Vasopressin
It has been suggested that Vasopressin receptor antagonist be merged into this article. (Discuss) Proposed since May 2011. |
Arginine vasopressin (AVP), also known as vasopressin, argipressin or antidiuretic hormone (ADH), is a neurohypophysial hormone found in most mammals. Vasopressin is responsible for increasing water absorption in the collecting ducts of the kidney nephron.[1] Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels in the kidney nephron collecting duct plasma membrane.[2] Vasopressin is a peptide hormone that controls the reabsorption of molecules in the tubules of the kidneys by affecting the tissue's permeability. It also increases peripheral vascular resistance, which in turn increases arterial blood pressure. It plays a key role in homeostasis, by the regulation of water, glucose, and salts in the blood. It is derived from a preprohormone precursor that is synthesized in the hypothalamus and stored in vesicles at the posterior pituitary. Most of it is stored in the posterior pituitary to be released into the bloodstream; however, some AVP is also released directly into the brain, where it plays an important role in social behavior and bonding.
Physiology
Function
One of the most important roles of AVP is to regulate the body's retention of water; it is released when the body is dehydrated and causes the kidneys to conserve water, thus concentrating the urine and reducing urine volume. At high concentrations, it also raises blood pressure by inducing moderate vasoconstriction. In addition, it has a variety of neurological effects on the brain, having been found, for example, to influence pair-bonding in voles. The high-density distributions of vasopressin receptor AVPr1a in prairie vole ventral forebrain regions have been shown to facilitate and coordinate reward circuits during partner preference formation, critical for pair bond formation.[3]
A very similar substance, lysine vasopressin (LVP) or lypressin, has the same function in pigs and is often used in human therapy.
Kidney
Vasopressin has two effects by which it contributes to increased urine osmolality (increased concentration) and decreased water excretion. These are:
1.) Increasing the water permeability of distal tubule and collecting duct cells in the kidney, thus allowing water reabsorption and excretion of more concentrated urine, i.e., antidiuresis. This occurs through insertion of water channels (Aquaporin-2) into the apical membrane of distal tubule and collecting duct epithelial cells. Aquaporins allow water to move down their osmotic gradient and out of the nephron, increasing the amount of water re-absorbed from the filtrate (forming urine) back into the bloodstream.
V2 receptors, which are G protein-coupled receptors on the basolateral plasma membrane of the epithelial cells, couple to the heterotrimeric G-protein Gs, which activates adenylyl cyclases III and VI to convert ATP into cAMP, plus 2 inorganic phosphates. The rise in cAMP then triggers the insertion of aquaporin-2 water channels by exocytosis of intracellular vesicles, recycling endosomes. Vasopressin also increases the concentration of calcium in the collecting duct cells, by episodic release from intracellular stores. Vasopressin, acting through cAMP, also increases transcription of the aquaporin-2 gene, thus increasing the total number of aquaporin-2 molecules in collecting duct cells.
Cyclic-AMP activates protein kinase A (PKA) by binding to its regulatory subunits and allowing them to detach from the catalytic subunits. Detachment exposes the catalytic site in the enzyme, allowing it to add phosphate groups to proteins (including the aquaporin-2 protein), which alters their functions.
2.) Increasing permeability of the inner medullary portion of the collecting duct to urea by regulating the cell surface expression of urea transporters[4], which facilitates its reabsorption into the medullary interstitium as it travels down the concentration gradient created by removing water from the connecting tubule, cortical collecting duct, and outer medullary collecting duct.
Cardiovascular system
Vasopressin increases peripheral vascular resistance (vasoconstriction) and thus increases arterial blood pressure. This effect appears small in healthy individuals; however it becomes an important compensatory mechanism for restoring blood pressure in hypovolemic shock such as that which occurs during hemorrhage.
Central nervous system
Vasopressin released within the brain has many actions:
- It has been implicated in memory formation, including delayed reflexes, image, short- and long-term memory, though the mechanism remains unknown; these findings are controversial. However, the synthetic vasopressin analogue desmopressin has come to interest as a likely nootropic.
- Vasopressin is released into the brain in a circadian rhythm by neurons of the supraoptic nucleus.
