Steel
| Steels |
|---|
 |
| Phases |
| Microstructures |
| Classes |
| Other iron-based materials |
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten.[1] Carbon and other elements act as a hardening agent, preventing dislocations in the iron atom crystal lattice from sliding past one another. Varying the amount of alloying elements and the form of their presence in the steel (solute elements, precipitated phase) controls qualities such as the hardness, ductility, and tensile strength of the resulting steel. Steel with increased carbon content can be made harder and stronger than iron, but such steel is also less ductile than iron.
Alloys with a higher than 2.1% carbon content are known as cast iron because of their lower melting point and castability.[1] Steel is also distinguishable from wrought iron, which can contain a small amount of carbon, but it is included in the form of slag inclusions. Two distinguishing factors are steel's increased rust resistance and better weldability.
Though steel had been produced by various inefficient methods long before the Renaissance, its use became more common after more-efficient production methods were devised in the 17th century. With the invention of the Bessemer process in the mid-19th century, steel became an inexpensive mass-produced material. Further refinements in the process, such as basic oxygen steelmaking (BOS), further lowered the cost of production while increasing the quality of the metal. Today, steel is one of the most common materials in the world, with more than 1.3 billion tons produced annually. It is a major component in buildings, infrastructure, tools, ships, automobiles, machines, appliances, and weapons. Modern steel is generally identified by various grades defined by assorted standards organizations.

Material properties

Iron, like most metals, is found in the Earth's crust only in the form of an ore, i.e., combined with other elements such as oxygen or sulfur.[2] Typical iron-containing minerals include Fe2O3—the form of iron oxide found as the mineral hematite, and FeS2—pyrite (fool's gold).[3] Iron is extracted from ore by removing oxygen and combining the ore with a preferred chemical partner such as carbon. This process, known as smelting, was first applied to metals with lower melting points, such as tin, which melts at approximately 250 °C (482 °F) and copper, which melts at approximately 1,000 °C (1,830 °F). In comparison, cast iron melts at approximately 1,370 °C (2,500 °F). All of these temperatures could be reached with ancient methods that have been used since the Bronze Age. Since the oxidation rate itself increases rapidly beyond 800 °C, it is important that smelting take place in a low-oxygen environment. Unlike copper and tin, liquid iron dissolves carbon quite readily. Smelting results in an alloy (pig iron) containing too much carbon to be called steel.[4] The excess carbon and other impurities are removed in a subsequent step.
Other materials are often added to the iron/carbon mixture to produce steel with desired properties. Nickel and manganese in steel add to its tensile strength and make austenite more chemically stable, chromium increases hardness and melting temperature, and vanadium also increases hardness while reducing the effects of metal fatigue. To prevent corrosion, at least 11% chromium is added to steel so that a hard oxide forms on the metal surface; this is known as stainless steel. Tungsten interferes with the formation of cementite, allowing martensite to form with slower quench rates, resulting in high speed steel. On the other hand, sulfur, nitrogen, and phosphorus make steel more brittle, so these commonly found elements must be removed from the ore during processing.[5]
The density of steel varies based on the alloying constituents, but usually ranges between 7.75 and 8.05 g/cm3 (0.280–0.291 lb/in3).[6]
Even in the narrow range of concentrations which make up steel, mixtures of carbon and iron can form a number of different structures, with very different properties. Understanding such properties is essential to making quality steel. At room temperature, the most stable form of iron is the body-centered cubic (BCC) structure α-ferrite. It is a fairly soft metallic material that can dissolve only a small concentration of carbon, no more than 0.021 wt% at 723 °C (1,333 °F), and only 0.005% at 0 °C (32 °F). If the steel contains more than 0.021% carbon then it transforms into a face-centered cubic (FCC) structure, called austenite or γ-iron. It is also soft and metallic but can dissolve considerably more carbon, as much as 2.1%[7] carbon at 1,148 °C (2,098 °F), which reflects the upper carbon content of steel.[8]
When steels with less than 0.8% carbon, known as a hypoeutectoid steel, are cooled from an austenitic phase the mixture attempts to revert to the ferrite phase, resulting in an excess of carbon. One way for carbon to leave the austenite is for cementite to precipitate out of the mix, leaving behind iron that is pure enough to take the form of ferrite, resulting in a cementite-ferrite mixture. Cementite is a hard and brittle intermetallic compound with the chemical formula of Fe3C. At the eutectoid, 0.8% carbon, the cooled structure takes the form of pearlite, named after its resemblance to mother of pearl. For steels that have more than 0.8% carbon the cooled structure takes the form of pearlite and cementite.[9]
Perhaps the most important polymorphic form is martensite, a metastable phase which is significantly stronger than other steel phases. When the steel is in an austenitic phase and then quenched it forms into martensite, because the atoms "freeze" in place when the cell structure changes from FCC to BCC. Depending on the carbon content the martensitic phase takes different forms. Below approximately 0.2% carbon it takes an α ferrite BCC crystal form, but higher carbon contents take a body-centered tetragonal (BCT) structure. There is no thermal activation energy for the transformation from austenite to martensite. Moreover, there is no compositional change so the atoms generally retain their same neighbors.[10]
