Cancer
| Cancer | |
|---|---|
| Specialty | Oncology |
Cancer, also known as a malignant tumor or malignant neoplasm, is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body.[1][2] Not all tumors are cancerous; benign tumors do not spread to other parts of the body.[2] Possible signs and symptoms include: a new lump, abnormal bleeding, a prolonged cough, unexplained weight loss, and a change in bowel movements among others.[3] While these symptoms may indicate cancer, they may also occur due to other issues.[3] There are over 100 different known cancers that affect humans.[2]
Tobacco use is the cause of about 22% of cancer deaths.[1] Another 10% is due to obesity, a poor diet, lack of physical activity, and consumption of alcohol.[1][4] Other factors include certain infections, exposure to ionizing radiation, and environmental pollutants.[5] In the developing world nearly 20% of cancers are due to infections such as hepatitis B, hepatitis C, and human papillomavirus (HPV).[1] These factors act, at least partly, by changing the genes of a cell.[6] Typically many such genetic changes are required before cancer develops.[6] Approximately 5–10% of cancers are due to genetic defects inherited from a person's parents.[7] Cancer can be detected by certain signs and symptoms or screening tests.[1] It is then typically further investigated by medical imaging and confirmed by biopsy.[8]
Many cancers can be prevented by not smoking, maintaining a healthy weight, not drinking too much alcohol, eating plenty of vegetables, fruits and whole grains, being vaccinated against certain infectious diseases, not eating too much processed and red meat, and avoiding too much exposure to sunlight.[9][10] Early detection through screening is useful for cervical and colorectal cancer.[11] The benefits of screening in breast cancer are controversial.[11][12] Cancer is often treated with some combination of radiation therapy, surgery, chemotherapy, and targeted therapy.[1][13] Pain and symptom management are an important part of care. Palliative care is particularly important in those with advanced disease.[1] The chance of survival depends on the type of cancer and extent of disease at the start of treatment.[6] In children under 15 at diagnosis the five-year survival rate in the developed world is on average 80%.[14] For cancer in the United States the average five-year survival rate is 66%.[15]
In 2012 about 14.1 million new cases of cancer occurred globally (not including skin cancer other than melanoma).[6] It caused about 8.2 million deaths or 14.6% of all human deaths.[6][16] The most common types of cancer in males are lung cancer, prostate cancer, colorectal cancer, and stomach cancer, and in females, the most common types are breast cancer, colorectal cancer, lung cancer, and cervical cancer.[6] If skin cancer other than melanoma were included in total new cancers each year it would account for around 40% of cases.[17][18] In children, acute lymphoblastic leukaemia and brain tumors are most common except in Africa where non-Hodgkin lymphoma occurs more often.[14] In 2012, about 165,000 children under 15 years of age were diagnosed with cancer. The risk of cancer increases significantly with age and many cancers occur more commonly in developed countries.[6] Rates are increasing as more people live to an old age and as lifestyle changes occur in the developing world.[19] The financial costs of cancer have been estimated at $1.16 trillion US dollars per year as of 2010.[20]
Definitions
Cancers are a large family of diseases that involve abnormal cell growth with the potential to invade or spread to other parts of the body.[1][2] They form a subset of neoplasms. A neoplasm or tumor is a group of cells that have undergone unregulated growth, and will often form a mass or lump, but may be distributed diffusely.[21][22]
All tumor cells show the six hallmarks of cancer. These are characteristics that the cancer cells need to produce a malignant tumor. They include:[23]
Sustained growth and division
Typically, cells of the body require hormones and other molecules that act as signals for them to grow and divide. Cancer cells, however, have the ability to grow without these external signals. There are multiple ways in which cancer cells can do this: by producing these signals themselves, known as autocrine signalling; by permanently activating the signalling pathways that respond to these signals; or by destroying 'off switches' that prevents excessive growth from these signals (negative feedback). In addition, cell division in normal, non-cancerous cells is tightly controlled. In cancer cells, these processes are deregulated because the proteins that control them are altered, leading to increased growth and cell division within the tumor.[24][25]
Preventing growth suppression
To tightly control cell division, cells have processes within them that prevent cell growth and division. These processes are orchestrated by proteins known as tumor supressor genes. These genes take information from the cell to ensure that it is ready to divide, and will halt division if not (when the DNA is damaged, for example). In cancer, these tumour suppressor proteins are altered so that they don't effectively prevent cell division, even when the cell has severe abnormalities. Another way cells prevent over-division is that normal cells will also stop dividing when the cells fill up the space they are in and touch other cells; known as contact inhibition. Cancer cells do not have contact inhibition, and so will continue to grow and divide, regardless of their surroundings.[24][26]
Resisting cell death
Cells have the ability to 'self-destruct'; a process known as apoptosis. This is required for organisms to grow and develop properly, for maintaining tissues of the body, and is also initiated when a cell is damaged or infected. Cancer cells, however, lose this ability; even though cells may become grossly abnormal, they do not apoptose. The cancer cells may do this by altering the mechanisms that detect the damage or abnormalities. This means that proper signalling cannot occur, thus apoptosis cannot activate. They may also have defects in the downstream signalling itself, or the proteins involved in apoptosis, each of which will also prevent proper apoptosis.[24][27]
Immortalisation
Cells of the body don't normally have the ability to divide indefinitely. They have a limited number of divisions before the cells become unable to divide (senescence), or die (crisis). The cause of these barriers is primarily due to the DNA at the end of chromosomes, known as telomeres. Telomeric DNA shortens with every cell division, until it becomes so short it activates senescence, so the cell stops dividing. Cancer cells bypass this barrier by manipulating enzymes that increase the length of telomeres. Thus, they can divide indefinitely, without initiating senescence.[24][28]
Developing blood vessels
Normal tissues of the body have blood vessels running through them that deliver oxygen from the lungs. Cells must be close to the blood vessels to get enough oxygen for them to survive. New blood vessels are formed during the development of embryos, during wound repair and during the female reproductive cycle. An expanding tumour requires new blood vessels to deliver adequate oxygen to the cancer cells, and thus exploits these normal physiological processes for its benefit. To do this, the cancer cells acquire the ability to orchestrate production of new vasculature by activating the 'angiogenic switch'. In doing so, they control non-cancerous cells that are present in the tumor that can form blood vessels by reducing the production of factors that inhibit blood vessel production, and increasing the production of factors that promote blood vessel formation.[24][29]
Metastasis and invasion
One of the most well known properties of cancer cells is their ability to invade neighboring tissues. It is what dictates whether the tumor is benign or malignant, and is the reason for their dissemination around the body. The cancer cells have to undergo a multitude of changes in order for them to aquire the ability to metastasize. It is a multistep process that starts with local invasion of the cells into the surrounding tissues. They then have to invade blood vessels, survive in the harsh environment of the circulatory system, exit this system and then start dividing in the new tissue.[24][30]
Signs and symptoms

When cancer begins, it invariably produces no symptoms. Signs and symptoms only appear as the mass continues to grow or ulcerates. The findings that result depend on the type and location of the cancer. Few symptoms are specific, with many of them also frequently occurring in individuals who have other conditions. Cancer is the new "great imitator". Thus, it is not uncommon for people diagnosed with cancer to have been treated for other diseases, which were assumed to be causing their symptoms.[31]
Local effects
Local symptoms may occur due to the mass of the tumor or its ulceration. For example, mass effects from lung cancer can cause blockage of the bronchus resulting in cough or pneumonia; esophageal cancer can cause narrowing of the esophagus, making it difficult or painful to swallow; and colorectal cancer may lead to narrowing or blockages in the bowel, resulting in changes in bowel habits. Masses in breasts or testicles may be easily felt. Ulceration can cause bleeding that, if it occurs in the lung, will lead to coughing up blood, in the bowels to anemia or rectal bleeding, in the bladder to blood in the urine, and in the uterus to vaginal bleeding. Although localized pain may occur in advanced cancer, the initial swelling is usually painless. Some cancers can cause a buildup of fluid within the chest or abdomen.[31]
Systemic symptoms
General symptoms occur due to distant effects of the cancer that are not related to direct or metastatic spread. These may include: unintentional weight loss, fever, being excessively tired, and changes to the skin.[32] Hodgkin disease, leukemias, and cancers of the liver or kidney can cause a persistent fever of unknown origin.[31]
Some cancers may cause specific groups of systemic symptoms, termed paraneoplastic phenomena. Examples include the appearance of myasthenia gravis in thymoma and clubbing in lung cancer.[31]
Metastasis
Cancer can spread from its original site by local spread, lymphatic spread to regional lymph nodes or by blood (haematogenous spread) to distant sites, known as metastasis. When cancer spreads by a haematogenous route, it usually spreads all over the body. However, cancer 'seeds' grow in certain selected site only ('soil') as hypothesized in the soil and seed hypothesis of cancer metastasis. The symptoms of metastatic cancers depend on the location of the tumor, and can include enlarged lymph nodes (which can be felt or sometimes seen under the skin and are typically hard), enlarged liver or enlarged spleen, which can be felt in the abdomen, pain or fracture of affected bones, and neurological symptoms.[31]
Causes
The great majority of cancers, some 90–95% of cases, are due to environmental factors. The remaining 5–10% are due to inherited genetics.[5] Environmental, as used by cancer researchers, means any cause that is not inherited genetically, such as lifestyle, economic and behavioral factors, and not merely pollution.[33] Common environmental factors that contribute to cancer death include tobacco (25–30%), diet and obesity (30–35%), infections (15–20%), radiation (both ionizing and non-ionizing, up to 10%), stress, lack of physical activity, and environmental pollutants.[5]
It is nearly impossible to prove what caused a cancer in any individual, because most cancers have multiple possible causes. For example, if a person who uses tobacco heavily develops lung cancer, then it was probably caused by the tobacco use, but since everyone has a small chance of developing lung cancer as a result of air pollution or radiation, then there is a small chance that the cancer developed because of air pollution or radiation. Excepting the rare transmissions that occur with pregnancies and only a marginal few organ donors, cancer is generally not a transmissible disease.[34]
Chemicals

Exposure to particular substances have been linked to specific types of cancer. These substances are called carcinogens. Tobacco smoking, for example, causes 90% of lung cancer.[35] It also causes cancer in the larynx, head, neck, stomach, bladder, kidney, esophagus and pancreas.[36] Tobacco smoke contains over fifty known carcinogens, including nitrosamines and polycyclic aromatic hydrocarbons.[37] Tobacco is responsible for about one in three of all cancer deaths in the developed world,[38] and about one in five worldwide.[37] Lung cancer death rates in the United States have mirrored smoking patterns, with increases in smoking followed by dramatic increases in lung cancer death rates and, more recently, decreases in smoking rates since the 1950s followed by decreases in lung cancer death rates in men since 1990.[39][40]
In Western Europe, 10% of cancers in males and 3% of all cancers in females are attributed to alcohol exposure, especially cancer of the liver and of the digestive tract.[41] Cancer related to substance exposures at work is believed to represent between 2–20% of all cases.[42] Every year, at least 200,000 people die worldwide from cancer related to their workplaces.[43] Millions of workers run the risk of developing cancers such as lung cancer and mesothelioma from inhaling tobacco smoke or asbestos fibers on the job, or leukemia from exposure to benzene at their workplaces.[43]
Diet and exercise
Diet, physical inactivity, and obesity are related to up to 30–35% of cancer deaths.[5][44] In the United States excess body weight is associated with the development of many types of cancer and is a factor in 14–20% of all cancer deaths.[44] Correspondingly, a UK study including data on over 5 million people showed higher body mass index to be related to at least 10 types of cancer, and responsible for around 12,000 cases each year in that country.[45] Physical inactivity is believed to contribute to cancer risk, not only through its effect on body weight but also through negative effects on the immune system and endocrine system.[44] More than half of the effect from diet is due to overnutrition (eating too much), rather than from eating too few vegetables or other healthful foods.