- Vasopressin released from centrally projecting hypothalamic neurons is involved in aggression, blood pressure regulation and temperature regulation.
- Selective AVPr1a blockade in the ventral pallidum has been shown to prevent partner preference, suggesting that these receptors in this ventral forebrain region are crucial for pair bonding.[3]
- Recent evidence suggests that vasopressin may have analgesic effects. The analgesia effects of vasopressin were found to be dependent on both stress and gender.[5]
Evidence for this comes from experimental studies in several species, which indicate that the precise distribution of vasopressin and vasopressin receptors in the brain is associated with species-typical patterns of social behavior. In particular, there are consistent differences between monogamous species and promiscuous species in the distribution of AVP receptors, and sometimes in the distribution of vasopressin-containing axons, even when closely related species are compared.[6] Moreover, studies involving either injecting AVP agonists into the brain or blocking the actions of AVP support the hypothesis that vasopressin is involved in aggression toward other males. There is also evidence that differences in the AVP receptor gene between individual members of a species might be predictive of differences in social behavior. One study has suggested that genetic variation in male humans affects pair-bonding behavior. The brain of males uses vasopressin as a reward for forming lasting bonds with a mate, and men with one or two of the genetic alleles are more likely to experience marital discord. The partners of the men with two of the alleles affecting vasopressin reception state disappointing levels of satisfaction, affection, and cohesion.[7] Vasopressin receptors distributed along the reward circuit pathway, to be specific in the ventral pallidum, are activated when AVP is released during social interactions such as mating, in monogamous prairie voles. The activation of the reward circuitry reinforces this behavior, leading to conditioned partner preference, and thereby initiates the formation of a pair bond.[8]
Control
Vasopressin is secreted from the posterior pituitary gland in response to reductions in plasma volume, in response to increases in the plasma osmolality, and in response to cholecystokinin secreted by the small intestine:
- Secretion in response to reduced plasma volume is activated by pressure receptors in the veins, atria, and carotids.
- Secretion in response to increases in plasma osmotic pressure is mediated by osmoreceptors in the hypothalamus.
- Secretion in response to increases in plasma cholecystokinin is mediated by an unknown pathway.
The neurons that make AVP, in the hypothalamic supraoptic nuclei (SON) and paraventricular nuclei (PVN), are themselves osmoreceptors, but they also receive synaptic input from other osmoreceptors located in regions adjacent to the anterior wall of the third ventricle. These regions include the organum vasculosum of the lamina terminalis and the subfornical organ.
Many factors influence the secretion of vasopressin:
- Ethanol (alcohol) reduces the calcium-dependent secretion of AVP by blocking voltage-gated calcium channels in neurohypophyseal nerve terminals.[9]
- Angiotensin II stimulates AVP secretion, in keeping with its general pressor and pro-volemic effects on the body.[10]
- Atrial natriuretic peptide inhibits AVP secretion, in part by inhibiting Angiotensin II-induced stimulation of AVP secretion.[10]
Secretion
The main stimulus for secretion of vasopressin is increased osmolality of plasma. Reduced volume of extracellular fluid also has this effect, but is a less sensitive mechanism.
The AVP that is measured in peripheral blood is almost all derived from secretion from the posterior pituitary gland (except in cases of AVP-secreting tumours). However there are two other sources of AVP with important local effects:
- Vasopressin is produced by magnocellular neurosecretory neurons and parvocellular neurosecretory neurons in the PVN and SON. AVP produced by magnocellular neurons travels down the axon through the infundibulum within neurosecretory granules that are found within Herring bodies, localized swellings of the axons and nerve terminals. These carry the peptide directly to the posterior pituitary gland, where it is stored until released into the blood. AVP synthesized by parvocellular neurosecretory neurons at the PVN is released at the median eminence, which then travels through the Hypophyseal portal system to the anterior pituitary where it stimulates corticotrope cells.
- Vasopressin is also released into the brain by several different populations of smaller neurons (see below).