Martensite has a lower density than austenite does, so that transformation between them results in a change of volume. In this case, expansion occurs. Internal stresses from this expansion generally take the form of compression on the crystals of martensite and tension on the remaining ferrite, with a fair amount of shear on both constituents. If quenching is done improperly, the internal stresses can cause a part to shatter as it cools. At the very least, they cause internal work hardening and other microscopic imperfections. It is common for quench cracks to form when water quenched, although they may not always be visible.[11]
Heat treatment
There are many types of heat treating processes available to steel. The most common are annealing and quenching and tempering. Annealing is the process of heating the steel to a sufficiently high temperature to soften it. This process occurs through three phases: recovery, recrystallization, and grain growth. The temperature required to anneal steel depends on the type of annealing and the constituents of the alloy.[12]
Quenching and tempering first involves heating the steel to the austenite phase, then quenching it in water or oil. This rapid cooling results in a hard and brittle martensitic structure.[10] The steel is then tempered, which is just a specialized type of annealing. In this application the annealing (tempering) process transforms some of the martensite into cementite or spheroidite to reduce internal stresses and defects, which ultimately results in a more ductile and fracture-resistant metal.[13]
Steel production

When iron is smelted from its ore by commercial processes, it contains more carbon than is desirable. To become steel, it must be melted and reprocessed to reduce the carbon to the correct amount, at which point other elements can be added. This liquid is then continuously cast into long slabs or cast into ingots. Approximately 96% of steel is continuously cast, while only 4% is produced as cast steel ingots.[citation needed] The ingots are then heated in a soaking pit and hot rolled into slabs, blooms, or billets. Slabs are hot or cold rolled into sheet metal or plates. Billets are hot or cold rolled into bars, rods, and wire. Blooms are hot or cold rolled into structural steel, such as I-beams and rails. In modern foundries these processes often occur in one assembly line, with ore coming in and finished steel coming out.[14] Sometimes after a steel's final rolling it is heat treated for strength, however this is relatively rare.[15]
History of steelmaking

Ancient steel
Life during the Industrial Revolution
The Industrial Revolution caused great changes in people's way of life. The first changes appeared locally. But by the early 1800's, most of the British people knew they were in the midst of a nationwide economic and social revolution. Educational and political privileges, which once had belonged largely to the upper class, spread to the growing middle class. Some workers were displaced by machines, but others found new job opportunities working with machinery. Both workers and employers had to adjust to a new cold and impersonal relationship. In addition, most workers lived and worked under harsh conditions in the expanding industrial cities.
The working class. Under the domestic system, many employers had a close relationship with their workers and felt some responsibility for them. But such relationships became impossible in the large factories of the Industrial Revolution. Industrialists employed many workers and could not deal with them personally. The working day probably was no longer under industrialism than under the domestic system--about 12 to 14 hours a day for six days a week. But in the factories, the machines forced workers to work faster and without rest. Jobs became more specialized, and the work monotonous.
Factory wages were low. Some employers kept them low deliberately. Many people agreed with the English writer Arthur Young, who wrote: "Everyone but an idiot knows that the lower classes must be kept poor, or they will never be industrious." Women and children worked as unskilled laborers and made only a small fraction of men's low wages. Children--many of them under 10 years of age--worked from 10 to 14 hours a day. Some were deformed by their work or crippled by unsafe machines. See Child labor.
Most factory workers, like other types of workers, were desperately poor and could not read or write. Housing in the growing industrial cities could not keep up with the migration of workers from rural areas. Severe overcrowding resulted, and many people lived in extremely unsanitary conditions that led to outbreaks of disease.
Until the early 1800's, British employers usually held the advantages in relations with their employees. Workers were not permitted to vote and could do little legally to improve their condition. British law forbade trade unions, and workers who joined a union could be imprisoned.
However, some workers did form trade unions. Many workers also went on strike or rioted. In the riots, unemployed workers destroyed machinery in an attempt to gain revenge against the employers they blamed for depriving them of jobs. Even employed workers took part in the riots and wrecked the machines as a protest against their low wages and terrible working conditions. In 1769, Parliament passed a law making the destruction of some kinds of machinery punishable by death. But workers continued to riot against machines. In 1811, organized bands of employed and unemployed workers called Luddites began rioting against textile machines. Historians do not agree on the origin of the term Luddites. Luddite riots broke out from time to time for about two years.
