available. Most tubes have an inflatable cuff to seal the trachea and bronchial tree against air leakage and aspiration of gastric contents, blood, secretions, and other fluids. Uncuffed tubes are also available, though their use is limited mostly to pediatric patients (in small children, the cricoid cartilage, the narrowest portion of the pediatric airway, often provides an adequate seal for mechanical ventilation).

Types of tracheal tube include oral or nasal, cuffed or un-cuffed, preformed (e.g. RAE tube), reinforced tubes, double-lumen tubes and tracheostomy tubes. For human use, tubes range in size from 2-10.5 mm in internal diameter (ID). The size is chosen based on the patient's body size, with the smaller sizes being used for pediatric and neonatal patients. Tubes larger than 6 mm ID usually have an inflatable cuff. Originally made from red rubber, most modern tubes are made from polyvinyl chloride. Those placed in a laser field may be flexometallic. Robertshaw (and others) developed double-lumen endo-bronchial tubes for intra-thoracic surgery. These allow single-lung ventilation whilst the other lung is collapsed to make surgery easier. The deflated lung is re-inflated as surgery finishes to check for fistulas (tears). Another type of endotracheal tube has a small second lumen opening above the inflatable cuff, which can be used for suction of the nasopharngeal area and above the cuff to aid extubation (removal). This allows suctioning of secretions which sit above the cuff which helps reduce the risk of chest infections in long-term intubated patients. A shortened tube, a tracheostomy tube, can be inserted through an opening in the neck (a tracheostomy) into the trachea. This is often a temporary stoma, but patients can live with them permanently.
The "armored" endotracheal tubes are cuffed, wire-reinforced, silicone rubber tubes which are quite flexible but yet difficult to compress or kink. This can make them useful for situations in which the trachea is anticipated to remain intubated for a prolonged duration, or if the neck is to remain flexed during surgery. Polyvinyl chloride tubes are relatively stiff in comparison. Preformed tubes (such as the oral and nasal RAE tubes, named after the inventors Ring, Adair and Elwyn) are also widely available for special applications. These may also be constructed of polyvinyl chloride or wire-reinforced silicone rubber. Other tubes (such as the Bivona® Fome-Cuf® tube) are designed specifcally for use in laser surgery in and around the airway. Various types of double-lumen endotracheal (actually, endobronchial) tubes have been developed (Carlens,[76] White, Robertshaw, etc.) for ventilating each lung independently—this is useful during pulmonary and other thoracic operations.
A tracheostomy tube is a 2-3 inch-long curved metal or plastic tube that may be inserted into a tracheostomy stoma to maintain patency of the lumen. Several types of tracheostomy tube are available, depending on the requirements of the patient, including Shiley, Bivona (a silicon tube with metal rings that are good for airways with damage to the tracheal rings or otherwise not straight), and fenestrated.
A tracheal button is a rigid plastic cannula about 1 inch in length that can be placed into the tracheostomy after removal of a tracheostomy tube, to maintain patency of the lumen. It is generally used in people with obstructive sleep apnea, who wear it during during waking hours and remove it while sleeping to ensure a patent airway and reduce the risk of asphyxiation. Since the tube does not extend far into the trachea, it is easy to breathe and speak with the device in place.
Methods to confirm tube placement
No single method for confirming tracheal tube placement has been shown to be 100% reliable. Accordingly, the use of multiple methods for confirmation of correct tube placement is now widely considered to be the standard of care. Such methods include direct visualization of the tip of the tube as it passes through the glottis. Additionally, one should be able to hear equal bilateral breath sounds on auscultation of the chest, and no sound upon auscultation of the epigastrium. Equal bilateral rise and fall of the chest wall should be evident with ventilatory excursions. A small amount of water vapor should also be evident within the lumen of the tube with each exhalation, and there should be no gastric contents in the tube at any time.
Ideally, at least one of the methods utilized for confirming tracheal tube placement should be an instrument. Waveform capnography has emerged as the gold standard for the confirmation of tube placement within the trachea. Other methods relying on instruments include the use of a colorimetric end-tidal carbon dioxide detector, a self-inflating esophageal bulb, or an esophageal detection device.[77] Pulse oximetry is also widely used as a tertiary confirmation measure, but this technique has important limitations, most notably a significant delay in the decrease in oxygen saturation, especially if the subject has been pre-oxygenated.
Tracheal tube maintenance
The tube is secured in place with tape or a tracheal tube holder. A cervical collar is sometimes used to prevent motion of the airway. Tube placement should be confirmed after each physical move of the patient and after any unexplained change in his/her clinical status. Continuous pulse oximetry and continuous waveform capnography are often used to monitor the tube's correct placement.
An excessive leak can sometimes be corrected through the placement of a larger (0.5 mm larger in internal diameter) tracheal tube, and in difficult-to-ventilate pediatric patients children it is often necessary to use cuffed tubes to allow for high pressure ventilation if the leak is too great to overcome with the ventilator.[78]
Special situations
Emergency intubation
Personnel experienced in direct laryngoscopy are not always immediately available in certain settings that require emergency tracheal intubation. For this reason, specialized devices have been designed to act as bridges to a definitive airway. Such devices include the laryngeal mask airway, cuffed oropharyngeal airway, and the Combitube.[79] Other devices such as rigid stylets, the lightwand (a blind technique) and indirect fiberoptic rigid stylets, such as the Bullard scope, Upsher scope, and the WuScope can also be used as alternatives to direct laryngoscopy. Each of these devices have its own unique set of benefits and drawbacks, and none of them is effective under all circumstances.