Receptors
Below is a table summarizing some of the actions of AVP at its four receptors, differently expressed in different tissues and exerting different actions:
| Type | Second messenger system | Locations | Actions |
| AVPR1A | Phosphatidylinositol/calcium | Liver, kidney, peripheral vasculature, brain | Vasoconstriction, gluconeogenesis, platelet aggregation, and release of factor VIII and von Willebrand factor; social recognition,[11] circadian tau[12] |
| AVPR1B or AVPR3 | Phosphatidylinositol/calcium | Pituitary gland, brain | Adrenocorticotropic hormone secretion in response to stress;[13] social interpretation of olfactory cues[14] |
| AVPR2 | Adenylate cyclase/cAMP | Basolateral membrane of the cells lining the collecting ducts of the kidneys (especially the cortical and outer medullary collecting ducts) | Insertion of aquaporin-2 (AQP2) channels (water channels). This allows water to be reabsorbed down an osmotic gradient, and so the urine is more concentrated. Release of von Willebrand factor and surface expression of P-selectin through exocytosis of Weibel-Palade bodies from endothelial cells[15][16] |
| VACM-1 | Phosphatidylinositol/calcium | Vascular endothelium and renal collecting tubules | Increases cytosolic calcium and acts as an inverse agonist of cAMP accumulation[17] |
Structure and relation to oxytocin

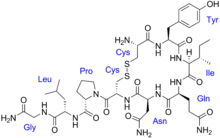
The vasopressins are peptides consisting of nine amino acids (nonapeptides). (NB: the value in the table above of 164 amino acids is that obtained before the hormone is activated by cleavage). The amino acid sequence of arginine vasopressin is Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly, with the cysteine residues forming a disulfide bond. Lysine vasopressin has a lysine in place of the arginine.
The structure of oxytocin is very similar to that of the vasopressins: It is also a nonapeptide with a disulfide bridge and its amino acid sequence differs at only two positions (see table below). The two genes are located on the same chromosome separated by a relatively small distance of less than 15,000 bases in most species. The magnocellular neurons that make vasopressin are adjacent to magnocellular neurons that make oxytocin, and are similar in many respects. The similarity of the two peptides can cause some cross-reactions: oxytocin has a slight antidiuretic function, and high levels of AVP can cause uterine contractions.[18][19]
Below is a table showing the superfamily of vasopressin and oxytocin neuropeptides:
| Vertebrate Vasopressin Family | ||
|---|---|---|
| Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2 | Argipressin (AVP, ADH) | Most mammals |
| Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Lys-Gly-NH2 | Lypressin (LVP) | Pigs, hippos, warthogs, some marsupials |
| Cys-Phe-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2 | Phenypressin | Some marsupials |
| Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Arg-Gly-NH2 | Vasotocin† | Non-mammals |
| Vertebrate Oxytocin Family | ||
| Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH2 | Oxytocin (OXT) | Most mammals, ratfish |
| Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Pro-Gly-NH2 | Prol-Oxytocin | Some New World monkeys, northern tree shrews |
| Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Ile-Gly-NH2 | Mesotocin | Most marsupials, all birds, reptiles, amphibians, lungfishes, coelacanths |
| Cys-Tyr-Ile-Gln-Ser-Cys-Pro-Ile-Gly-NH2 | Seritocin | Frogs |
| Cys-Tyr-Ile-Ser-Asn-Cys-Pro-Ile-Gly-NH2 | Isotocin | Bony fishes |
| Cys-Tyr-Ile-Ser-Asn-Cys-Pro-Gln-Gly-NH2 | Glumitocin | Skates |
| Cys-Tyr-Ile-Asn/Gln-Asn-Cys-Pro-Leu/Val-Gly-NH2 | Various tocins | Sharks |
| Invertebrate VP/OT Superfamily | ||
| Cys-Leu-Ile-Thr-Asn-Cys-Pro-Arg-Gly-NH2 | Diuretic Hormone | Locust |
| Cys-Phe-Val-Arg-Asn-Cys-Pro-Thr-Gly-NH2 | Annetocin | Earthworm |
| Cys-Phe-Ile-Arg-Asn-Cys-Pro-Lys-Gly-NH2 | Lys-Connopressin | Geography & imperial cone snail, pond snail, sea hare, leech |
| Cys-Ile-Ile-Arg-Asn-Cys-Pro-Arg-Gly-NH2 | Arg-Connopressin | Striped cone snail |
| Cys-Tyr-Phe-Arg-Asn-Cys-Pro-Ile-Gly-NH2 | Cephalotocin | Octopus |
| Cys-Phe-Trp-Thr-Ser-Cys-Pro-Ile-Gly-NH2 | Octopressin | Octopus |
| †Vasotocin is the evolutionary progenitor of all the vertebrate neurohypophysial hormones.[20] | ||
Role in disease
Decreased vasopressin release or decreased renal sensitivity to AVP leads to diabetes insipidus, a condition featuring hypernatremia (increased blood sodium concentration), polyuria (excess urine production), and polydipsia (thirst).