Some specific foods are linked to specific cancers. A high-salt diet is linked to gastric cancer.[46] Aflatoxin B1, a frequent food contaminate, causes liver cancer.[46] Betel nut chewing causes oral cancer.[46] The differences in dietary practices may partly explain differences in cancer incidence in different countries. For example, gastric cancer is more common in Japan due to its high-salt diet[47] and colon cancer is more common in the United States. Immigrants develop the risk of their new country, often within one generation, suggesting a substantial link between diet and cancer.[48]
Infection
Worldwide approximately 18% of cancer deaths are related to infectious diseases.[5] This proportion varies in different regions of the world from a high of 25% in Africa to less than 10% in the developed world.[5] Viruses are the usual infectious agents that cause cancer but cancer bacteria and parasites may also have an effect.
A virus that can cause cancer is called an oncovirus. These include human papillomavirus (cervical carcinoma), Epstein–Barr virus (B-cell lymphoproliferative disease and nasopharyngeal carcinoma), Kaposi's sarcoma herpesvirus (Kaposi's sarcoma and primary effusion lymphomas), hepatitis B and hepatitis C viruses (hepatocellular carcinoma), and human T-cell leukemia virus-1 (T-cell leukemias). Bacterial infection may also increase the risk of cancer, as seen in Helicobacter pylori-induced gastric carcinoma.[49] Parasitic infections strongly associated with cancer include Schistosoma haematobium (squamous cell carcinoma of the bladder) and the liver flukes, Opisthorchis viverrini and Clonorchis sinensis (cholangiocarcinoma).[50]
Radiation
Up to 10% of invasive cancers are related to radiation exposure, including both ionizing radiation and non-ionizing ultraviolet radiation.[5] Additionally, the vast majority of non-invasive cancers are non-melanoma skin cancers caused by non-ionizing ultraviolet radiation, mostly from sunlight. Sources of ionizing radiation include medical imaging and radon gas.
Ionizing radiation is not a particularly strong mutagen.[51] Residential exposure to radon gas, for example, has similar cancer risks as passive smoking.[51] Radiation is a more potent source of cancer when it is combined with other cancer-causing agents, such as radon gas exposure plus smoking tobacco.[51] Radiation can cause cancer in most parts of the body, in all animals, and at any age. Children and adolescents are twice as likely to develop radiation-induced leukemia as adults; radiation exposure before birth has ten times the effect.[51]
Medical use of ionizing radiation is a small but growing source of radiation-induced cancers. Ionizing radiation may be used to treat other cancers, but this may, in some cases, induce a second form of cancer.[51] It is also used in some kinds of medical imaging.[52]
Prolonged exposure to ultraviolet radiation from the sun can lead to melanoma and other skin malignancies.[53] Clear evidence establishes ultraviolet radiation, especially the non-ionizing medium wave UVB, as the cause of most non-melanoma skin cancers, which are the most common forms of cancer in the world.[53]
Non-ionizing radio frequency radiation from mobile phones, electric power transmission, and other similar sources have been described as a possible carcinogen by the World Health Organization's International Agency for Research on Cancer.[54] However, studies have not found a consistent link between cell phone radiation and cancer risk.[55]
Heredity
The vast majority of cancers are non-hereditary ("sporadic cancers"). Hereditary cancers are primarily caused by an inherited genetic defect. Less than 0.3% of the population are carriers of a genetic mutation that has a large effect on cancer risk and these cause less than 3–10% of all cancer.[56] Some of these syndromes include: certain inherited mutations in the genes BRCA1 and BRCA2 with a more than 75% risk of breast cancer and ovarian cancer,[56] and hereditary nonpolyposis colorectal cancer (HNPCC or Lynch syndrome), which is present in about 3% of people with colorectal cancer,[57] among others.
Physical agents
Some substances cause cancer primarily through their physical, rather than chemical, effects on cells.[58] A prominent example of this is prolonged exposure to asbestos, naturally occurring mineral fibers that are a major cause of mesothelioma, which is a cancer of the serous membrane, usually the serous membrane surrounding the lungs.[58] Other substances in this category, including both naturally occurring and synthetic asbestos-like fibers, such as wollastonite, attapulgite, glass wool, and rock wool, are believed to have similar effects.[58] Non-fibrous particulate materials that cause cancer include powdered metallic cobalt and nickel, and crystalline silica (quartz, cristobalite, and tridymite).[58] Usually, physical carcinogens must get inside the body (such as through inhaling tiny pieces) and require years of exposure to develop cancer.[58]
Physical trauma resulting in cancer is relatively rare.[59] Claims that breaking bones resulted in bone cancer, for example, have never been proven.[59] Similarly, physical trauma is not accepted as a cause for cervical cancer, breast cancer, or brain cancer.[59] One accepted source is frequent, long-term application of hot objects to the body. It is possible that repeated burns on the same part of the body, such as those produced by kanger and kairo heaters (charcoal hand warmers), may produce skin cancer, especially if carcinogenic chemicals are also present.[59] Frequently drinking scalding hot tea may produce esophageal cancer.[59] Generally, it is believed that the cancer arises, or a pre-existing cancer is encouraged, during the process of repairing the trauma, rather than the cancer being caused directly by the trauma.[59] However, repeated injuries to the same tissues might promote excessive cell proliferation, which could then increase the odds of a cancerous mutation.
It is controversial whether chronic inflammation can directly cause mutation.[59][60] It is recognized, however, that inflammation can contribute to proliferation, survival, angiogenesis and migration of cancer cells by influencing the microenvironment around tumors.[61][62] Furthermore, oncogenes are known to build up an inflammatory pro-tumorigenic microenvironment.[63]
Hormones
Some hormones play a role in the development of cancer by promoting cell proliferation.[64] Insulin-like growth factors and their binding proteins play a key role in cancer cell proliferation, differentiation and apoptosis, suggesting possible involvement in carcinogenesis.[65]
Hormones are important agents in sex-related cancers, such as cancer of the breast, endometrium, prostate, ovary, and testis, and also of thyroid cancer and bone cancer.[64] For example, the daughters of women who have breast cancer have significantly higher levels of estrogen and progesterone than the daughters of women without breast cancer. These higher hormone levels may explain why these women have higher risk of breast cancer, even in the absence of a breast-cancer gene.[64] Similarly, men of African ancestry have significantly higher levels of testosterone than men of European ancestry, and have a correspondingly much higher level of prostate cancer.[64] Men of Asian ancestry, with the lowest levels of testosterone-activating androstanediol glucuronide, have the lowest levels of prostate cancer.[64]
Other factors are also relevant: obese people have higher levels of some hormones associated with cancer and a higher rate of those cancers.[64] Women who take hormone replacement therapy have a higher risk of developing cancers associated with those hormones.[64] On the other hand, people who exercise far more than average have lower levels of these hormones, and lower risk of cancer.[64] Osteosarcoma may be promoted by growth hormones.[64] Some treatments and prevention approaches leverage this cause by artificially reducing hormone levels, and thus discouraging hormone-sensitive cancers.[64]
Pathophysiology
Genetics
Cancer is fundamentally a disease of tissue growth regulation failure. In order for a normal cell to transform into a cancer cell, the genes that regulate cell growth and differentiation must be altered.[66]
The affected genes are divided into two broad categories. Oncogenes are genes that promote cell growth and reproduction. Tumor suppressor genes are genes that inhibit cell division and survival. Malignant transformation can occur through the formation of novel oncogenes, the inappropriate over-expression of normal oncogenes, or by the under-expression or disabling of tumor suppressor genes. Typically, changes in many genes are required to transform a normal cell into a cancer cell.[67]
Genetic changes can occur at different levels and by different mechanisms. The gain or loss of an entire chromosome can occur through errors in mitosis. More common are mutations, which are changes in the nucleotide sequence of genomic DNA.