Rapid-sequence induction and intubation
Rapid-sequence induction and intubation (RSI) refers to a special method of induction of general anesthesia, commonly employed in emergency operations, and other situations where patients are assumed to have a "full stomach". RSI involves pre-oxygenating the lungs with a tightly-fitting oxygen mask, followed by the sequential administration of an intravenous hypnotic agent and a rapidly-acting neuromuscular-blocking drug, prior to intubation of the trachea. One important difference between RSI and routine tracheal intubation is that the practitoner does not ventilate the lungs after the onset of general anesthesia and apnea.
Another key feature of RSI is the application of manual pressure to the cricoid cartilage, often referred to as the Sellick maneuver, prior to instrumentation of the airway and intubation of the trachea. Named for British anaesthetist Brian Arthur Sellick (1918–1996) who first described the procedure in 1961,[80] the Sellick maneuver (sometimes referred to as the application of cricoid pressure) is a method of preventing regurgitation and pulmonary aspiration of gastric contents, in addition to helping to bring the glottis into view during laryngoscopy and tracheal intubation. Pulmonary aspiration of gastric contents can result in a severe chemical aspiration pneumonitis that has a high mortality rate; proper use of the Sellick maneuver can minimize the occurrence of this dreaded complication. Cricoid pressure has been widely used during rapid sequence intubation for decades, despite a lack of compelling evidence to support this practice.[81][82] The initial article by Sellick was based on a small sample size at a time when high tidal volumes, head-down positioning, and barbiturate anesthesia were the rule.[83] Recent studies have shown that incorrect application of cricoid pressure may displace the esophagus laterally, instead of compressing it as described by Sellick.[84][85] Several studies demonstrate some degree of glottic compression,[86][87][88] reduction in tidal volume, and increase in peak pressures.[89][90][91][92][93][94]
Nasotracheal intubation

Difficult intubation
Many individuals have unusual airway anatomy, such as those who have limited range of motion of the cervical spine or temporomandibular joint, or who have oropharyngeal tumors, hematomas, angioedema, micrognathia, retrognathia, or excess adipose tissue of the face and neck. Using conventional laryngoscopic techniques, intubation of the trachea can be difficult in such people. Use of the flexible fiberoptic bronchoscope and similar devices has become among the preferred techniques in the management of such cases. Among the drawbacks of these devices are their high cost of purchase, maintenance and repair.[95][96] Another drawback is that intubation with one of these devices can take considerably longer than that achieved using conventional laryngoscopy; this limits their use somewhat in urgent and emergent situations.
Cricothyrotomy
A cricothyrotomy is an incision made through the skin and cricothyroid membrane to establish a patent airway during certain life-threatening situations, such as airway obstruction by a foreign body, angioedema, or massive facial trauma. Cricothyrotomy is nearly always performed as a last resort in cases where orotracheal and nasotracheal intubation are impossible or contraindicated. Cricothyrotomy is easier and quicker to perform than tracheotomy, does not require manipulation of the cervical spine, and is associated with fewer complications.[97]
The quickest and easiest method of to perform this technique is the needle cricothyrotomy, in which a large-bore (12-14 gauge) intravenous catheter is used to puncture the cricothyroid membrane. Oxygen can then be administered through this catheter via jet insufflation. However, while needle cricothyrotomy may be life-saving in extreme circumstances, this technique is only intended to be a temporizing measure until a definitive airway can be established.[45] In practice, needle cricothyrotomy is little better than apneic oxygenation inasmuch as the small diameter of an intravenous catheter allows for adequate oxygenation but not for elimination of carbon dioxide (ventilation). After one hour of apneic oxygenation through a needle cricothyrotomy, one can expect a PaCO2 of greater than 250 millimeters of mercury and an arterial pH of less than 6.72, despite an oxygen saturation of 98% or greater.[98] A more definitive airway can be established by performing a surgical cricothyrotomy, in which a 5-6 mm endotracheal tube or tracheostomy tube can be inserted through a larger incision.
Tracheotomy
Pediatric patients
Most of the general principles of anesthesia can be applied to children, but there are some significant anatomical and physiological differences between children and adults that can cause problems, especially in neonates and children weighing less than 15 kg. For infants and young children, oral intubation is easier than nasal. Nasal route carries risk of dislodgement of adenoid tissue and epistaxis, but advantages include good fixation of tube. Because of good fixation, nasal route is preferable to oral route in children undergoing intensive care and requiring prolonged intubation. The position of the tube is checked by auscultation (equal air entry on each side and, in long-term intubation, by chest X-ray). Because the airway of a child is narrow, a small amount of oedema can produce severe obstruction. Edema can easily be caused by forcing in a tracheal tube that is too tight. (If length of the tube is suspected to be large, immediate changing it to the smaller size is suggestible.)