High levels of AVP secretion (syndrome of inappropriate antidiuretic hormone, SIADH) and resultant hyponatremia (low blood sodium levels) occurs in brain diseases and conditions of the lungs (Small cell lung carcinoma). In the perioperative period, the effects of surgical stress and some commonly used medications (e.g., opiates, syntocinon, anti-emetics) lead to a similar state of excess vasopressin secretion. This may cause mild hyponatremia for several days.
Hyponatremia can be treated pharmaceutically through the use of vasopressin receptor antagonists. These include the approved drug Vaprisol and the phase III drug lixivaptan.
Pharmacology
Vasopressin analogues
Vasopressin agonists are used therapeutically in various conditions, and its long-acting synthetic analogue desmopressin is used in conditions featuring low vasopressin secretion, as well as for control of bleeding (in some forms of von Willebrand disease and in mild haemophilia A) and in extreme cases of bedwetting by children. Terlipressin and related analogues are used as vasoconstrictors in certain conditions. Use of vasopressin analogues for esophageal varices commenced in 1970.[21]
Vasopressin infusion has also been used as a second line of management in septic shock patients not responding to high dose of inotropes (e.g., dopamine or norepinephrine).
The role of vasopressin analogues in cardiac arrest
Injection of vasopressors for the treatment of cardiac arrest was first suggested in the literature in 1896 when Austrian scientist Dr. R. Gottlieb described the vasopressor epinephrine as an "infusion of a solution of suprarenal extract [that] would restore circulation when the blood pressure had been lowered to unrecordable levels by chloral hydrate."[22] Modern interest in vasopressors as a treatment for cardiac arrest stem mostly from canine studies performed in the 1960's by anesthesiologists Dr. John W. Pearson and Dr. Joseph Stafford Redding in which they demonstrated improved outcomes with the use of adjunct intracardiac epinephrine injection during resuscitation attempts after induced cardiac arrest.[22] Also contributing to the idea that vasopressors may be useful treatments in cardiac arrest are studies performed in the early to mid 1990's that found significantly higher levels of endogenous serum vasopressin in adults after successful resuscitation from out-of-hospital cardiac arrest compared to those who did not live.[23][24] Results of animal models have supported the use of either vasopressin or epinephrine in cardiac arrest resuscitation attempts, showing improved coronary perfusion pressure[25] and overall improvement in short-term survival as well as neurological outcomes.[26]
Vasopressin vs. epinephrine
| RR (95% CI) | |
|---|---|
| Failure of ROSC | 0.81 (0.58-1.12) |
| Death before hospital admission | 0.72 (0.38-1.39) |
| Death within 24 hours | 0.74 (0.38-1.43) |
| Death before hospital discharge | 0.96 (0.87-1.05) |
| Number of deaths and neurologically impaired survivors | 1.00 (0.94-1.07) |
Although both vasopressors, vasopressin and epinephrine differ in that vasopressin does not have direct effects on cardiac contractility as epinephrine does.[26] Thus, vasopressin is theorized to be of increased benefit over epinephrine in cardiac arrest due to its properties of not increasing myocardial and cerebral oxygen demands.[26] This idea has led to the advent of several studies searching for the presence of a clinical difference in benefit of these two treatment choices. Initial small studies demonstrated improved outcomes with vasopressin in comparison to epinephrine.[27] However, subsequent studies have not all been in agreement. Several randomized controlled trials have been unable to reproduce positive results with vasopressin treatment in both return of spontaneous circulation (ROSC) and survival to hospital discharge,[27][28][29][30] including a systematic review and meta-analysis completed in 2005 that found no evidence of a significant difference with vasopressin in five studied outcomes (see Table 1).[25]