The working and living conditions of the working class improved gradually during the 1800's. Parliament, which had largely represented only the upper class, began to act in the interests of the middle and working classes. It repealed the law forbidding trade unions and passed other laws regulating factory conditions. In 1832, a Reform Bill gave most middle-class men the right to vote. Another Reform Bill, passed in 1867, granted the right to vote to many city workers and owners of small farms.
The middle and upper classes. Although the workers did not at first share in the prosperity of the Industrial Revolution, members of the middle and upper classes prospered from the beginning. Many people made fortunes during the period. The revolution made available products that provided new comforts and conveniences to those who could afford them. The middle class, which consisted of business and professional people, won political and educational benefits. As the middle class gained in power, it became increasingly important politically. By the mid-1800's, business interests largely controlled British government policies.
Before the Industrial Revolution, England had only two universities, Oxford and Cambridge. But the revolution created a need for engineers and for clerical and professional workers. As a result, education became vital, and some libraries, schools, and universities were founded by private persons or groups.
The Industrial Revolution indirectly helped increase Britain's population. As people of the middle and upper classes enjoyed better diets and lived in more sanitary housing, they suffered less from disease and lived longer. The material condition of the working class also improved. Partly as a result of these improved conditions, the population grew rapidly. In 1750, Britain had about 61/2 million persons. By 1830, the population had increased to about 14 million.
Spread of the Industrial Revolution
The techniques of industrialization began to spread from Great Britain to other countries soon after the Industrial Revolution started. Great Britain tried to maintain a monopoly of its discoveries and skills. British law prohibited the emigration of craftworkers until 1824 and prohibited the export of machinery until 1843. Nevertheless, hundreds of skilled workers and manufacturers left Great Britain, taking knowledge of industrialization with them.
In 1750, John Holker, a Lancashire manufacturer, settled in France, where he helped modernize spinning techniques in the textile industry. In 1789, Samuel Slater, a Derbyshire textile worker, emigrated to the United States and built a spinning mill in Rhode Island. William Cockerill, a Lancashire carpenter, moved to Belgium in 1799 and began to manufacture textile machinery. In 1817, Cockerill's son John established factories near Liege that produced bridge materials, cannon, locomotives, and steam engines.
Some manufacturers in Great Britain permitted people from other countries to inspect their factories. From 1810 to 1812, Francis Cabot Lowell, an American businessman, visited Lancashire textile mills. Lowell returned to the United States and established a textile factory in Waltham, Mass. This factory was one of the first in the world to combine under one roof all the processes for manufacturing cotton cloth. In 1838, the famous German industrialist Alfred Krupp went to Sheffield, where he learned the most up-to-date processes for making steel.
The export of British capital became even more important than the export of people and machines in the spread of the Industrial Revolution. For hundreds of years, British merchants had extended credit and made loans to customers in other countries. As the Industrial Revolution grew, the flow of British capital to other countries increased. The flow became a flood with the coming of the railroad. British companies financed the export of locomotives, iron for rails, and experts to build and operate railroads in many countries throughout the world.
Belgium became the second country to industrialize. Between 1830 and 1870, the nation rapidly developed its heavy industry with much financial support from the government. Textile making, which had been important in Belgium for many years, was industrialized. The cities of Ghent, Liege, and Verviers developed into major textile-manufacturing centers.
France began to industrialize during the mid-1700's. But progress stopped in the late 1700's and early 1800's because of the French Revolution and the wars of France's ruler, Napoleon Bonaparte. In 1850, more than half of France's iron production still came from old-fashioned and expensive charcoal furnaces. After 1850, however, coke rapidly replaced charcoal for smelting and puddling.
A poor transportation system crippled French industry during most of the 1800's. The transportation system had fallen into bad condition during the French Revolution and the Napoleonic Wars. Although the government deepened and widened many rivers and canals, these improvements did not meet the needs of growing industries in France. In 1842, the government also approved the building of a national railway system, but many complications forced long delays in its construction. France remained largely a country of farms and small businesses. After World War II (1939-1945), the French government began a series of national plans to modernize the economy.
Germany had the natural resources needed for industrialization, but political and social obstacles held the country back. Until Germany was unified in 1871, it was a collection of separate states that often failed to cooperate with one another in economic matters. In addition, a small group of landowners controlled much of the land. In the early 1800's, the German government gradually took steps to provide for the industrial development of the land and its minerals. At the same time, the state of Prussia succeeded in arranging agreements among the German states on common tariffs.