The appropriate length for the endotracheal tube can be estimated by doubling the distance from the corner of the child's mouth to the ear canal. The tip of the tube should be at midtrachea, between the clavicles on an AP chest X-ray. The correct diameter of the tube is that which results in a small leak at a pressure of about 25 cm of water. The appropriate inner diameter for the endotracheal tube is roughly the same diameter as the child's little finger. For normally nourished children 2 years of age and older, the internal diameter of the tube can be calculated using the following formula:
- Internal diameter of tube (mm) = (patient's age in years + 16) / 4
For neonates, 3 mm internal diameter is accepted while for premature infants 2.5 mm internal diameter is more appropriate.
As with adults, there are a number of devices specially designed for assistance with difficult tracheal intubation in pediatric patients.[99][100]
Complications
Tracheal intubation is an invasive procedure that requires a great deal of clinical experience to master.[101] When performed improperly (e.g., unrecognized esophageal intubation), the associated complications may be rapidly fatal.[102] Consequently, in recent editions of its Guidelines for Cardiopulmonary Resuscitation the American Heart Association has de-emphasized the role of tracheal intubation in advanced airway maintenance, in favor of more basic techniques like bag-valve-mask ventilation.[103] Despite these concerns, tracheal intubation is still considered the definitive technique for airway management, as it allows the most reliable means of oxygenation and ventilation, while providing the highest level of protection against vomitus and regurgitation.
Although the conventional laryngoscope has proven effective across a wide variety of settings and patients, its use and misuse can result in serious complications (e.g., trauma to oropharyngeal and dental structures). Newer technologies such as flexible fiberoptic laryngoscopy have fared better in reducing the incidence of such complications, though the most common cause of intubation trauma remains a lack of skill on the part of the laryngoscopist.
Even when properly performed, significant complications may result as a result of tracheal intubation, especially for prolonged duration. Such complications include dental trauma, vocal cord paresis, tracheoinnominate fistula, tracheomalacia, tracheoesophageal fistula, or even frank rupture of the trachea.
The cuff pressure must be monitored carefully in order to avoid complications from over-inflation, many of which can be traced to excessive cuff pressure causing ischemia of the tracheal mucosa.[104]
See also
References
- ^ a b c Steven E. Sittig and James E. Pringnitz (February 2001). "Tracheostomy: evolution of an airway" (PDF). AARC Times: 48–51. Retrieved 25 July 2010.
- ^ a b c d Alfio Ferlito, Alessandra Rinaldo, Ashok R. Shaha, Patrick J. Bradley (December 2003). "Percutaneous Tracheotomy". Acta Otolaryngologica. 123 (9): 1008–1012. doi:10.1080/00016480310000485. PMID 14710900. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f O. Rajesh & R. Meher (2006). "Historical Review Of Tracheostomy". The Internet Journal of Otorhinolaryngology. 4 (2). ISSN 1528-8420. Retrieved 25 July 2010.
- ^ Patricia Skinner (2008). "Unani-tibbi". In Laurie J. Fundukian (ed.). The Gale Encyclopedia of Alternative Medicine (3rd ed.). Farmington Hills, Michigan: Gale Cengage. ISBN 9781414448725. Retrieved 25 July 2010.
{{cite book}}: External link in|publisher= - ^ Mostafa Shehata (April 2003). "The Ear, Nose and Throat in Islamic Medicine" (PDF). Journal of the International Society for the History of Islamic Medicine. 2 (3): 2–5. ISSN 1303-667x. Retrieved 25 July 2010.
{{cite journal}}: Check|issn=value (help) - ^ a b John J. Conley (September 1952). "Tracheotomy". The American Journal of Nursing. 52 (9): 1078–1081. doi:10.2307/3459556. PMID 12985608. Retrieved 25 July 2010.
- ^ Armand Trousseau (1833). "Mémoire sur un cas de tracheotomie pratiquée dans la période extrème de croup". Journal des connaissances médico-chirurgicales. 1 (5): 41.
{{cite journal}}:|access-date=requires|url=(help) - ^ Armand Trousseau (1852). "Nouvelles recherches sur la trachéotomie pratiquée dans la période extrême du croup". In Jean Lequime and J. de Biefve (ed.). Annales de médecine belge et étrangère. Brussels: Imprimerie et Librairie Société Encyclographiques des Sciences Médicales. pp. 279–288. Retrieved 25 July 2010.
- ^ a b Koltai PJ, Nixon RE (July 1989). "The story of the laryngoscope". Ear Nose Throat J. 68 (7): 494–502. PMID 2676465. Retrieved 25 July 2010.
- ^ a b Byron Bailey (August 1996). "Laryngoscopy and laryngoscopes: Who's first? The forefathers/four fathers of laryngology". Laryngoscope. 106 (8): 939–943. doi:10.1097/00005537-199608000-00005. PMID 8699905. Retrieved 25 July 2010.
- ^ William Beaumont and Andrew Combe (1838). Experiments and observations on the gastric juice, and the physiology of digestion. Edinburgh: MacLachlan & Stewart. p. 319. Retrieved 25 July 2010.
- ^ William Stanley Sykes (1961). "The cheerful centenarian, or the founder of laryngoscopy". In William Stanley Sykes (ed.). Essays on the first hundred years of anaesthesia (Vol. 2). Edinburgh: E & S Livingstone Ltd. pp. 95–113. ISBN 9780443026584.