Vasopressin and epinephrine vs. epinephrine alone
| RR (95% CI) | p value | |
|---|---|---|
| ROSC[30] | 1.42 (1.14-1.77) | |
| Survival to hospital admission[31] | 1.42 (1.02-2.04) | 0.05 |
| In subgroup: PEA[31] | 1.30 (0.90-2.06) | 0.02 |
| In subgroup: Collapse to ED arrival time of 15-30 minutes[31] | 1.22 (1.01-1.49) | 0.05 |
| In subgroup: Collapse to ED arrival time of 30-45 minutes[31] | 1.11 (1.00-1.24) | 0.05 |
| Survival to hospital discharge[30] | 3.69 (1.52-8.95) |
There is no current evidence of significant survival benefit with improved neurological outcomes in patients given combinations of both epinephrine and vasopressin during cardiac arrest.[25][28][32][33] A systematic review from 2008 did, however, find one study that showed a statistically significant improvement in ROSC and survival to hospital discharge with this combination treatment; unfortunately, those patients that survived to hospital discharge had overall poor outcomes and many suffered permanent, severe neurological damage. [33][30] A more recently published clinical trial out of Singapore has shown similar results, finding combination treatment to only improve the rate of survival to hospital admission, especially in the subgroup analysis of patients with longer "collapse to emergency department" arrival times of 15 to 45 minutes.[31] Table 2 lists all statistically significant findings of a correlation between combined treatment and positive outcomes found in these two studies.
2010 American Heart Association Guidelines
The 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care recommend the consideration of vasopressor treatment in the form of epinephrine in adults with cardiac arrest (Class IIb, LOE A recommendation).[34] Due to the absence of evidence that vasopressin administered instead of or in addition to epinephrine has significant positive outcomes, the guidelines do not currently contain vasopressin as a part of the cardiac arrest algorithms.[34] It does, however, allow for one dose of vasopressin to replace either the first or second dose of epinephrine in the treatment of cardiac arrest (Class IIb, LOE A recommendation).[34]
Vasopressin receptor inhibition
A vasopressin receptor antagonist is an agent that interferes with action at the vasopressin receptors. They can be used in the treatment of hyponatremia.[35]
See also
References
- ^ Caldwell HK, Young WS III (2006). "Oxytocin and Vasopressin: Genetics and Behavioral Implications". In Lajtha A, Lim R (ed.). Handbook of Neurochemistry and Molecular Neurobiology: Neuroactive Proteins and Peptides (3rd ed.). Berlin: Springer. pp. 573–607. ISBN 0-387-30348-0.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA (1995). "Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane". Proc. Natl. Acad. Sci. U.S.A. 92 (4): 1013–7. doi:10.1073/pnas.92.4.1013. PMC 42627. PMID 7532304.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Lim MM, Young LJ (2004). "Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole". Neuroscience. 125 (1): 35–45. doi:10.1016/j.neuroscience.2003.12.008. PMID 15051143.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21686211, please use {{cite journal}} with
|pmid=21686211instead. - ^ Wiltshire, Tim (2011). "Relax, you won't feel the pain". Nature Neuroscience. 14: 1496–1497. doi:10.1038/nn.2987. PMID 22119947.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Young LJ (2009). "The neuroendocrinology of the social brain". Front Neuroendocrinol. 30 (4): 425–8. doi:10.1016/j.yfrne.2009.06.002. PMID 19596026.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P (2008). "Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans". Proc. Natl. Acad. Sci. U.S.A. 105 (37): 14153–6. doi:10.1073/pnas.0803081105. PMC 2533683. PMID 18765804.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ (2001). "Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole". J. Neurosci. 21 (18): 7392–6. PMID 11549749.