Large-scale mutations involve the deletion or gain of a portion of a chromosome. Genomic amplification occurs when a cell gains many copies (often 20 or more) of a small chromosomal locus, usually containing one or more oncogenes and adjacent genetic material. Translocation occurs when two separate chromosomal regions become abnormally fused, often at a characteristic location. A well-known example of this is the Philadelphia chromosome, or translocation of chromosomes 9 and 22, which occurs in chronic myelogenous leukemia, and results in production of the BCR-abl fusion protein, an oncogenic tyrosine kinase.
Small-scale mutations include point mutations, deletions, and insertions, which may occur in the promoter region of a gene and affect its expression, or may occur in the gene's coding sequence and alter the function or stability of its protein product. Disruption of a single gene may also result from integration of genomic material from a DNA virus or retrovirus, leading to the expression of viral oncogenes in the affected cell and its descendants.
Replication of the enormous amount of data contained within the DNA of living cells will probabilistically result in some errors (mutations). Complex error correction and prevention is built into the process, and safeguards the cell against cancer. If significant error occurs, the damaged cell can "self-destruct" through programmed cell death, termed apoptosis. If the error control processes fail, then the mutations will survive and be passed along to daughter cells.
Some environments make errors more likely to arise and propagate. Such environments can include the presence of disruptive substances called carcinogens, repeated physical injury, heat, ionising radiation, or hypoxia.[68]
The errors that cause cancer are self-amplifying and compounding, for example:
- A mutation in the error-correcting machinery of a cell might cause that cell and its children to accumulate errors more rapidly.
- A further mutation in an oncogene might cause the cell to reproduce more rapidly and more frequently than its normal counterparts.
- A further mutation may cause loss of a tumor suppressor gene, disrupting the apoptosis signalling pathway and resulting in the cell becoming immortal.
- A further mutation in signaling machinery of the cell might send error-causing signals to nearby cells.
The transformation of normal cell into cancer is akin to a chain reaction caused by initial errors, which compound into more severe errors, each progressively allowing the cell to escape the controls that limit normal tissue growth. This rebellion-like scenario becomes an undesirable survival of the fittest, where the driving forces of evolution work against the body's design and enforcement of order. Once cancer has begun to develop, this ongoing process, termed clonal evolution, drives progression towards more invasive stages.[69] Clonal evolution leads to intra-tumour heterogeneity that complicates designing effective treatment strategies.
Characteristic abilities developed by cancers are divided into a number of categories. Six categories were originally proposed, in a 2000 article called "The Hallmarks of Cancer" by Douglas Hanahan and Robert Weinberg: evasion of apoptosis, self-sufficiency in growth signals, insensitivity to anti-growth signals, sustained angiogenesis, limitless replicative potential, and metastasis. Based on further work, the same authors added two more categories in 2011: reprogramming of energy metabolism and evasion of immune destruction.[70][71]
Epigenetics

Classically, cancer has been viewed as a set of diseases that are driven by progressive genetic abnormalities that include mutations in tumor-suppressor genes and oncogenes, and chromosomal abnormalities. However, it has become apparent that cancer is also driven by epigenetic alterations.[72]
Epigenetic alterations refer to functionally relevant modifications to the genome that do not involve a change in the nucleotide sequence. Examples of such modifications are changes in DNA methylation (hypermethylation and hypomethylation) and histone modification[73] and changes in chromosomal architecture (caused by inappropriate expression of proteins such as HMGA2 or HMGA1).[74] Each of these epigenetic alterations serves to regulate gene expression without altering the underlying DNA sequence. These changes may remain through cell divisions, last for multiple generations, and can be considered to be epimutations (equivalent to mutations).
Epigenetic alterations occur frequently in cancers. As an example, Schnekenburger and Diederich[75] listed protein coding genes that were frequently altered in their methylation in association with colon cancer. These included 147 hypermethylated and 27 hypomethylated genes. Of the hypermethylated genes, 10 were hypermethylated in 100% of colon cancers, and many others were hypermethylated in more than 50% of colon cancers.
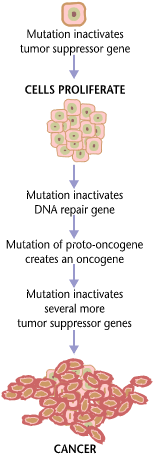
While large numbers of epigenetic alterations are found in cancers, the epigenetic alterations in DNA repair genes, causing reduced expression of DNA repair proteins, may be of particular importance. Such alterations are thought to occur early in progression to cancer and to be a likely cause of the genetic instability characteristic of cancers.[76][77][78][79]
Reduced expression of DNA repair genes causes deficient DNA repair. This is shown in the figure at the 4th level from the top. (In the figure, red wording indicates the central role of DNA damage and defects in DNA repair in progression to cancer.) When DNA repair is deficient DNA damages remain in cells at a higher than usual level (5th level from the top in figure), and these excess damages cause increased frequencies of mutation and/or epimutation (6th level from top of figure). Mutation rates increase substantially in cells defective in DNA mismatch repair[80][81] or in homologous recombinational repair (HRR).[82] Chromosomal rearrangements and aneuploidy also increase in HRR defective cells.[83]
Higher levels of DNA damage not only cause increased mutation (right side of figure), but also cause increased epimutation. During repair of DNA double strand breaks, or repair of other DNA damages, incompletely cleared sites of repair can cause epigenetic gene silencing.[84][85]
Deficient expression of DNA repair proteins due to an inherited mutation can cause an increased risk of cancer. Individuals with an inherited impairment in any of 34 DNA repair genes (see article DNA repair-deficiency disorder) have an increased risk of cancer, with some defects causing up to a 100% lifetime chance of cancer (e.g. p53 mutations).[86] Germ line DNA repair mutations are noted in a box on the left side of the figure, with an arrow indicating their contribution to DNA repair deficiency. However, such germline mutations (which cause highly penetrant cancer syndromes) are the cause of only about 1 percent of cancers.[87]
In sporadic cancers, deficiencies in DNA repair are occasionally caused by a mutation in a DNA repair gene, but are much more frequently caused by epigenetic alterations that reduce or silence expression of DNA repair genes. This is indicated in the figure at the 3rd level from the top. Many studies of heavy metal-induced carcinogenesis show that such heavy metals cause reduction in expression of DNA repair enzymes, some through epigenetic mechanisms. In some cases, DNA repair inhibition is proposed to be a predominant mechanism in heavy metal-induced carcinogenicity. In addition, there are frequent epigenetic alterations of the DNA sequences coding for small RNAs called microRNAs (or miRNAs). MiRNAs do not code for proteins, but can “target” protein-coding genes and reduce their expression.
Cancers usually arise from an assemblage of mutations and epimutations that confer a selective advantage leading to clonal expansion (see Field defects in progression to cancer). Mutations, however, may not be as frequent in cancers as epigenetic alterations. An average cancer of the breast or colon can have about 60 to 70 protein-altering mutations, of which about three or four may be “driver” mutations, and the remaining ones may be “passenger” mutations.[88]
As pointed out above under genetic alterations, cancer is caused by failure to regulate tissue growth, when the genes that regulate cell growth and differentiation are altered. It has become clear that these alterations are caused by both DNA sequence mutation in oncogenes and tumor suppressor genes as well as by epigenetic alterations. The epigenetic deficiencies in expression of DNA repair genes, in particular, likely cause an increased frequency of mutations, some of which then occur in oncogenes and tumor suppressor genes.
Metastasis
Metastasis is the spread of cancer to other locations in the body. The new tumors are called metastatic tumors, while the original is called the primary tumor. Almost all cancers can metastasize.[89] Most cancer deaths are due to cancer that has spread from its primary site to other organs (metastasized).[90]
Metastasis is very common in the late stages of cancer, and it can occur via the blood or the lymphatic system or both. The typical steps in metastasis are local invasion, intravasation into the blood or lymph, circulation through the body, extravasation into the new tissue, proliferation, and angiogenesis. Different types of cancers tend to metastasize to particular organs, but overall the most common places for metastases to occur are the lungs, liver, brain, and the bones.[89]
Diagnosis

Most cancers are initially recognized either because of the appearance of signs or symptoms or through screening. Neither of these lead to a definitive diagnosis, which requires the examination of a tissue sample by a pathologist. People with suspected cancer are investigated with medical tests. These commonly include blood tests, X-rays, CT scans and endoscopy.