{{cite book}}:|access-date=requires|url=(help) - ^ Wolfgang G. Mouton, Justin R. Bessell, Guy J. Maddern (December 1998). "Looking Back to the Advent of Modern Endoscopy: 150th Birthday of Maximilian Nitze" (PDF). World Journal of Surgery. 22 (12): 1256–1258. doi:10.1007/s002689900555. ISSN 1432-2323. PMID 9841754. Retrieved 26 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jan Mikulicz-Radecki (1881). "Über Gastroskopie und Ösophagoskopie". Wiener Medizinische Presse. 22: 1405–1408, 1437–1443, 1473–1475, 1505–1507, 1537–1541, 1573–1577, 1629–1631.
{{cite journal}}:|access-date=requires|url=(help) - ^ Schramm H, Jan Mikulicz-Radecki (1881). "Gastroskopia i ezofagoskopia". Przeglad Lekarski. 20: 610.
{{cite journal}}:|access-date=requires|url=(help) - ^ Wojciech Kielan, Bogdan Lazarkiewicz, Zygmunt Grzebieniak, Adam Skalski and Piotr Zukrowski (March 2005). "Jan Mikulicz-Radecki: one of the creators of world surgery" (PDF). Keio J Med. 54 (1): 1–7. doi:10.2302/kjm.54.1. PMID 15832074. Retrieved 26 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ N.P. Hirsch, G.B. Smith, and P.O. Hirsch (January 1986). "Alfred Kirstein: Pioneer of direct laryngoscopy". Anaesthesia. 41 (1): 42–45. doi:10.1111/j.1365-2044.1986.tb12702.x. PMID 3511764. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Christopher M. Burkle, Fernando A. Zepeda, Douglas R. Bacon, Steven H. Rose (April 2004). "A Historical Perspective on Use of the Laryngoscope as a Tool in Anesthesiology". Anesthesiology. 100 (4): 1003–1006. doi:10.1097/00000542-200404000-00034. PMID 15087639. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chevalier Jackson (1913). "The technique of insertion of intratracheal insufflation tubes". Surg Gynecol Obstet. 17: 507–509.
{{cite journal}}:|access-date=requires|url=(help) - ^ a b Steven M. Zeitels (March 1998). "Chevalier Jackson's contributions to direct laryngoscopy". J Voice. 12 (1): 1–6. doi:10.1016/S0892-1997(98)80069-6. PMID 9619973. Retrieved 25 July 2010.
- ^ Henry H. Janeway (1913). "Intra-tracheal anesthesia from the standpoint of the nose, throat and oral surgeon with a description of a new instrument for catheterizing the trachea". The Laryngoscope. 23 (11): 1082–1090. doi:10.1288/00005537-191311000-00009. Retrieved 25 July 2010.
- ^ E. Stanley Rowbotham and Ivan Magill (1921). "Anaesthetics in the plastic surgery of the face and jaws". Proc R Soc Med. 14 (1): 17–27.
{{cite journal}}:|access-date=requires|url=(help) - ^ Ivan Magill (1923). "The provision for expiration in endotracheal insufflations anaesthesia". The Lancet. 202 (5211): 68–69. doi:10.1016/S0140-6736(01)37756-5.
{{cite journal}}:|access-date=requires|url=(help) - ^ Ivan Magill (1928). "Endotracheal anaesthesia". Proc R Soc Med. 22 (2): 85–88.
{{cite journal}}:|access-date=requires|url=(help) - ^ Ivan Magill (1930). "Technique in endotracheal anaesthesia". BMJ. 2: 817–819. doi:10.1136/bmj.2.1243.817-a.
{{cite journal}}:|access-date=requires|url=(help) - ^ Thomas KB (July–August 1978). "Sir Ivan Whiteside Magill, KCVO, DSc, MB, BCh, BAO, FRCS, FFARCS (Hon), FFARCSI (Hon), DA. A review of his publications and other references to his life and work". Anaesthesia. 33 (7): 628–634. PMID 356665. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: date format (link) - ^ Gail McLachlan (September 2008). "Sir Ivan Magill KCVO, DSc, MB, BCh, BAO, FRCS, FFARCS (Hon), FFARCSI (Hon), DA, (1888-1986)" (PDF). Ulster Med J. 77 (3): 146–152. PMC 2604469. PMID 18956794. Retrieved 25 July 2010.
- ^ Ivan Magill (1920). "Forceps for intratracheal anaesthesia". BMJ. 2: 670. doi:10.1136/bmj.2.571.670.
{{cite journal}}:|access-date=requires|url=(help) - ^ Ivan Magill (06 March 1926). "An improved laryngoscope for anaesthetists". The Lancet. 207 (5349): 500. doi:10.1016/S0140-6736(01)17109-6. Retrieved 27 July 2010.
{{cite journal}}: Check date values in:|date=(help) - ^ Ivan Magill (30 April 1921). "A Portable Apparatus for Tracheal Insufflation Anaesthesia". The Lancet. 197 (5096): 918. doi:10.1016/S0140-6736(00)55592-5. Retrieved 27 July 2010.
- ^ Ivan Magill (11 June 1921). "Warming Ether Vapour for Inhalation". The Lancet. 197 (5102): 1270. doi:10.1016/S0140-6736(01)24908-3. Retrieved 27 July 2010.