Between 1830 and 1850, the coal production in Germany doubled. About 1850, iron ore mining in Germany began to increase sharply. As a result, the number of furnaces fueled by coke also increased rapidly. Foreign investors and new German investment banks provided money for the booming iron industry. Germany's steel production also began to grow rapidly in the late 1800's. By 1900, its steel production exceeded that of Great Britain and ranked second to that of the United States.
The United States. The first industrialization outside Europe occurred in the British colonies that became the United States. The colonies had a wide range of industries. The most successful was shipbuilding. By the time the colonies declared their independence in 1776, about a third of Britain's ships were being built in America. Iron manufacturing was also a major industry, and a few American companies exported iron to Great Britain.
By the early 1800's, the small arms industry in the United States had developed machines and machine tools that could produce standard parts that were required for mass production. Industrial production, especially of textiles and light metals, began to increase sharply in the United States in the 1820's. The greatest increases in manufacturing took place in New England. Industrialization also benefited from improvements made in rivers and canals. These improvements reduced the cost of transporting goods to and from the interior of the country.
Beginning in the 1830's, industrialization increased rapidly throughout the Eastern United States. The iron industry in Pennsylvania made especially great advances as iron was adapted for agricultural tools, railroad track, and a variety of structural uses. By the 1850's, the quality and price of American iron enabled U.S. ironmakers to compete with Great Britain's ironmakers in the international market.
During the mid-1800's, the agricultural, construction, and mining industries expanded as the population spread westward. Manufacturing accounted for less than a fifth of all U.S. production in 1840. By 1860, it accounted for a third. However, agricultural products made up more than two-thirds of the value of all U.S. exports in 1860, and the country still imported more manufactured goods than it exported. But by the late 1800's, the United States had become the largest and most competitive industrial nation in the world.
By 1870, the main trends of the Industrial Revolution were clearly marked in all industrialized countries. Industry had advanced faster than agriculture. Goods were being made by power-driven machinery and assembled in factories, where management planned operations and the workers did little more than tend the machines. Capital controlled industrial production, but labor was being allowed to organize to fight for higher wages, shorter hours, and better working conditions. The railroad, the improved sailing ship, the steamship, and the telegraph had reduced the cost and time of transportation and communication. Living standards of the workers in industrial countries were higher than they had ever been. Populations grew rapidly, and more people lived in cities than ever before.
Wherever the Industrial Revolution spread, it destroyed a traditional way of life. But as the revolution progressed in each country, more and more workers came to accept the routines and disciplines of industrialization.
Contributor: Eric Edwin Lampard, Ph.D., Prof. of History, State Univ. of New York at Stony Brook; Former Prof. of History, Univ. of Wisconsin, Madison.
Best matches for STEEL INDUSTRY IN THE LATE 1800'S Between 1830 and 1870, the nation rapidly developed its heavy industry with... Jump to text » Germany's steel production also began to grow rapidly in the late 1800's. Jump to text »
Steel was known in antiquity, and may have been produced by managing bloomeries, iron-smelting facilities, where the bloom contained carbon.[16]
The earliest known production of steel is a piece of ironware excavated from an archaeological site in Anatolia (Kaman-Kalehoyuk) and is about 4,000 years old.[17] Other ancient steel comes from East Africa, dating back to 1400 BC.[18] In the 4th century BC steel weapons like the Falcata were produced in the Iberian Peninsula, while Noric steel was used by the Roman military.[19] The Chinese of the Warring States (403–221 BC) had quench-hardened steel,[20] while Chinese of the Han Dynasty (202 BC – 220 AD) created steel by melting together wrought iron with cast iron, gaining an ultimate product of a carbon-intermediate steel by the 1st century AD.[21][22] The Haya people of East Africa discovered a type of high-heat blast furnace which allowed them to forge carbon steel at 3,275 °F (1,802 °C) nearly 2,000 years ago.[23] This ability was not duplicated until centuries later in Europe during the Industrial Revolution.