Most people are distressed to learn that they have cancer. They may become extremely anxious and depressed. The risk of suicide in people with cancer is approximately double the normal risk.[91]
Classification
Cancers are classified by the type of cell that the tumor cells resemble and is therefore presumed to be the origin of the tumor. These types include:
- Carcinoma: Cancers derived from epithelial cells. This group includes many of the most common cancers, particularly in the aged, and include nearly all those developing in the breast, prostate, lung, pancreas, and colon.
- Sarcoma: Cancers arising from connective tissue (i.e. bone, cartilage, fat, nerve), each of which develops from cells originating in mesenchymal cells outside the bone marrow.
- Lymphoma and leukemia: These two classes of cancer arise from hematopoietic (blood-forming) cells that leave the marrow and tend to mature in the lymph nodes and blood, respectively. Leukemia is the most common type of cancer in children accounting for about 30%.[92]
- Germ cell tumor: Cancers derived from pluripotent cells, most often presenting in the testicle or the ovary (seminoma and dysgerminoma, respectively).
- Blastoma: Cancers derived from immature "precursor" cells or embryonic tissue. Blastomas are more common in children than in older adults.
Cancers are usually named using -carcinoma, -sarcoma or -blastoma as a suffix, with the Latin or Greek word for the organ or tissue of origin as the root. For example, cancers of the liver parenchyma arising from malignant epithelial cells is called hepatocarcinoma, while a malignancy arising from primitive liver precursor cells is called a hepatoblastoma, and a cancer arising from fat cells is called a liposarcoma. For some common cancers, the English organ name is used. For example, the most common type of breast cancer is called ductal carcinoma of the breast. Here, the adjective ductal refers to the appearance of the cancer under the microscope, which suggests that it has originated in the milk ducts.
Benign tumors (which are not cancers) are named using -oma as a suffix with the organ name as the root. For example, a benign tumor of smooth muscle cells is called a leiomyoma (the common name of this frequently occurring benign tumor in the uterus is fibroid). Confusingly, some types of cancer use the -noma suffix, examples including melanoma and seminoma.
Some types of cancer are named for the size and shape of the cells under a microscope, such as giant cell carcinoma, spindle cell carcinoma, and small-cell carcinoma.
Pathology
The tissue diagnosis given by the pathologist indicates the type of cell that is proliferating, its histological grade, genetic abnormalities, and other features of the tumor. Together, this information is useful to evaluate the prognosis of the patient and to choose the best treatment. Cytogenetics and immunohistochemistry are other types of testing that the pathologist may perform on the tissue specimen. These tests may provide information about the molecular changes (such as mutations, fusion genes, and numerical chromosome changes) that have happened in the cancer cells, and may thus also indicate the future behavior of the cancer (prognosis) and best treatment.
-
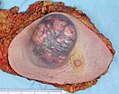
An invasive ductal carcinoma of the breast (pale area at the center) surrounded by spikes of whitish scar tissue and yellow fatty tissue
-
An invasive colorectal carcinoma (top center) in a colectomy specimen
-
A squamous-cell carcinoma (the whitish tumor) near the bronchi in a lung specimen
-
A large invasive ductal carcinoma in a mastectomy specimen
Prevention
Cancer prevention is defined as active measures to decrease the risk of cancer.[93] The vast majority of cancer cases are due to environmental risk factors, and many, but not all, of these environmental factors are controllable lifestyle choices. Thus, cancer is considered a largely preventable disease.[94] Greater than 30% of cancer deaths could be prevented by avoiding risk factors including: tobacco, overweight / obesity, an insufficient diet, physical inactivity, alcohol, sexually transmitted infections, and air pollution.[95] Not all environmental causes are controllable, such as naturally occurring background radiation, and other cases of cancer are caused through hereditary genetic disorders, and thus it is not possible to prevent all cases of cancer.
Dietary
While many dietary recommendations have been proposed to reduce the risk of cancer, the evidence to support them is not definitive.[9][96] The primary dietary factors that increase risk are obesity and alcohol consumption; with a diet low in fruits and vegetables and high in red meat being implicated but not confirmed.[97][98] A 2014 meta-analysis did not find a relationship between fruits and vegetables and cancer.[99] Consumption of coffee is associated with a reduced risk of liver cancer.[100] Studies have linked excessive consumption of red or processed meat to an increased risk of breast cancer, colon cancer, and pancreatic cancer, a phenomenon that could be due to the presence of carcinogens in meats cooked at high temperatures.[101][102] This was confirmed in 2015 by the IARC of the World Health Organization, which determined that eating processed meat (e.g., bacon, ham, hot dogs, sausages) and, to a lesser degree, red meat was linked to some cancers.[103][104]
Dietary recommendations for cancer prevention typically include an emphasis on vegetables, fruit, whole grains, and fish, and an avoidance of processed and red meat (beef, pork, lamb), animal fats, and refined carbohydrates.[9][96]
Medication
The concept that medications can be used to prevent cancer is attractive, and evidence supports their use in a few defined circumstances.[105] In the general population, NSAIDs reduce the risk of colorectal cancer, however due to the cardiovascular and gastrointestinal side effects they cause overall harm when used for prevention.[106] Aspirin has been found to reduce the risk of death from cancer by about 7%.[107] COX-2 inhibitor may decrease the rate of polyp formation in people with familial adenomatous polyposis, however it is associated with the same adverse effects as NSAIDs.[108] Daily use of tamoxifen or raloxifene has been demonstrated to reduce the risk of developing breast cancer in high-risk women.[109] The benefit versus harm for 5-alpha-reductase inhibitor such as finasteride is not clear.[110]
Vitamins have not been found to be effective at preventing cancer,[111] although low blood levels of vitamin D are correlated with increased cancer risk.[112][113] Whether this relationship is causal and vitamin D supplementation is protective is not determined.[114] Beta-Carotene supplementation has been found to increase lung cancer rates in those who are high risk.[115] Folic acid supplementation has not been found effective in preventing colon cancer and may increase colon polyps.[116] It is unclear if selenium supplementation has an effect.[117]
Vaccination
Vaccines have been developed that prevent infection by some carcinogenic viruses.[118] Human papillomavirus vaccine (Gardasil and Cervarix) decreases the risk of developing cervical cancer.[118] The hepatitis B vaccine prevents infection with hepatitis B virus and thus decreases the risk of liver cancer.[118] The administration of human papillomavirus and hepatitis B vaccinations is recommended when resources allow.[119]
Screening
Unlike diagnosis efforts prompted by symptoms and medical signs, cancer screening involves efforts to detect cancer after it has formed, but before any noticeable symptoms appear.[120] This may involve physical examination, blood or urine tests, or medical imaging.[120]
Cancer screening is currently not possible for many types of cancers, and even when tests are available, they may not be recommended for everyone. Universal screening or mass screening involves screening everyone.[121] Selective screening identifies people who are known to be at higher risk of developing cancer, such as people with a family history of cancer.[121] Several factors are considered to determine whether the benefits of screening outweigh the risks and the costs of screening.[120] These factors include:
- Possible harms from the screening test: for example, X-ray images involve exposure to potentially harmful ionizing radiation.
- The likelihood of the test correctly identifying cancer.
- The likelihood of cancer being present: Screening is not normally useful for rare cancers.
- Possible harms from follow-up procedures.
- Whether suitable treatment is available.
- Whether early detection improves treatment outcomes.
- Whether the cancer will ever need treatment.
- Whether the test is acceptable to the people: If a screening test is too burdensome (for example, being extremely painful), then people will refuse to participate.[121]
- Cost of the test.
Recommendations
The U.S. Preventive Services Task Force (USPSTF) strongly recommends cervical cancer screening in women who are sexually active and have a cervix at least until the age of 65.[122] They recommend that Americans be screened for colorectal cancer via fecal occult blood testing, sigmoidoscopy, or colonoscopy starting at age 50 until age 75.[123] There is insufficient evidence to recommend for or against screening for skin cancer,[124] oral cancer,[125] lung cancer,[126] or prostate cancer in men under 75.[127] Routine screening is not recommended for bladder cancer,[128] testicular cancer,[129] ovarian cancer,[130] pancreatic cancer,[131] or prostate cancer.[132]
The USPSTF recommends mammography for breast cancer screening every two years for those 50–74 years old; however, they do not recommend either breast self-examination or clinical breast examination.[133] A 2011 Cochrane review came to slightly different conclusions with respect to breast cancer screening stating that routine mammography may do more harm than good.[134]
Japan screens for gastric cancer using photofluorography due to the high incidence there.[19]
Genetic testing
| Gene | Cancer types |
|---|---|
| BRCA1, BRCA2 | Breast, ovarian, pancreatic |
| HNPCC, MLH1, MSH2, MSH6, PMS1, PMS2 | Colon, uterine, small bowel, stomach, urinary tract |
Genetic testing for individuals at high-risk of certain cancers is recommended.[119][135] Carriers of these mutations may then undergo enhanced surveillance, chemoprevention, or preventative surgery to reduce their subsequent risk.[135]
Management
Many treatment options for cancer exist, with the primary ones including surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy and palliative care. Which treatments are used depends on the type, location, and grade of the cancer as well as the person's health and wishes. The treatment intent may be curative or not curative.