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wang X, Dayanithi G, Lemos JR, Nordmann JJ, Treistman SN (1991). "Calcium currents and peptide release from neurohypophysial terminals are inhibited by ethanol". The Journal of Pharmacology and Experimental Therapeutics. 259 (2): 705–11. PMID 1941619.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Matsukawa T, Miyamoto T (2011). "Angiotensin II-stimulated secretion of arginine vasopressin is inhibited by atrial natriuretic peptide in humans". Am. J. Physiol. Regul. Integr. Comp. Physiol. 300 (3): R624–9. doi:10.1152/ajpregu.00324.2010. PMID 21123762.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ (2004). "Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice". Neuropsychopharmacology. 29 (3): 483–93. doi:10.1038/sj.npp.1300360. PMID 14647484.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS (2007). "Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression". Genes Brain Behav. 6 (6): 540–51. doi:10.1111/j.1601-183X.2006.00281.x. PMID 17083331.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lolait SJ, Stewart LQ, Jessop DS, Young WS, O'Carroll AM (2007). "The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors". Endocrinology. 148 (2): 849–56. doi:10.1210/en.2006-1309. PMC 2040022. PMID 17122081.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O'Carroll AM, Young WS (2004). "Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task". Horm Behav. 46 (5): 638–45. doi:10.1016/j.yhbeh.2004.07.004. PMID 15555506.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kanwar S, Woodman RC, Poon MC, Murohara T, Lefer AM, Davenpeck KL, Kubes P (1 October 1995). "Desmopressin induces endothelial P-selectin expression and leukocyte rolling in postcapillary venules". Blood. 86 (7): 2760–6. PMID 7545469.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kaufmann JE, Oksche A, Wollheim CB, Günther G, Rosenthal W, Vischer UM (2000). "Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP". J. Clin. Invest. 106 (1): 107–16. doi:10.1172/JCI9516. PMC 314363. PMID 10880054.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Buchwalter A, Van Dort C, Schultz S, Smith R, Le IP, Abbott JL, Oosterhouse E, Johnson AE, Hansen-Smith F, Burnatowska-Hledin M (2008). "Expression of VACM-1/cul5 mutant in endothelial cells induces MAPK phosphorylation and maspin degradation and converts cells to the angiogenic phenotype". Microvasc. Res. 75 (2): 155–68. doi:10.1016/j.mvr.2007.08.004. PMID 17950367.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Li C, Wang W, Summer SN, Westfall TD, Brooks DP, Falk S, Schrier RW (2008). "Molecular mechanisms of antidiuretic effect of oxytocin". J. Am. Soc. Nephrol. 19 (2): 225–32. doi:10.1681/ASN.2007010029. PMC 2396735. PMID 18057218.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Joo KW, Jeon US, Kim GH, Park J, Oh YK, Kim YS, Ahn C, Kim S, Kim SY, Lee JS, Han JS (2004). "Antidiuretic action of oxytocin is associated with increased urinary excretion of aquaporin-2". Nephrol. Dial. Transplant. 19 (10): 2480–6. doi:10.1093/ndt/gfh413. PMID 15280526.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Acher R, Chauvet J (1995). "The neurohypophysial endocrine regulatory cascade: precursors, mediators, receptors, and effectors". Front Neuroendocrinol. 16 (3): 237–89. doi:10.1006/frne.1995.1009. PMID 7556852.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Baum S, Nusbaum M, Tumen HJ (1970). "The control of gastrointestinal hemorrhage by selective mesenteric infusion of pitressin". Gastroenterology. 58: 926.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Pearson JW, Redding JS (1963). "The role of epinephrine in cardiac resuscitation". Anesth. Analg. 42: 599–606. PMID 14061643.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Lindner KH, Strohmenger HU, Ensinger H, Hetzel WD, Ahnefeld FW, Georgieff M (1992). "Stress hormone response during and after cardiopulmonary resuscitation". Anesthesiology. 77 (4): 662–8. PMID 1329579.