- ^ Ivan Magill (04 August 1923). "An apparatus for the administration of nitrous oxide, oxygen, and ether". The Lancet. 202 (5214): 228. doi:10.1016/S0140-6736(01)22460-X. Retrieved 27 July 2010.
{{cite journal}}: Check date values in:|date=(help) - ^ Robert Reynolds Macintosh (1943). "A new laryngoscope". The Lancet. 1: 205.
{{cite journal}}:|access-date=requires|url=(help) - ^ a b Scott J, Baker PA (July 2009). "How did the Macintosh laryngoscope become so popular?". Pediatric Anesthesia. 19 (s1): 24–29. doi:10.1111/j.1460-9592.2009.03026.x. PMID 19572841. Retrieved 25 July 2010.
- ^ P.K. Schäfer and T. Sauerbruch (2004). "Rudolf Schindler (1888 - 1968) - Father of Gastroscopy". Z Gastroenterol. 42 (6): 550–556. doi:10.1055/s-2004-813178. PMID 15190453. Retrieved 25 July 2010.
- ^ Olympus Corporation (2010). "History of endoscopes. Volume 2: Birth of gastrocameras". Shinjuku, Tokyo, Japan: Olympus Corporation. Retrieved 25 July 2010.
- ^ Olympus Corporation (2010). "History of endoscopes. Volume 3: Birth of fiberscopes". Shinjuku, Tokyo, Japan: Olympus Corporation. Retrieved 25 July 2010.
- ^ Fernando Alves Martin (30 June 2009). "Fernando Alves Martins: A vida e a obra de um homem discreto. Inventor, compositor, curioso, um homem à frente do seu tempo". Figueira da Foz, Portugal: Blogger. Retrieved 25 July 2010.
{{cite web}}: External link in|publisher= - ^ Peter Murphy (1967). "A fibre-optic endoscope used for nasal intubation". Anaesthesia. 22 (3): 489–491. doi:10.1111/j.1365-2044.1967.tb02771.x. PMID 4951601. Retrieved 25 July 2010.
- ^ Chevalier Jackson (1909). "Tracheostomy". Laryngoscope. 19: 285–290.
{{cite journal}}:|access-date=requires|url=(help) - ^ Powell DM, Price PD, Forrest LA (February 1998). "Review of percutaneous tracheostomy". Laryngoscope. 108 (2): 170–177. doi:10.1097/00005537-199802000-00004. PMID 9473064. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ José M Añón, Vicente Gómez, Mª Paz Escuela, Vicente De Paz, Luis F Solana, Rosa M De La Casa, Juan C Pérez, Eugenio Zeballos and Luis Navarro (2000). "Percutaneous tracheostomy: comparison of Ciaglia and Griggs techniques" (PDF). Critical Care. 4 (2): 124–128. doi:10.1186/cc667. PMC 29040. PMID 11056749. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b c F. Agrò, G. Barzoi and F. Montecchia (2003). "Tracheal intubation using a Macintosh laryngoscope or a GlideScope® in 15 patients with cervical spine immobilization". British Journal of Anaesthesia. 90 (5): 705–706. doi:[https://doi.org/10.1093%2Fbja%2Faeg560%0A12697606 10.1093/bja/aeg560
12697606]. Retrieved 25 July 2010.
{{cite journal}}: Check|doi=value (help); line feed character in|doi=at position 19 (help) - ^ a b Cooper RM, Pacey JA, Bishop MJ, McCluskey SA (February 2005). "Early clinical experience with a new videolaryngoscope (GlideScope) in 728 patients". Can J Anaesth. 52 (2): 191–198. PMID 15684262. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b American College of Surgeons Committee on Trauma (October 2004). "Airway and ventilatory management". ATLS: Advanced Trauma Life Support Program for Doctors (7th ed.). Chicago, Illinois: American College of Surgeons. pp. 41–68. ISBN 978-1-880696-31-6. Retrieved 25 July 2010.
{{cite book}}: External link in|author= - ^ a b P.R.F. Davies, S.Q.M. Tighe, G.L. Greenslade, G.H. Evans (20 October 1990). "Laryngeal mask airway and tracheal tube insertion by unskilled personnel". The Lancet. 336 (8721): 977–979. doi:10.1016/0140-6736(90)92429-L. PMID 1978159. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cormack RS, Lehane J (November 1984). "Difficult tracheal intubation in obstetrics". Anaesthesia. 39 (11): 1105–1111. doi:10.1111/j.1365-2044.1984.tb08932.x. PMID 6507827. Retrieved 25 July 2010.
- ^ Ernst Zadrobilek (28 October 2009). "The Cormack-Lehane classification: twenty-fifth anniversary of the first published description". Internet Journal of Airway Management. 5. Retrieved 25 July 2010.