Wootz steel and Damascus steel
Evidence of the earliest production of high carbon steel in the Indian Subcontinent was found in Samanalawewa area in Sri Lanka.[24] Wootz steel was produced in India by about 300 BC.[25] Along with their original methods of forging steel, the Chinese had also adopted the production methods of creating Wootz steel, an idea imported into China from India by the 5th century AD.[26] In Sri Lanka, this early steel-making method employed the unique use of a wind furnace, blown by the monsoon winds, that was capable of producing high-carbon steel.[27] Also known as Damascus steel, wootz is famous for its durability and ability to hold an edge. It was originally created from a number of different materials including various trace elements. It was essentially a complicated alloy with iron as its main component. Recent studies have suggested that carbon nanotubes were included in its structure, which might explain some of its legendary qualities, though given the technology available at that time, they were produced by chance rather than by design.[28] Natural wind was used where the soil containing iron was heated up with the use of wood. The ancient Sinhalese managed to extract a ton of steel for every 2 tons of soil[citation needed], a remarkable feat at the time. One such furnace was found in Samanalawewa and archaeologists were able to produce steel as the ancients did long ago.[27][29]
Crucible steel, formed by slowly heating and cooling pure iron and carbon (typically in the form of charcoal) in a crucible, was produced in Merv by the 9th to 10th century AD.[25] In the 11th century, there is evidence of the production of steel in Song China using two techniques: a "berganesque" method that produced inferior, inhomogeneous steel and a precursor to the modern Bessemer process that utilized partial decarbonization via repeated forging under a cold blast.[30]
Modern steelmaking

Since the 17th century the first step in European steel production has been the smelting of iron ore into pig iron in a blast furnace.[31] Originally using charcoal, modern methods use coke, which has proven to be a great deal cheaper.[32][33][34]
Processes starting from bar iron
In these processes pig iron was "fined" in a finery forge to produce bar iron (wrought iron), which was then used in steel-making.[31]
The production of steel by the cementation process was described in a treatise published in Prague in 1574 and was in use in Nuremberg from 1601. A similar process for case hardening armour and files was described in a book published in Naples in 1589. The process was introduced to England in about 1614.[35] It was produced by Sir Basil Brooke at Coalbrookdale during the 1610s. The raw material for this were bars of wrought iron. During the 17th century it was realised that the best steel came from oregrounds iron from a region of Sweden, north of Stockholm. This was still the usual raw material in the 19th century, almost as long as the process was used.[36][37]
Crucible steel is steel that has been melted in a crucible rather than being forged, with the result that it is more homogeneous. Most previous furnaces could not reach high enough temperatures to melt the steel. The early modern crucible steel industry resulted from the invention of Benjamin Huntsman in the 1740s. Blister steel (made as above) was melted in a crucible or in a furnace, and cast (usually) into ingots.[37][38]
Processes starting from pig iron


The modern era in steelmaking began with the introduction of Henry Bessemer's Bessemer process in 1858. His raw material was pig iron.[39] This enabled steel to be produced in large quantities cheaply, thus mild steel is now used for most purposes for which wrought iron was formerly used.[40] The Gilchrist-Thomas process (or basic Bessemer process) was an improvement to the Bessemer process, lining the converter with a basic material to remove phosphorus. Another improvement in steelmaking was the Siemens-Martin process, which complemented the Bessemer process.[37]
These were rendered obsolete by the Linz-Donawitz process of basic oxygen steelmaking (BOS), developed in the 1950s, and other oxygen steelmaking processes. Basic oxygen steelmaking is superior to previous steelmaking methods because the oxygen pumped into the furnace limits impurities.[41] Now, electric arc furnaces (EAF) are a common method of reprocessing scrap metal to create new steel. They can also be used for converting pig iron to steel, but they use a great deal of electricity (about 440 kWh per metric ton), and are thus generally only economical when there is a plentiful supply of cheap electricity.[42]
Steel industry

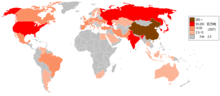
It is common today to talk about "the iron and steel industry" as if it were a single entity, but historically they were separate products. The steel industry is often considered to be an indicator of economic progress, because of the critical role played by steel in infrastructural and overall economic development.[43]
The economic boom in China and India has caused a massive increase in the demand for steel in recent years. Between 2000 and 2005, world steel demand increased by 6%. Since 2000, several Indian [44] and Chinese steel firms have risen to prominence like Tata Steel (which bought Corus Group in 2007), Shanghai Baosteel Group Corporation and Shagang Group. ArcelorMittal is however the world's largest steel producer.