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lindner KH, Haak T, Keller A, Bothner U, Lurie KG (1996). "Release of endogenous vasopressors during and after cardiopulmonary resuscitation". Heart. 75 (2): 145–50. PMC 484250. PMID 8673752.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Aung K, Htay T (2005). "Vasopressin for cardiac arrest: a systematic review and meta-analysis". Arch. Intern. Med. 165 (1): 17–24. doi:10.1001/archinte.165.1.17. PMID 15642869.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Williamson K, Breed M, Alibertis K, Brady WJ (2012). "The impact of the code drugs: cardioactive medications in cardiac arrest resuscitation". Emerg. Med. Clin. North Am. 30 (1): 65–75. doi:10.1016/j.emc.2011.09.008. PMID 22107975.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Lee SW (2011). "Drugs in resuscitation: an update". Singapore Med J. 52 (8): 596–602. PMID 21879219.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Callaway CW, Hostler D, Doshi AA, Pinchalk M, Roth RN, Lubin J, Newman DH, Kelly LJ (2006). "Usefulness of vasopressin administered with epinephrine during out-of-hospital cardiac arrest". Am. J. Cardiol. 98 (10): 1316–21. doi:10.1016/j.amjcard.2006.06.022. PMID 17134621.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Stiell IG, Hébert PC, Wells GA, Vandemheen KL, Tang AS, Higginson LA, Dreyer JF, Clement C, Battram E, Watpool I, Mason S, Klassen T, Weitzman BN (2001). "Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial". Lancet. 358 (9276): 105–9. doi:10.1016/S0140-6736(01)05328-4. PMID 11463411.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Wenzel V, Krismer AC, Arntz HR, Sitter H, Stadlbauer KH, Lindner KH (2004). "A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation". N. Engl. J. Med. 350 (2): 105–13. doi:10.1056/NEJMoa025431. PMID 14711909.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Ong ME, Tiah L, Leong BS, Tan EC, Ong VY, Tan EA, Poh BY, Pek PP, Chen Y (2012). "A randomised, double-blind, multi-centre trial comparing vasopressin and adrenaline in patients with cardiac arrest presenting to or in the Emergency Department". Resuscitation. doi:10.1016/j.resuscitation.2012.02.005. PMID 22353644.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Gueugniaud PY, David JS, Chanzy E, Hubert H, Dubien PY, Mauriaucourt P, Bragança C, Billères X, Clotteau-Lambert MP, Fuster P, Thiercelin D, Debaty G, Ricard-Hibon A, Roux P, Espesson C, Querellou E, Ducros L, Ecollan P, Halbout L, Savary D, Guillaumée F, Maupoint R, Capelle P, Bracq C, Dreyfus P, Nouguier P, Gache A, Meurisse C, Boulanger B, Lae C, Metzger J, Raphael V, Beruben A, Wenzel V, Guinhouya C, Vilhelm C, Marret E (2008). "Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation". N. Engl. J. Med. 359 (1): 21–30. doi:10.1056/NEJMoa0706873. PMID 18596271.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Sillberg VA, Perry JJ, Stiell IG, Wells GA (2008). "Is the combination of vasopressin and epinephrine superior to repeated doses of epinephrine alone in the treatment of cardiac arrest-a systematic review". Resuscitation. 79 (3): 380–6. doi:10.1016/j.resuscitation.2008.07.020. PMID 18951676.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ (2010). "Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 122 (18 Suppl 3): S729–67. doi:10.1161/CIRCULATIONAHA.110.970988. PMID 20956224.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Palm C, Pistrosch F, Herbrig K, Gross P (2006). "Vasopressin antagonists as aquaretic agents for the treatment of hyponatremia". Am. J. Med. 119 (7 Suppl 1): S87–92. doi:10.1016/j.amjmed.2006.05.014. PMID 16843091.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
Further reading
- Rector, Floyd C.; Brenner, Barry M. (2004). Brenner & Rector's the kidney (7th ed.). Philadelphia: Saunders. ISBN 0-7216-0164-2.
{{cite book}}: CS1 maint: multiple names: authors list (link)
External links
- Molecular neurobiology of social bonding: Implications for autism spectrum disorders a lecture by Prof. Larry Young, Jan. 4, 2010.