Chemotherapy
Chemotherapy is the treatment of cancer with one or more cytotoxic anti-neoplastic drugs (chemotherapeutic agents) as part of a standardized regimen. The term encompasses any of a large variety of different anticancer drugs, which are divided into broad categories such as alkylating agents and antimetabolites.[136] Traditional chemotherapeutic agents act by killing cells that divide rapidly, one of the main properties of most cancer cells.
Targeted therapy is a form of chemotherapy that targets specific molecular differences between cancer and normal cells. The first targeted therapies to be developed blocked the estrogen receptor molecule, inhibiting the growth of breast cancer. Another common example is the class of Bcr-Abl inhibitors, which are used to treat chronic myelogenous leukemia (CML).[137] Currently, there are targeted therapies for breast cancer, multiple myeloma, lymphoma, prostate cancer, melanoma and other cancers.[138]
The efficacy of chemotherapy depends on the type of cancer and the stage. In combination with surgery, chemotherapy has proven useful in a number of different cancer types including: breast cancer, colorectal cancer, pancreatic cancer, osteogenic sarcoma, testicular cancer, ovarian cancer, and certain lung cancers.[139] The overall effectiveness ranges from being curative for some cancers, such as some leukemias,[140][141] to being ineffective, such as in some brain tumors,[142] to being needless in others, like most non-melanoma skin cancers.[143] The effectiveness of chemotherapy is often limited by toxicity to other tissues in the body. Even when it is impossible for chemotherapy to provide a permanent cure, chemotherapy may be useful to reduce symptoms like pain or to reduce the size of an inoperable tumor in the hope that surgery will be possible in the future.
Radiation
Radiation therapy involves the use of ionizing radiation in an attempt to either cure or improve the symptoms of cancer. It works by damaging the DNA of cancerous tissue leading to cellular death. To spare normal tissues (such as skin or organs, which radiation must pass through to treat the tumor), shaped radiation beams are aimed from several angles of exposure to intersect at the tumor, providing a much larger absorbed dose there than in the surrounding, healthy tissue. As with chemotherapy, different cancers respond differently to radiation therapy.[144][145][146]
Radiation therapy is used in about half of all cases and the radiation can be from either internal sources in the form of brachytherapy or external radiation sources. The radiation is most commonly low energy x-rays for treating skin cancers while higher energy x-ray beams are used in the treatment of cancers within the body.[147] Radiation is typically used in addition to surgery and or chemotherapy but for certain types of cancer, such as early head and neck cancer, may be used alone.[148] For painful bone metastasis, it has been found to be effective in about 70% of people.[148]
Surgery
Surgery is the primary method of treatment of most isolated solid cancers and may play a role in palliation and prolongation of survival. It is typically an important part of making the definitive diagnosis and staging the tumor as biopsies are usually required. In localized cancer surgery typically attempts to remove the entire mass along with, in certain cases, the lymph nodes in the area. For some types of cancer this is all that is needed to eliminate the cancer.[139]
Palliative care
Palliative care refers to treatment that attempts to make the person feel better and may or may not be combined with an attempt to treat the cancer. Palliative care includes action to reduce the physical, emotional, spiritual, and psycho-social distress experienced by people with cancer. Unlike treatment that is aimed at directly killing cancer cells, the primary goal of palliative care is to improve the person's quality of life.
People at all stages of cancer treatment should have some kind of palliative care to provide comfort. In some cases, medical specialty professional organizations recommend that people and physicians respond to cancer only with palliative care and not with cure-directed therapy.[149] This includes:[150]
- people with low performance status, corresponding with limited ability to care for themselves[149]
- people who received no benefit from prior evidence-based treatments[149]
- people who are not eligible to participate in any appropriate clinical trial[149]
- people for whom the physician sees no strong evidence that treatment would be effective[149]
Palliative care is often confused with hospice and therefore only involved when people approach end of life. Like hospice care, palliative care attempts to help the person cope with the immediate needs and to increase the person's comfort. Unlike hospice care, palliative care does not require people to stop treatment aimed at prolonging their lives or curing the cancer.
Multiple national medical guidelines recommend early palliative care for people whose cancer has produced distressing symptoms (pain, shortness of breath, fatigue, nausea) or who need help coping with their illness. In people who have metastatic disease when first diagnosed, oncologists should consider a palliative care consult immediately. Additionally, an oncologist should consider a palliative care consult in any person they feel has less than 12 months of life even if continuing aggressive treatment.[151][152][153]
Immunotherapy
A variety of therapies using immunotherapy, stimulating or helping the immune system to fight cancer, have come into use since 1997, and this continues to be an area of very active research.[154]
Alternative medicine
Complementary and alternative cancer treatments are a diverse group of health care systems, practices, and products that are not part of conventional medicine.[155] "Complementary medicine" refers to methods and substances used along with conventional medicine, while "alternative medicine" refers to compounds used instead of conventional medicine.[156] Most complementary and alternative medicines for cancer have not been rigorously studied or tested. Some alternative treatments have been investigated and shown to be ineffective but still continue to be marketed and promoted. Cancer researcher Andrew J. Vickers has stated: "The label 'unproven' is inappropriate for such therapies; it is time to assert that many alternative cancer therapies have been 'disproven'."[157]
Prognosis
Cancer has a reputation as a deadly disease. Taken as a whole, about half of people receiving treatment for invasive cancer (excluding carcinoma in situ and non-melanoma skin cancers) die from cancer or its treatment.[19] Survival is worse in the developing world,[19] partly because the types of cancer that are most common there are at present harder to treat than those associated with the lifestyle of developed countries.[158] However, the survival rates vary dramatically by type of cancer, and by the stage at which it is diagnosed, with the range running from the great majority of people surviving to almost no one surviving as long as five years after diagnosis. Once a cancer has metastasized or spread beyond its original site, the prognosis normally becomes much worse.
Those who survive cancer are at increased risk of developing a second primary cancer at about twice the rate of those never diagnosed with cancer.[159] The increased risk is believed to be primarily due to the same risk factors that produced the first cancer, partly due to the treatment for the first cancer, and potentially related to better compliance with screening.[159]
Predicting either short-term or long-term survival is difficult and depends on many factors. The most important factors are the particular kind of cancer and the patient's age and overall health. People who are frail with many other health problems have lower survival rates than otherwise healthy people. A centenarian is unlikely to survive for five years even if the treatment is successful. People who report a higher quality of life tend to survive longer.[160] People with lower quality of life may be affected by major depressive disorder and other complications from cancer treatment and/or disease progression that both impairs their quality of life and reduces their quantity of life. Additionally, patients with worse prognoses may be depressed or report a lower quality of life directly because they correctly perceive that their condition is likely to be fatal.
People with cancer, even those who are walking on their own, have an increased risk of blood clots in veins. The use of heparin appears improve survival and decrease the risk of blood clots.[161]
Epidemiology

In 2008, approximately 12.7 million cancers were diagnosed (excluding non-melanoma skin cancers and other non-invasive cancers),[19] and in 2010 nearly 7.98 million people died.[163] Cancers as a group account for approximately 13% of all deaths each year with the most common being: lung cancer (1.4 million deaths), stomach cancer (740,000 deaths), liver cancer (700,000 deaths), colorectal cancer (610,000 deaths), and breast cancer (460,000 deaths).[164] This makes invasive cancer the leading cause of death in the developed world and the second leading cause of death in the developing world.[19] Over half of cases occur in the developing world.[19]
Deaths from cancer were 5.8 million in 1990[163] and rates have been increasing primarily due to an aging population and lifestyle changes in the developing world.[19] The most significant risk factor for developing cancer is old age.[165] Although it is possible for cancer to strike at any age, most people who are diagnosed with invasive cancer are over the age of 65.[165] According to cancer researcher Robert A. Weinberg, "If we lived long enough, sooner or later we all would get cancer."[166] Some of the association between aging and cancer is attributed to immunosenescence,[167] errors accumulated in DNA over a lifetime,[168] and age-related changes in the endocrine system.[169] The effect of aging on cancer is complicated with a number of factors such as DNA damage and inflammation promoting it and a number of factors such as vascular aging and endocrine changes inhibiting it.[170]
Some slow-growing cancers are particularly common. Autopsy studies in Europe and Asia have shown that up to 36% of people have undiagnosed and apparently harmless thyroid cancer at the time of their deaths, and that 80% of men develop prostate cancer by age 80.[171][172] As these cancers did not cause the person's death, identifying them would have represented overdiagnosis rather than useful medical care.