In 2005, the British Geological Survey stated China was the top steel producer with about one-third of the world share; Japan, Russia, and the US followed respectively.[45]
In 2008, steel started to be traded as a commodity in the London Metal Exchange. At the end of 2008, the steel industry faced a sharp downturn that led to many cut-backs.[46]
Recycling
Contemporary steel
Modern steels are made with varying combinations of alloy metals to fulfill many purposes.[5] Carbon steel, composed simply of iron and carbon, accounts for 90% of steel production.[1] High strength low alloy steel has small additions (usually < 2% by weight) of other elements, typically 1.5% manganese, to provide additional strength for a modest price increase.[47] Low alloy steel is alloyed with other elements, usually molybdenum, manganese, chromium, or nickel, in amounts of up to 10% by weight to improve the hardenability of thick sections.[1] Stainless steels and surgical stainless steels contain a minimum of 11% chromium, often combined with nickel, to resist corrosion (rust). Some stainless steels are magnetic, while others are nonmagnetic.[48]
Some more modern steels include tool steels, which are alloyed with large amounts of tungsten and cobalt or other elements to maximize solution hardening. This also allows the use of precipitation hardening and improves the alloy's temperature resistance.[1] Tool steel is generally used in axes, drills, and other devices that need a sharp, long-lasting cutting edge. Other special-purpose alloys include weathering steels such as Cor-ten, which weather by acquiring a stable, rusted surface, and so can be used un-painted.[49]
Many other high-strength alloys exist, such as dual-phase steel, which is heat treated to contain both a ferritic and martensitic microstructure for extra strength.[50] Transformation Induced Plasticity (TRIP) steel involves special alloying and heat treatments to stabilize amounts of austentite at room temperature in normally austentite-free low-alloy ferritic steels. By applying strain to the metal, the austentite undergoes a phase transition to martensite without the addition of heat.[51] Maraging steel is alloyed with nickel and other elements, but unlike most steel contains almost no carbon at all. This creates a very strong but still malleable metal.[52] Twinning Induced Plasticity (TWIP) steel uses a specific type of strain to increase the effectiveness of work hardening on the alloy.[53] Eglin Steel uses a combination of over a dozen different elements in varying amounts to create a relatively low-cost metal for use in bunker buster weapons. Hadfield steel (after Sir Robert Hadfield) or manganese steel contains 12–14% manganese which when abraded forms an incredibly hard skin which resists wearing. Examples include tank tracks, bulldozer blade edges and cutting blades on the jaws of life.[54]
Most of the more commonly used steel alloys are categorized into various grades by standards organizations. For example, the Society of Automotive Engineers has a series of grades defining many types of steel.[55] The American Society for Testing and Materials has a separate set of standards, which define alloys such as A36 steel, the most commonly used structural steel in the United States.[56]
Though not an alloy, galvanized steel is a commonly used variety of steel which has been hot-dipped or electroplated in zinc for protection against rust.[57]
Uses

Iron and steel are used widely in the construction of roads, railways, other infrastructure, applicances, and buildings. Most large modern structures, such as stadiums and skyscrapers, bridges, and airports, are supported by a steel skeleton. Even those with a concrete structure will employ steel for reinforcing. In addition to widespread use in major appliances and cars. Despite growth in usage of aluminium, it is still the main material for car bodies. Steel is used in a variety of other construction materials, such as bolts, nails, and screws.[58] Other common applications include shipbuilding, pipeline transport, mining, offshore construction, aerospace, white goods (e.g. washing machines), heavy equipment such as bulldozers, office furniture, steel wool, tools, and armour in the form of personal vests or vehicle armour (better known as rolled homogeneous armour in this role).
Historical

Before the introduction of the Bessemer process and other modern production techniques, steel was expensive and was only used where no cheaper alternative existed, particularly for the cutting edge of knives, razors, swords, and other items where a hard, sharp edge was needed. It was also used for springs, including those used in clocks and watches.[37] With the advent of speedier and thriftier production methods, steel has been easier to obtain and much cheaper. It has replaced wrought iron for a multitude of purposes. However, the availability of plastics in the latter part of the 20th century allowed these materials to replace steel due to their lower cost and weight.[59]
Long steel

- As reinforcing bars and mesh in reinforced concrete
- Railroad tracks
- Structural steel in modern buildings and bridges
- Wires
Flat carbon steel
- Major appliances
- Magnetic cores
- The inside and outside body of automobiles, trains, and ships.
Stainless steel

- Cutlery
- Rulers
- Surgical equipment
- Wrist watches
See also
|
References
- ^ a b c d e Ashby, Michael F. (1992) [1986]. Engineering Materials 2 (with corrections ed.). Oxford: Pergamon Press. ISBN 0-08-032532-7.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Winter, Mark. "Periodic Table: Iron". The University of Sheffield. Retrieved 2007-02-28.
- ^ F. Brookins, Theo (1899). "Common Minerals and Valuable Ores". Birds and All Nature. 6 (4). A. W. Mumford. Retrieved 2007-02-28.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|month=ignored (help) - ^ "Smelting". Britannica. Encyclopedia Britannica. 2007.
{{cite encyclopedia}}:|access-date=requires|url=(help) - ^ a b "Alloying of Steels". Metallurgical Consultants. 2006-06-28. Retrieved 2007-02-28.
- ^ Elert, Glenn. "Density of Steel". Retrieved 2009-04-23.
- ^ Sources differ on this value so it has been rounded to 2.1%, however the exact value is rather academic because plain-carbon steel is very rarely made with this level of carbon. See:
- Smith & Hashemi 2006, p. 363—2.08%.