- ^ Adnet F, Borron SW, Racine SX, Clemessy JL, Fournier JL, Plaisance P, Lapandry C (1997). "The intubation difficulty scale (IDS): proposal and evaluation of a new score characterizing the complexity of endotracheal intubation". Anesthesiology. 87 (6): 1290–1297. doi:10.1097/00000542-199712000-00005. PMID 9416711. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ S. Rao Mallampati, Stephen P. Gatt, Laverne D. Gugino, Sukumar P. Desai, Barbara Waraksa, Dubravka Freiberger, Philip L. Liu (July 1985). "A clinical sign to predict difficult tracheal intubation: a prospective study" (PDF). Canadian Journal of Anesthesia. 32 (4): 429–434. doi:10.1007/BF03011357. PMID 4027773. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ R. Krage, C. van Rijn, D. van Groeningen, S.A. Loer, L.A. Schwarte and P. Schober (16 June 2010). "Cormack–Lehane classification revisited". British Journal of Anaesthesia. 105 (2): 220–227. doi:10.1093/bja/aeq136. PMID 20554633. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Robert A. Miller (May 1941). "A new laryngoscope". Anesthesiology. 2 (3): 317–320. doi:10.1097/00000542-194105000-00008. Retrieved 25 July 2010.
- ^ Somchai Amornyotin, Ungkab Prakanrattana, Phongthara Vichitvejpaisal, Thantima Vallisut, Neunghathai Kunanont, and Ladda Permpholprasert (2010). "Comparison of the Clinical Use of Macintosh and Miller Laryngoscopes for Orotracheal Intubation by Second-Month Nurse Students in Anesthesiology" (PDF). Anesthesiology Research and Practice. 2010: 1–5. doi:10.1155/2010/432846. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Gorback MS (01 November 1991). "Management of the challenging airway with the Bullard laryngoscope". J Clin Anesth. 3 (6): 473–477. doi:10.1016/0952-8180(91)90096-6. PMID 1760171. Retrieved 25 July 2010.
{{cite journal}}: Check date values in:|date=(help) - ^ Pearce AC, Shaw S, Macklin S (1996). "Evaluation of the UpsherScope: a new rigid fibrescope". Anaesthesia. 51 (6): 561–564. doi:10.1111/j.1365-2044.1996.tb12565.x. ISSN 0003-2409. PMID 8694210. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Peter Fridrich, Michael Frass, Claus G. Krenn, Christian Weinstabl, Jonathan L. Benumof, and Peter Krafft (December 1997). "The UpsherScope in routine and difficult airway management: a randomized, controlled clinical trial". Anesth Analg. 85 (6): 1377–1381. doi:10.1097/00000539-199712000-00036. PMID 9390612. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tzu-lang Wu and Hsiu-chin Chou (October 1994). "A new laryngoscope: the combination intubating device". Anesthesiology. 81 (4): 1085–1087. doi:10.1097/00000542-199410000-00044. PMID 7943825. Retrieved 25 July 2010.
- ^ B. Shippey, D. Ray, D. McKeown (27 January 2008). "Use of the McGrath® Videolaryngoscope in the Management of Difficult and Failed Tracheal Intubation". British Journal of Anaesthesia. 100 (1): 116–119. doi:10.1093/bja/aem303. PMID 17959584. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Daiken Medical Co., Ltd. (June 2007). "Coopdech video laryngoscope portable VLP-100" (PDF). Osaka, Japan: Daiken Medical Co., Ltd. Retrieved 25 July 2010.
- ^ Boedeker BH, Berg BW, Bernhagen MA, Murray WB (2009). "Endotracheal intubation comparing a prototype Storz CMAC and a glidescope videolaryngoscope in a medical transport helicopter - a pilot study". Stud Health Technol Inform. 142: 37–39. PMID 19377109. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pentax Medical Company (06 July 2006). "Airway Scope AWS-S100, Rigid Video Laryngoscope for Intubation". Montvale, New Jersey: Pentax Medical Company. Retrieved 25 July 2010.
{{cite web}}: Check date values in:|date=(help); External link in|publisher= - ^ Pentax Medical Company. "Pentax AWS" (PDF). Montvale, New Jersey: Pentax Medical Company. Retrieved 25 July 2010.
- ^ M.A. Malik, C. O'Donoghue, J. Carney, C.H. Maharaj, B.H. Harte and J.G. Laffey (2009). "Comparison of the Glidescope®, the Pentax AWS®, and the Truview EVO2® with the Macintosh laryngoscope in experienced anaesthetists: a manikin study". British Journal of Anaesthesia. 102 (1): 128–134. doi:10.1093/bja/aen342. PMID 19059923. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Takashi Asai, Yoshiro Enomoto, Keiko Shimizu, Koh Shingu, Yasuhisa Okuda (January 2008). "The Pentax-AWS Video-Laryngoscope: The First Experience in One Hundred Patients" (PDF). Anesth Analg. 106 (1): 257–259. doi:10.1213/01.ane.0000287647.46165.bc. PMID 18165587. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marshal B. Kaplan, Denham S. Ward, George Berci (December 2002). "A New Video Laryngoscope - An Aid in Intubation and Teaching" (PDF). J Clin Anesth. 14 (8): 620–626. PMID 12565125. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ D. Low, D. Healy and N. Rasburn (February 2008). "The use of the BERCI DCI® Video Laryngoscope for teaching novices direct laryngoscopy and tracheal intubation". Anaesthesia. 63 (2): 195–201. doi:10.1111/j.1365-2044.2007.05323.x. PMID 18211452. Retrieved 25 July 2010.
- ^ Zbinden S, Schüpfer G (March 1989). "The Tube-Stat: a useful aid in difficult intubation". Anaesthesist. 38 (3): 140–143. PMID 2719227. Retrieved 25 July 2010.