The three most common childhood cancers are leukemia (34%), brain tumors (23%), and lymphomas (12%).[173] In the United States cancer affects about 1 in 285 children.[174] Rates of childhood cancer have increased by 0.6% per year between 1975 to 2002 in the United States[175] and by 1.1% per year between 1978 and 1997 in Europe.[173] Death from childhood cancer have decreased by half since 1975 in the United States.[174]
History

Cancer has existed for all of human history.[176] The earliest written record regarding cancer is from circa 1600 BC in the Egyptian Edwin Smith Papyrus and describes cancer of the breast.[176] Hippocrates (ca. 460 BC – ca. 370 BC) described several kinds of cancer, referring to them with the Greek word καρκίνος karkinos (crab or crayfish).[176] This name comes from the appearance of the cut surface of a solid malignant tumor, with "the veins stretched on all sides as the animal the crab has its feet, whence it derives its name".[177] Galen stated that "cancer of the breast is so called because of the fancied resemblance to a crab given by the lateral prolongations of the tumor and the adjacent distended veins".[178]: 738 Celsus (ca. 25 BC – 50 AD) translated karkinos into the Latin cancer, also meaning crab and recommended surgery as treatment.[176] Galen (2nd century AD) disagreed with the use of surgery and recommended purgatives instead.[176] These recommendations largely stood for 1000 years.[176]
In the 15th, 16th and 17th centuries, it became acceptable for doctors to dissect bodies to discover the cause of death.[179] The German professor Wilhelm Fabry believed that breast cancer was caused by a milk clot in a mammary duct. The Dutch professor Francois de la Boe Sylvius, a follower of Descartes, believed that all disease was the outcome of chemical processes, and that acidic lymph fluid was the cause of cancer. His contemporary Nicolaes Tulp believed that cancer was a poison that slowly spreads, and concluded that it was contagious.[180]
The physician John Hill described tobacco snuff as the cause of nose cancer in 1761.[179] This was followed by the report in 1775 by British surgeon Percivall Pott that chimney sweeps' carcinoma, a cancer of the scrotum, was a common disease among chimney sweeps.[181] With the widespread use of the microscope in the 18th century, it was discovered that the 'cancer poison' spread from the primary tumor through the lymph nodes to other sites ("metastasis"). This view of the disease was first formulated by the English surgeon Campbell De Morgan between 1871 and 1874.[182]
Society and culture
Though many diseases (such as heart failure) may have a worse prognosis than most cases of cancer, cancer is the subject of widespread fear and taboos. The euphemism "after a long illness" is still commonly used (2012), reflecting an apparent stigma.[183] This deep belief that cancer is necessarily a difficult and usually deadly disease is reflected in the systems chosen by society to compile cancer statistics: the most common form of cancer—non-melanoma skin cancers, accounting for about one-third of all cancer cases worldwide, but very few deaths[184][185]—are excluded from cancer statistics specifically because they are easily treated and almost always cured, often in a single, short, outpatient procedure.[186]
Cancer is regarded as a disease that must be "fought" to end the "civil insurrection"; a War on Cancer has been declared. Military metaphors are particularly common in descriptions of cancer's human effects, and they emphasize both the parlous state of the affected individual's health and the need for the individual to take immediate, decisive actions himself, rather than to delay, to ignore, or to rely entirely on others caring for him. The military metaphors also help rationalize radical, destructive treatments.[187][188]
In the 1970s, a relatively popular alternative cancer treatment was a specialized form of talk therapy, based on the idea that cancer was caused by a bad attitude.[189] People with a "cancer personality"—depressed, repressed, self-loathing, and afraid to express their emotions—were believed to have manifested cancer through subconscious desire. Some psychotherapists said that treatment to change the patient's outlook on life would cure the cancer.[189] Among other effects, this belief allows society to blame the victim for having caused the cancer (by "wanting" it) or having prevented its cure (by not becoming a sufficiently happy, fearless, and loving person).[190] It also increases patients' anxiety, as they incorrectly believe that natural emotions of sadness, anger or fear shorten their lives.[190] The idea was excoriated by the notoriously outspoken Susan Sontag, who published Illness as Metaphor while recovering from treatment for breast cancer in 1978.[189] Although the original idea is now generally regarded as nonsense, the idea partly persists in a reduced form with a widespread, but incorrect, belief that deliberately cultivating a habit of positive thinking will increase survival.[190] This notion is particularly strong in breast cancer culture.[190]
One idea about why people with cancer are blamed or stigmatized, called the just-world hypothesis, is that blaming cancer on the patient's actions or attitudes allows the blamers to regain a sense of control. This is based upon the blamers' belief that the world is fundamentally just, and so any dangerous illness, like cancer, must be a type of punishment for bad choices, because in a just world, bad things would not happen to good people.[191]
Economic effect
In 2007, the overall costs of cancer in the U.S. — including treatment and indirect mortality expenses (such as lost productivity in the workplace) — was estimated to be $226.8 billion. In 2009, 32% of Hispanics and 10% of children 17 years old or younger lacked health insurance; "uninsured patients and those from ethnic minorities are substantially more likely to be diagnosed with cancer at a later stage, when treatment can be more extensive and more costly."[192]
Research
Because cancer is a class of diseases,[193][194] it is unlikely that there will ever be a single "cure for cancer" any more than there will be a single treatment for all infectious diseases.[195] Angiogenesis inhibitors were once thought to have potential as a "silver bullet" treatment applicable to many types of cancer, but this has not been the case in practice.[196] It is more likely that angiogenesis inhibitors and other cancer therapeutics will be used in combination to reduce cancer morbidity and mortality.[197]
Experimental cancer treatments are treatments that are being studied to see whether they work. Typically, these are studied in clinical trials to compare the proposed treatment to the best existing treatment. They may be entirely new treatments, or they may be treatments that have been used successfully in one type of cancer, and are now being tested to see whether they are effective in another type.[198] More and more, such treatments are being developed alongside companion diagnostic tests to target the right drugs to the right patients, based on their individual biology.[199]
Cancer research is the intense scientific effort to understand disease processes and discover possible therapies.
Research about cancer causes focuses on the following issues:
- Agents (e.g. viruses) and events (e.g. mutations) that cause or facilitate genetic changes in cells destined to become cancer.
- The precise nature of the genetic damage, and the genes that are affected by it.
- The consequences of those genetic changes on the biology of the cell, both in generating the defining properties of a cancer cell, and in facilitating additional genetic events that lead to further progression of the cancer.
The improved understanding of molecular biology and cellular biology due to cancer research has led to a number of new treatments for cancer since U.S. President Nixon declared the "War on Cancer" in 1971. Since then, the U.S. has spent over $200 billion on cancer research, including resources from the public and private sectors and foundations.[200] During that time, the country has seen a five percent decrease in the cancer death rate (adjusting for size and age of the population) between 1950 and 2005.[201]
Hypercompetition for the financial resources that are required to conduct science appears to suppress the creativity, cooperation, risk-taking, and original thinking required to make fundamental discoveries, unduly favoring low-risk research into small incremental advancements over innovative research that might discover radically new and dramatically improved therapy. Other consequences of the highly pressured competition for research resources appear to be a substantial number of research publications whose results cannot be replicated, and perverse incentives in research funding that encourage grantee institutions to grow without making sufficient investments in their own faculty and facilities.[202][203][204][205]
Pregnancy
Because cancer is largely a disease of older adults, it is not common in pregnant women. Cancer affects approximately 1 in 1,000 pregnant women.[206] The most common cancers found during pregnancy are the same as the most common cancers found in non-pregnant women during childbearing ages: breast cancer, cervical cancer, leukemia, lymphoma, melanoma, ovarian cancer, and colorectal cancer.[206]
Diagnosing a new cancer in a pregnant woman is difficult, in part because any symptoms are commonly assumed to be a normal discomfort associated with pregnancy.[206] As a result, cancer is typically discovered at a somewhat later stage than average in many pregnant or recently pregnant women. Some imaging procedures, such as MRIs (magnetic resonance imaging), CT scans, ultrasounds, and mammograms with fetal shielding are considered safe during pregnancy; some others, such as PET scans are not.[206]
Treatment is generally the same as for non-pregnant women.[206] However, radiation and radioactive drugs are normally avoided during pregnancy, especially if the fetal dose might exceed 100 cGy. In some cases, some or all treatments are postponed until after birth if the cancer is diagnosed late in the pregnancy. Early deliveries to speed the start of treatment are not uncommon. Surgery is generally safe, but pelvic surgeries during the first trimester may cause miscarriage. Some treatments, especially certain chemotherapy drugs given during the first trimester, increase the risk of birth defects and pregnancy loss (spontaneous abortions and stillbirths).[206]
Elective abortions are not required and, for the most common forms and stages of cancer, do not improve the likelihood of the mother surviving or being cured.[206] In a few instances, such as advanced uterine cancer, the pregnancy cannot be continued, and in others, such as an acute leukemia discovered early in pregnancy, the pregnant woman may choose to have an abortion so that she can begin aggressive chemotherapy without worrying about birth defects.[206]
Some treatments may interfere with the mother's ability to give birth vaginally or to breastfeed her baby.[206] Cervical cancer may require birth by Caesarean section. Radiation to the breast reduces the ability of that breast to produce milk and increases the risk of mastitis. Also, when chemotherapy is being given after birth, many of the drugs pass through breast milk to the baby, which could harm the baby.[206]
Other animals
Veterinary oncology, concentrating mainly on cats and dogs, is a growing specialty in wealthy countries, and the major forms of human treatment such as surgery and radiotherapy may be offered. The most common types of cancer differ, but the cancer burden seems at least as high in pets as in humans. Animals, typically rodents, are often used in cancer research, and studies of natural cancers in larger animals may benefit research into human cancer.[207]
In non-humans, a few types of transmissible cancer have been described, wherein the cancer spreads between animals by transmission of the tumor cells themselves. This phenomenon is seen in dogs with Sticker's sarcoma, also known as canine transmissible venereal tumor,[208] as well as devil facial tumor disease in Tasmanian devils.
Notes
- ^ a b c d e f g h "Cancer Fact sheet N°297". World Health Organization. February 2014. Retrieved 10 June 2014.