- Degarmo, Black & Kohser 2003, p. 75—2.11%.
- Ashby & Jones 1992—2.14%.
- ^ Smith & Hashemi 2006, p. 363.
- ^ Smith & Hashemi 2006, p. 365–372.
- ^ a b Smith & Hashemi 2006, pp. 373–378.
- ^ "Quench hardening of steel". Retrieved 2009-07-19.
- ^ Smith & Hashemi 2006, p. 249.
- ^ Smith & Hashemi 2006, p. 388.
- ^ Smith & Hashemi 2006, pp. 361–362.
- ^ Bugayev et al. 2001, p. 225
- ^ Wagner, Donald B. "Early iron in China, Korea, and Japan". Retrieved 2007-02-28.
- ^ "Ironware piece unearthed from Turkey found to be oldest steel". Retrieved 2009-03-27.
- ^ "Civilizations in Africa: The Iron Age South of the Sahara". Washington State University. Retrieved 2007-08-14.
- ^ "Noricus ensis," Horace, Odes, i. 16.9
- ^ Wagner, Donald B. (1993). Iron and Steel in Ancient China: Second Impression, With Corrections. Leiden: E.J. Brill. p. 243. ISBN 9004096329.
- ^ Needham, Joseph (1986). Science and Civilization in China: Volume 4, Part 3, Civil Engineering and Nautics. Taipei: Caves Books, Ltd. p. 563.
- ^ Gernet, 69.
- ^ Africa's Ancient Steelmakers. Time Magazine, Sept. 25, 1978.
- ^ Wilford, John Noble (1996-02-06). "Ancient Smelter Used Wind To Make High-Grade Steel". The New York Times.
- ^ a b Ann Feuerbach, 'An investigation of the varied technology found in swords, sabres and blades from the Russian Northern Caucasus' IAMS 25 for 2005, 27-43 at 29, apparently ultimately from the writings of Zosimos of Panopolis.
- ^ Needham, Volume 4, Part 1, 282.
- ^ a b G. Juleff (1996). "An ancient wind powered iron smelting technology in Sri Lanka". Nature. 379 (3): 60–63. doi:10.1038/379060a0.
{{cite journal}}: Invalid|ref=harv(help) - ^ Sanderson, Katharine (2006-11-15). "Sharpest cut from nanotube sword". News nature. Nature. doi:10.1038/news061113-11.
{{cite journal}}:|access-date=requires|url=(help); Invalid|ref=harv(help) - ^ Wayman, M L and Juleff, G (1999). "Crucible Steelmaking in Sri Lanka". Historical Metallurgy. 33 (1): 26.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Robert Hartwell (966). "Markets, Technology and the Structure of Enterprise in the Development of the Eleventh Century Chinese Iron and Steel Industry". Journal of Economic History. 26: 53–54.
{{cite journal}}: Invalid|ref=harv(help) - ^ a b R. F. Tylecote, A history of metallurgy 2 edn, Institute of Materials, London 1992, 95-99 and 102-105.
- ^ A. Raistrick, A Dynasty of Ironfounders (1953; York 1989)
- ^ C. K. Hyde, Technological Change and the British iron industry (Princeton 1977)
- ^ B. Trinder, The Industrial Revolution in Shropshire (Chichester 2000)
- ^ K. C. Barraclough, Steel before Bessemer: I Blister Steel: the birth of an industry (The Metals Society, London, 1984), 48-52.
- ^ P. W. King (2003). "The Cartel in Oregrounds Iron: trading in the raw material for steel during the eighteenth century". Journal of Industrial History. 6 (1): 25–49.
{{cite journal}}: Invalid|ref=harv(help) - ^ a b c d "Iron and steel industry". Britannica. Encyclopaedia Britannica. 2007.
{{cite encyclopedia}}:|access-date=requires|url=(help) - ^ K. C. Barraclough, Steel before Bessemer: II Crucible Steel: the growth of technology (The Metals Society, London, 1984).
- ^ James Moore Swank (1892). History of the Manufacture of Iron in All Ages. ISBN 0833734636.
- ^ "Bessemer process". Britannica. Vol. 2. Encyclopedia Britannica. 2005. p. 168.
{{cite encyclopedia}}:|access-date=requires|url=(help) - ^ "Basic oxygen process". Britannica. Encyclopedia Britannica. 2007.
{{cite encyclopedia}}:|access-date=requires|url=(help) - ^ J.A.T. Jones, B. Bowman, P.A. Lefrank, Electric Furnace Steelmaking, in The Making, Shaping and Treating of Steel, 525–660. R.J. Fruehan, Editor. 1998, The AISE Steel Foundation: Pittsburgh.
- ^ "Steel Industry". Retrieved 2009-07-12.