- ^ Lionel Davis, Scott D. Cook-Sather, and Mark S. Schreiner (March 2000). "Lighted Stylet Tracheal Intubation: A Review". Anesth Analg. 90 (3): 745–756. PMID 10702469. Retrieved 28 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Maharaj CH, Costello JF, McDonnell JG, Harte BH, Laffey JG (June 2007). "The Airtraq as a rescue airway device following failed direct laryngoscopy: a case series". Anaesthesia. 62 (6): 598–601. doi:10.1111/j.1365-2044.2007.05036.x. ISSN 0003-2409. PMID 17506739. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Norman R. James (1950). "Blind Intubation". Anaesthesia. 5 (3): 159–160. doi:10.1111/j.1365-2044.1950.tb12674.x. Retrieved 25 July 2010.
- ^ Viswanathan S, Campbell C, Wood DG, Riopelle JM, Naraghi M (November–December 1992). "The Eschmann Tracheal Tube Introducer. (Gum elastic bougie)". Anesthesiol Rev. 19 (6): 29–34. PMID 10148170. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: date format (link) CS1 maint: multiple names: authors list (link) - ^ M. El-Orbany and M.R. Salem (October 2004). "The Eschmann Tracheal Tube Introducer Is Not an Airway Exchange Device". Anesth Analg. 99 (4): 1269–1270. doi:10.1213/01.ANE.0000133955.92363.B1. PMID 15385401. Retrieved 25 July 2010.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help); zero width space character in|doi=at position 9 (help) - ^ Brendan T. Finucane and Hilton L. Kupshik (March 1978). "A flexible stilette for replacing damaged tracheal tubes" (PDF). Canadian Journal of Anesthesia. 25 (2): 153–154. doi:10.1007/BF03005076. ISSN 0832-610X. PMID 638831. Retrieved 25 July 2010.
- ^ Hudson RCI (2002). "SHERIDAN® endotracheal tubes catalog" (PDF). Temecula, California: Hudson RCI. Retrieved 25 July 2010.
{{cite web}}: External link in|publisher= - ^ Eric P. Loudermilk, Maximilian Hartmannsgruber, Daniel P. Stoltzfus and Paul B. Langevin (June 1997). "A Prospective Study of the Safety of Tracheal Extubation Using a Pediatric Airway Exchange Catheter for Patients With a Known Difficult Airway" (PDF). Chest. 111 (6): 1660–1665. doi:10.1378/chest.111.6.1660. ISSN 0012-3692. PMID 9187190. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Carlens E (October 1949). "A new flexible double-lumen catheter for bronchospirometry". J Thorac Surg. 18 (5): 742–746. PMID 18149050. Retrieved 25 July 2010.
- ^ Tim Wolfe, M.D. (May 1998). "The Esophageal Detector Device: Summary of the current articles in the literature". Salt Lake City, Utah: Wolfe Tory Medical, Inc. Retrieved 25 July 2010.
{{cite web}}: External link in|publisher= - ^ Sheridan RL (May 2006). "Uncuffed endotracheal tubes should not be used in seriously burned children". Pediatr Crit Care Med. 7 (3): 258–259. doi:10.1097/01.PCC.0000216681.71594.04. PMID 16575345. Retrieved 25 July 2010.
- ^ Foley LJ, Ochroch EA (July 2000). "Bridges to establish an emergency airway and alternate intubating techniques". Critical Care Clinics. 16 (3): 429–444. doi:10.1016/S0749-0704(05)70121-4. PMID 10941582. Retrieved 25 July 2010.
- ^ Sellick, Brian A. (1961). "Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia". The Lancet. 278 (7199): 404–406. doi:10.1016/S0140-6736(61)92485-0.
- ^ Salem MR, Sellick BA, Elam JO. The historical background of cricoid pressure in anesthesia and resuscitation. Anesth Analg 1974;53(2):230-232.
- ^ American Heart Association (2006). Textbook of Advanced Cardiac Life Support. Dallas, TX: American Heart Association.
- ^ Maltby, J. M., & Berialt, M. T. (2002). Science, pseudoscience and Sellick. Canadian Journal of Anaesthesia, 49(5), 443-447
- ^ Smith, K. J., Dobranowski, J., Yip, G., Dauphin, A., & Choi, P. T. (2003). Cricoid pressure displaces the esophagus: an observational study using magnetic resonance imaging. Anesthesiology, 99(1), 60-64;
- ^ Smith, K. J., Ladak, S., Choi, Pt L., & Dobranowski, J. (2002). The cricoid cartilage and the oesophagus are not aligned in close to half of adult patients. Canadian journal of Anaesthesia, 49(5), 503-507.