- ^ a b c d "Defining Cancer". National Cancer Institute. Retrieved 10 June 2014.
- ^ a b "Cancer - Signs and symptoms". NHS Choices. Retrieved 10 June 2014.
- ^ "Obesity and Cancer Risk". National Cancer Institute. 3 January 2012. Retrieved 4 July 2015.
- ^ a b c d e f g Anand P, Kunnumakkara AB, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB (September 2008). "Cancer is a preventable disease that requires major lifestyle changes". Pharm. Res. 25 (9): 2097–116. doi:10.1007/s11095-008-9661-9. PMC 2515569. PMID 18626751.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 1.1. ISBN 9283204298.
- ^ "Heredity and Cancer". American Cancer Society. Retrieved 22 July 2013.
- ^ "How is cancer diagnosed?". American Cancer Society. 29 January 2013. Retrieved 10 June 2014.
- ^ a b c Kushi LH, Doyle C, McCullough M; et al. (2012). "American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity". CA Cancer J Clin. 62 (1): 30–67. doi:10.3322/caac.20140. PMID 22237782.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Parkin, DM; Boyd, L; Walker, LC (6 December 2011). "16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010". British journal of cancer. 105 Suppl 2: S77-81. doi:10.1038/bjc.2011.489. PMID 22158327.
- ^ a b World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 4.7. ISBN 9283204298.
- ^ Gøtzsche PC, Jørgensen KJ (4 June 2013). "Screening for breast cancer with mammography". The Cochrane database of systematic reviews. 6: CD001877. doi:10.1002/14651858.CD001877.pub5. PMID 23737396.
- ^ "Targeted Cancer Therapies". NCI. 25 April 2014. Retrieved 11 June 2014.
- ^ a b World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 1.3. ISBN 9283204298.
- ^ "SEER Stat Fact Sheets: All Cancer Sites". National Cancer Institute. Retrieved 18 June 2014.
- ^ "The top 10 causes of death Fact sheet N°310". WHO. May 2014. Retrieved 10 June 2014.
- ^ Dubas, LE; Ingraffea, A (February 2013). "Nonmelanoma skin cancer". Facial plastic surgery clinics of North America. 21 (1): 43–53. doi:10.1016/j.fsc.2012.10.003. PMID 23369588.
- ^ Cakir, BÖ; Adamson, P; Cingi, C (November 2012). "Epidemiology and economic burden of nonmelanoma skin cancer". Facial plastic surgery clinics of North America. 20 (4): 419–22. doi:10.1016/j.fsc.2012.07.004. PMID 23084294.
- ^ a b c d e f g h Jemal A, Bray, F, Center, MM, Ferlay, J, Ward, E, Forman, D (February 2011). "Global cancer statistics". CA: a cancer journal for clinicians. 61 (2): 69–90. doi:10.3322/caac.20107. PMID 21296855.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 6.7. ISBN 9283204298.
- ^ "Cancer Glossary". cancer.org. American Cancer Society. Retrieved 11 September 2013.
- ^ "What is cancer?". cancer.gov. National Cancer Institute. Retrieved 11 September 2013.
- ^ Hanahan, D; Weinberg, RA (7 January 2000). "The hallmarks of cancer". Cell. 100 (1): 57–70. PMID 10647931.
- ^ a b c d e f Hanahan, D; Weinberg, RA (4 March 2011). "Hallmarks of cancer: the next generation". Cell. 144 (5): 646–74. PMID 21376230.
- ^ Evan, GI; Vousden, KH (17 May 2001). "Proliferation, cell cycle and apoptosis in cancer". Nature. 411 (6835): 342–8. PMID 11357141.
- ^ McClatchey, AI; Yap, AS (October 2012). "Contact inhibition (of proliferation) redux". Current opinion in cell biology. 24 (5): 685–94. PMID 22835462.
- ^ Elmore, S (June 2007). "Apoptosis: a review of programmed cell death". Toxicologic pathology. 35 (4): 495–516. PMID 17562483.
- ^ Greenberg, RA (March 2005). "Telomeres, crisis and cancer". Current molecular medicine. 5 (2): 213–8. PMID 15974875.
- ^ Bergers, G; Benjamin, LE (June 2003). "Tumorigenesis and the angiogenic switch". Nature reviews. Cancer. 3 (6): 401–10. PMID 12778130.
- ^ van Zijl, F; Krupitza, G; Mikulits, W (2011). "Initial steps of metastasis: cell invasion and endothelial transmigration". Mutation research. 728 (1–2): 23–34. PMID 21605699.
- ^ a b c d e Holland Chp. 1
- ^ O'Dell, edited by Michael D. Stubblefield, Michael W. (2009). Cancer rehabilitation principles and practice. New York: Demos Medical. p. 983. ISBN 978-1-933864-33-4.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Kravchenko J, Akushevich I, Manton, KG (2009). Cancer mortality and morbidity patterns in the U. S. population: an interdisciplinary approach. Berlin: Springer. ISBN 0-387-78192-7.
The term environment refers not only to air, water, and soil but also to substances and conditions at home and at the workplace, including diet, smoking, alcohol, drugs, exposure to chemicals, sunlight, ionizing radiation, electromagnetic fields, infectious agents, etc. Lifestyle, economic and behavioral factors are all aspects of our environment.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Tolar J, Neglia JP (June 2003). "Transplacental and other routes of cancer transmission between individuals". J. Pediatr. Hematol. Oncol. 25 (6): 430–4. doi:10.1097/00043426-200306000-00002. PMID 12794519.
- ^ Biesalski HK, Bueno de Mesquita B, Chesson A, Chytil F, Grimble R, Hermus RJ, Köhrle J, Lotan R, Norpoth K, Pastorino U, Thurnham D (1998). "European Consensus Statement on Lung Cancer: risk factors and prevention. Lung Cancer Panel". CA Cancer J Clin. 48 (3): 167–76, discussion 164–6. doi:10.3322/canjclin.48.3.167. PMID 9594919.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kuper H, Boffetta P, Adami HO (September 2002). "Tobacco use and cancer causation: association by tumour type". Journal of Internal Medicine. 252 (3): 206–24. doi:10.1046/j.1365-2796.2002.01022.x. PMID 12270001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Kuper H, Adami HO, Boffetta P (June 2002). "Tobacco use, cancer causation and public health impact". Journal of Internal Medicine. 251 (6): 455–66. doi:10.1046/j.1365-2796.2002.00993.x. PMID 12028500.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sasco AJ, Secretan MB, Straif K (August 2004). "Tobacco smoking and cancer: a brief review of recent epidemiological evidence". Lung Cancer. 45 Suppl 2: S3–9. doi:10.1016/j.lungcan.2004.07.998. PMID 15552776.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thun MJ, Jemal A (October 2006). "How much of the decrease in cancer death rates in the United States is attributable to reductions in tobacco smoking?". Tob Control. 15 (5): 345–7. doi:10.1136/tc.2006.017749. PMC 2563648. PMID 16998161.
- ^ Dubey S, Powell CA (May 2008). "Update in lung cancer 2007". Am. J. Respir. Crit. Care Med. 177 (9): 941–6. doi:10.1164/rccm.200801-107UP. PMC 2720127. PMID 18434333.
- ^ Schütze M, Boeing H, Pischon T, Rehm J, Kehoe T, Gmel G, Olsen A, Tjønneland AM, Dahm CC, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Trichopoulou A, Benetou V, Zylis D, Kaaks R, Rohrmann S, Palli D, Berrino F, Tumino R, Vineis P, Rodríguez L, Agudo A, Sánchez MJ, Dorronsoro M, Chirlaque MD, Barricarte A, Peeters PH, van Gils CH, Khaw KT, Wareham N, Allen NE, Key TJ, Boffetta P, Slimani N, Jenab M, Romaguera D, Wark PA, Riboli E, Bergmann MM (2011). "Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort study". BMJ. 342: d1584. doi:10.1136/bmj.d1584. PMC 3072472. PMID 21474525.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Irigaray P, Newby JA, Clapp R, Hardell L, Howard V, Montagnier L, Epstein S, Belpomme D (December 2007). "Lifestyle-related factors and environmental agents causing cancer: an overview". Biomed. Pharmacother. 61 (10): 640–58. doi:10.1016/j.biopha.2007.10.006. PMID 18055160.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "WHO calls for prevention of cancer through healthy workplaces" (Press release). World Health Organization. 27 April 2007. Retrieved 13 October 2007.
- ^ a b c Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, Gansler T, Andrews KS, Thun MJ (2006). "American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity". CA Cancer J Clin. 56 (5): 254–81, quiz 313–4. doi:10.3322/canjclin.56.5.254. PMID 17005596.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bhaskaran, K (2014). "Body mass index and risk of 22 specific cancers". Lancet. 384 (9945): 755–765. doi:10.1016/S0140-6736(14)60892-8. PMID 25129328.
- ^ a b c Park S, Bae J, Nam BH, Yoo KY (2008). "Aetiology of cancer in Asia" (PDF). Asian Pac. J. Cancer Prev. 9 (3): 371–80. PMID 18990005.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brenner H, Rothenbacher D, Arndt V (2009). "Epidemiology of stomach cancer". Methods Mol. Biol. Methods in Molecular Biology. 472: 467–77. doi:10.1007/978-1-60327-492-0_23. ISBN 978-1-60327-491-3. PMID 19107449.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Buell P, Dunn JE (May 1965). "Cancer mortality among Japanese Issei and Nisei of California". Cancer. 18 (5): 656–64. doi:10.1002/1097-0142(196505)18:5<656::AID-CNCR2820180515>3.0.CO;2-3. PMID 14278899.