- ^ "India's steel industry steps onto world stage". Retrieved 2009-07-12.
- ^ "Long-term planning needed to meet steel demand". The News. 2008-03-01. Archived from the original on 2010-11-02. Retrieved 2010-11-02.
- ^ Uchitelle, Louis (2009-01-01). "Steel Industry, in Slump, Looks to Federal Stimulus". The New York Times. Retrieved 2009-07-19.
- ^ "High strength low alloy steels". Schoolscience.co.uk. Retrieved 2007-08-14.
- ^ "Steel Glossary". American Iron and Steel Institute (AISI). Retrieved 2006-07-30.
- ^ "Steel Interchange". American Institute of Steel Construction Inc. (AISC). Archived from the original on 2007-12-22. Retrieved 2007-02-28.
- ^ "Dual-phase steel". Intota Expert Knowledge Services. Retrieved 2007-03-01.
- ^ Werner, Prof. Dr. mont. Ewald. "Transformation Induced Plasticity in low alloyed TRIP-steels and microstructure response to a complex stress history". Retrieved 2007-03-01.
- ^ "Properties of Maraging Steels". Retrieved 2009-07-19.
- ^ Mirko, Centi. "Transformation Induced Plasticity (TRIP), Twinning Induced Plasticity (TWIP) and Dual-Phase (DP) Steels". Tampere University of Technology. Archived from the original on 2008-03-07. Retrieved 2007-03-01.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hadfield manganese steel. Answers.com. McGraw-Hill Dictionary of Scientific and Technical Terms, McGraw-Hill Companies, Inc., 2003. Retrieved on 2007-02-28.
- ^ Bringas, John E. (2004). Handbook of Comparative World Steel Standards: Third Edition (PDF) (3rd. ed.). ASTM International. p. 14. ISBN 0-8031-3362-6. Archived from the original (PDF) on 2007-01-27.
- ^ Steel Construction Manual, 8th Edition, second revised edition, American Institute of Steel Construction, 1986, ch. 1 page 1-5
- ^ "Galvanic protection". Britannica. Encyclopedia Britannica. 2007.
{{cite encyclopedia}}:|access-date=requires|url=(help) - ^ Ochshorn, Jonathan (2002-06-11). "Steel in 20th Century Architecture". Encyclopedia of Twentieth Century Architecture. Retrieved 2010-04-26.
- ^ "Materials science". Britannica. Encyclopedia Britannica. 2007.
{{cite encyclopedia}}:|access-date=requires|url=(help)
Bibliography
- Ashby, Michael F.; Jones, David Rayner Hunkin (1992). "An introduction to microstructures, processing and design" (Document). Butterworth-Heinemann.
{{cite document}}: Invalid|ref=harv(help)CS1 maint: postscript (link) - Bugayev, K.; Konovalov, Y.; Bychkov, Y.; Tretyakov, E.; Savin, Ivan V. (2001). Iron and Steel Production. The Minerva Group, Inc. ISBN 9780894991097. Retrieved 2009-07-19..
- Degarmo, E. Paul; Black, J T.; Kohser, Ronald A. (2003). Materials and Processes in Manufacturing (9th ed.). Wiley. ISBN 0-471-65653-4.
{{cite book}}: Invalid|ref=harv(help)CS1 maint: postscript (link) - Gernet, Jacques (1982). A History of Chinese Civilization. Cambridge: Cambridge University Press.
- Smith, William F.; Hashemi, Javad (2006). Foundations of Materials Science and Engineering (4th ed.). McGraw-Hill. ISBN 0-07-295358-6.
{{cite book}}: Invalid|ref=harv(help)CS1 maint: postscript (link)
Further reading
- Duncan Burn; The Economic History of Steelmaking, 1867–1939: A Study in Competition. Cambridge University Press, 1961.
- Harukiyu Hasegawa, The Steel Industry in Japan: A Comparison with Britain. 1996.
- J. C. Carr and W. Taplin, History of the British Steel Industry. Harvard University Press, 1962.
- H. Lee Scamehorn, Mill & Mine: The Cf&I in the Twentieth Century. University of Nebraska Press, 1992.
- Needham, Joseph (1986). Science and Civilization in China: Volume 4, Part 1 & Part 3. Taipei: Caves Books, Ltd.
- Warren, Kenneth, Big Steel: The First Century of the United States Steel Corporation, 1901–2001. University of Pittsburgh Press, 2001.
External links
- World Steel Association (worldsteel)
- steeluniversity.org: Online steel education resources from worldsteel and the University of Liverpool
- Extensive picture gallery of iron and steel production methods in North America and Europe
- Interactive knife steel composition chart and comparison graph builder
- Huge archive on steels, Cambridge University