- ^ Palmer, JHM, Ball, D.R. The effect of cricoids pressure on the cricoids cartilage and vocal cords: An endoscopic study in anaesthetized patients. Anaesthesia (2000): 55; 260-287
- ^ Hartsilver, E. L., Vanner, R. G. Airway obstruction with cricoids pressure. Anesthesia (2000): 55: 208-211
- ^ Haslam, N., Parker, L., and Duggan, J.E. Effect of cricoid pressure on the view at laryngoscopy. Anesthesia (2005): 60: 41-47
- ^ Hocking, G., Roberts, F.L., Thew, M.E. Airway obstruction with cricoids pressure and lateral tilt. Anesthesia (2001), 56; 825-828
- ^ Escott MEA, Owen H, Strahan AD, Plummer JL. Cricoid pressure training: how useful are descriptions of force? Anaesth Intensive Care 2003;31:388-391
- ^ Owen H, Follows V, Reynolds KJ, Burgess G, Plummer J. Learning to apply effective cricoid pressure using a part task trainer. Anaesthesia 2002;57(11):1098-1101
- ^ Walton S, Pearce A. Auditing the application of cricoid pressure. Anaesthesia 2000;55:1028-1029
- ^ Koziol CA, Cuddleford JD, Moos DD. Assessing the force generated with the application of cricoid pressure. AORN J 2000;72:1018-1030
- ^ Meek T, Gittins N, Duggan JE. Cricoid pressure: knowledge and performance amongst anaesthetic assistants. Anaesthesia 1999;54(1):59-62.
- ^ Kirkpatrick MB, Smith JR, Hoffman PJ, Middleton RM III (November 1992). "Bronchoscope damage and repair costs: results of a regional postal survey". Respir Care. 37 (11): 1256–1259. PMID 10145745. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ales Rozman, Stefan Duh, Marija Petrinec-Primozic, Nadja Triller (2009). "Flexible Bronchoscope Damage and Repair Costs in a Bronchoscopy Teaching Unit" (PDF). Respiration. 77 (3): 325–330. doi:10.1159/000188788. PMID 19122449. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ M. Gregory Katos and David Goldenberg (June 2007). "Emergency cricothyrotomy". Operative Techniques in Otolaryngology. 18 (2): 110–114. doi:10.1016/j.otot.2007.05.002. Retrieved 25 July 2010.
- ^ M.J. Frumin, R.M. Epstein and G. Cohen (November–December 1959). "Apneic oxygenation in man". Anesthesiology. 20 (6): 789–798. doi:10.1097/00000542-195911000-00007. PMID 13825447. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: date format (link) - ^ Borland LM, Caselbrant M (January 1990). "The Bullard laryngoscope: a new indirect oral laryngoscope (pediatric version)". Anesth Analg. 70 (1): 105–108. PMID 2297088. Retrieved 28 July 2010.
- ^ Rebecca S. Hackell, AB, Lisa D. Held, DO, Paul A. Stricker, MD and John E. Fiadjoe, MD (September 2009). "Management of the Difficult Infant Airway with the Storz Video Laryngoscope: A Case Series". Anesth Analg. 109 (3): 763–766. doi:10.1213/ANE.0b013e3181ad8a05. PMID 19690244. Retrieved 28 July 2010.
{{cite journal}}: zero width space character in|doi=at position 9 (help)CS1 maint: multiple names: authors list (link) - ^ von Goedecke A, Herff H, Paal P, Dörges V, Wenzel V (March 2007). "Field airway management disasters". Anesth Analg. 104 (3): 481–483. doi:10.1213/01.ane.0000255964.86086.63. PMID 17312190. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mazur, Glen (January 2004). Richard O. Cummins (ed.). ACLS: Principles And Practice. Dallas, Texas: American Heart Association. pp. 135–180. ISBN 978-0874933413.
{{cite book}}:|access-date=requires|url=(help) - ^ ECC Committee, Subcommittees and Task Forces of the American Heart Association (2005). "2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 112 (24 Suppl): IV51–7. doi:10.1161/CIRCULATIONAHA.105.166550. PMID 16314375.
{{cite journal}}:|chapter=ignored (help); Unknown parameter|month=ignored (help) - ^ Papiya Sengupta, Daniel I Sessler, Paul Maglinger, Spencer Wells, Alicia Vogt, Jaleel Durrani, and Anupama Wadhwa (2004). "Endotracheal tube cuff pressure in three hospitals, and the volume required to produce an appropriate cuff pressure". BMC Anesthesiology. 4 (1): 8. doi:10.1186/1471-2253-4-8. PMC 535565. PMID 15569386. Retrieved 25 July 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link)
Bibliography
- Kabrhel C, Thomsen TW, Setnik GS, Walls RM (26 April 2007). "Videos in clinical medicine. Orotracheal intubation". N Engl J Med. 356 (17): e15. doi:10.1056/NEJMvcm063574. PMID 17460222.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Ovassapian A (1994). "Conduct of anesthesia". In Shields TW (ed.). General thoracic surgery. Baltimore: Williams & Wilkins. pp. 307–23. ISBN 0-683-07716-3.
- Smith NT (December 2000). "Simulation in anesthesia: the merits of large simulators versus small simulators". Curr Opin Anaesthesiol. 13 (6): 659–665. doi:10.1097/00001503-200012000-00009. PMID 17016372.
- Armand Trousseau (1858). "Du tubage de la glotte et de la trachéotomie". Bulletin de L'Académie Impériale de Médecine. 23. ISSN 0001-4079.
{{cite journal}}:|access-date=requires|url=(help)
External links
- Videos of direct laryngoscopy recorded with the Airway Cam (TM) imaging system, a head mounted camera system that captures the perspective of the operator.
- Airway devices for intubation
- Portex Endotracheal tubes
- Mallincrodt Endotracheal tubes
- Other Endotracheal tubes & holders
- http://www.medstudents.com.br/anest/anest2f1.htm