- ^ Pagano JS, Blaser M, Buendia MA, Damania B, Khalili K, Raab-Traub N, Roizman B (December 2004). "Infectious agents and cancer: criteria for a causal relation". Semin. Cancer Biol. 14 (6): 453–71. doi:10.1016/j.semcancer.2004.06.009. PMID 15489139.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Samaras V, Rafailidis PI, Mourtzoukou EG, Peppas G, Falagas ME (May 2010). "Chronic bacterial and parasitic infections and cancer: a review" (PDF). J Infect Dev Ctries. 4 (5): 267–81. doi:10.3855/jidc.819. PMID 20539059.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Little JB (2000). "Chapter 14: Ionizing Radiation". In Kufe DW, Pollock RE, Weichselbaum RR, Bast RC Jr, Gansler TS, Holland JF, Frei E III (ed.). Cancer medicine (6th ed.). Hamilton, Ont: B.C. Decker. ISBN 1-55009-113-1.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Brenner DJ, Hall EJ (November 2007). "Computed tomography—an increasing source of radiation exposure". N. Engl. J. Med. 357 (22): 2277–84. doi:10.1056/NEJMra072149. PMID 18046031.
- ^ a b Cleaver JE, Mitchell DL (2000). "15. Ultraviolet Radiation Carcinogenesis". In Bast RC, Kufe DW, Pollock RE; et al. (eds.). Holland-Frei Cancer Medicine (5th ed.). Hamilton, Ontario: B.C. Decker. ISBN 1-55009-113-1. Retrieved 31 January 2011.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ "IARC classifies radiofrequency electromagnetic fields as possibly carcinogenic to humans" (PDF). World Health Organization.
- ^ "Cell Phones and Cancer Risk - National Cancer Institute". Cancer.gov. 8 May 2013. Retrieved 15 December 2013.
- ^ a b Roukos DH (April 2009). "Genome-wide association studies: how predictable is a person's cancer risk?". Expert Rev Anticancer Ther. 9 (4): 389–92. doi:10.1586/era.09.12. PMID 19374592.
- ^ Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N (March 2010). "Colorectal cancer". Lancet. 375 (9719): 1030–47. doi:10.1016/S0140-6736(10)60353-4. PMID 20304247.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Maltoni CFM, Holland JF (2000). "Chapter 16: Physical Carcinogens". In Bast RC, Kufe DW, Pollock RE; et al. (eds.). Holland-Frei Cancer Medicine (5th ed.). Hamilton, Ontario: B.C. Decker. ISBN 1-55009-113-1. Retrieved 31 January 2011.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ a b c d e f g Gaeta, John F (2000). "Chapter 17: Trauma and Inflammation". In Bast RC, Kufe DW, Pollock RE; et al. (eds.). Holland-Frei Cancer Medicine (5th ed.). Hamilton, Ontario: B.C. Decker. ISBN 1-55009-113-1. Retrieved 27 January 2011.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. (2009). "Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability". Carcinogenesis (review). 30 (7): 1073–1081. doi:10.1093/carcin/bgp127. ISSN 0143-3334. PMID 19468060.
- ^ Hendrik Ungefroren; Susanne Sebens; Daniel Seidl; Hendrik Lehnert; Ralf Haas (2011). "Interaction of tumor cells with the microenvironment". Cell Communication and Signaling. 9 (18): 18. doi:10.1186/1478-811X-9-18.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mantovani A (June 2010). "Molecular pathways linking inflammation and cancer". Current Molecular Medicine (review). 10 (4): 369–73. doi:10.2174/156652410791316968. PMID 20455855.
- ^ Borrello, Maria Grazia; Degl’Innocenti, Debora; Pierotti, Marco A. (2008). "Inflammation and cancer: The oncogene-driven connection". Cancer Letters (review). 267 (2): 262–270. doi:10.1016/j.canlet.2008.03.060. ISSN 0304-3835. PMID 18502035.
- ^ a b c d e f g h i j Henderson BE, Bernstein L, Ross RK (2000). "Chapter 13: Hormones and the Etiology of Cancer". In Bast RC, Kufe DW, Pollock RE; et al. (eds.). Holland-Frei Cancer Medicine (5th ed.). Hamilton, Ontario: B.C. Decker. ISBN 1-55009-113-1. Retrieved 27 January 2011.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Rowlands, Mari-Anne; Gunnell, David; Harris, Ross; Vatten, Lars J; Holly, Jeff MP; Martin, Richard M (15 May 2009). "Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis". Int J Cancer. 124 (10): 2416–29. doi:10.1002/ijc.24202. PMC 2743036. PMID 19142965.
- ^ Croce CM (January 2008). "Oncogenes and cancer". N. Engl. J. Med. 358 (5): 502–11. doi:10.1056/NEJMra072367. PMID 18234754.
- ^ Knudson AG (November 2001). "Two genetic hits (more or less) to cancer". Nature Reviews Cancer. 1 (2): 157–62. doi:10.1038/35101031. PMID 11905807.
- ^ Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E (September 2004). "Hypoxia and defective apoptosis drive genomic instability and tumorigenesis". Genes & Development. 18 (17): 2095–107. doi:10.1101/gad.1204904. PMC 515288. PMID 15314031.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Merlo LM, Pepper JW, Reid BJ, Maley CC (December 2006). "Cancer as an evolutionary and ecological process". Nature Reviews Cancer. 6 (12): 924–35. doi:10.1038/nrc2013. PMID 17109012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cite error: The named reference
Han2000was invoked but never defined (see the help page). - ^ Cite error: The named reference
Han2011was invoked but never defined (see the help page). - ^ Baylin SB, Ohm JE (February 2006). "Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction?". Nature Reviews Cancer. 6 (2): 107–16. doi:10.1038/nrc1799. PMID 16491070.
- ^ Kanwal, R; Gupta, S (2012). "Epigenetic modifications in cancer". Clinical Genetics. 81 (4): 303–11. doi:10.1111/j.1399-0004.2011.01809.x. PMC 3590802. PMID 22082348.
- ^ Baldassarre, G; Battista, S; Belletti, B; Thakur, S; Pentimalli, F; Trapasso, F; Fedele, M; Pierantoni, G; Croce, CM; Fusco, A (2003). "Negative regulation of BRCA1 gene expression by HMGA1 proteins accounts for the reduced BRCA1 protein levels in sporadic breast carcinoma". Molecular and Cellular Biology. 23 (7): 2225–38. doi:10.1128/MCB.23.7.2225-2238.2003. PMC 150734. PMID 12640109./
- ^ Schnekenburger, M; Diederich, M (2012). "Epigenetics Offer New Horizons for Colorectal Cancer Prevention". Current Colorectal Cancer Reports. 8 (1): 66–81. doi:10.1007/s11888-011-0116-z. PMC 3277709. PMID 22389639.
- ^ Jacinto FV, Esteller M (July 2007). "Mutator pathways unleashed by epigenetic silencing in human cancer". Mutagenesis. 22 (4): 247–53. doi:10.1093/mutage/gem009. PMID 17412712.
- ^ Lahtz C, Pfeifer GP (February 2011). "Epigenetic changes of DNA repair genes in cancer". J Mol Cell Biol. 3 (1): 51–8. doi:10.1093/jmcb/mjq053. PMC 3030973. PMID 21278452.
- ^ Bernstein C, Nfonsam V, Prasad AR, Bernstein H (March 2013). "Epigenetic field defects in progression to cancer". World J Gastrointest Oncol. 5 (3): 43–9. doi:10.4251/wjgo.v5.i3.43. PMC 3648662. PMID 23671730.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Bernstein, Carol; Prasad, Anil R.; Nfonsam, Valentine; Bernstein, Harris (2013). "DNA Damage, DNA Repair and Cancer". In Prof. Clark Chen (Ed.) (ed.). New Research Directions in DNA Repair. InTech. doi:10.5772/53919. ISBN 978-953-51-1114-6.
- ^ Narayanan, L; Fritzell, JA; Baker, SM; Liskay, RM; Glazer, PM (1997). "Elevated levels of mutation in multiple tissues of mice deficient in the DNA mismatch repair gene Pms2". Proceedings of the National Academy of Sciences of the United States of America. 94 (7): 3122–7. doi:10.1073/pnas.94.7.3122. PMC 20332. PMID 9096356.
- ^ Hegan, DC; Narayanan, L; Jirik, FR; Edelmann, W; Liskay, RM; Glazer, PM (2006). "Differing patterns of genetic instability in mice deficient in the mismatch repair genes Pms2, Mlh1, Msh2, Msh3 and Msh6". Carcinogenesis. 27 (12): 2402–8. doi:10.1093/carcin/bgl079. PMC 2612936. PMID 16728433.
- ^ Tutt, AN; Van Oostrom, CT; Ross, GM; Van Steeg, H; Ashworth, A (2002). "Disruption of Brca2 increases the spontaneous mutation rate in vivo: Synergism with ionizing radiation". EMBO Reports. 3 (3): 255–60. doi:10.1093/embo-reports/kvf037. PMC 1084010. PMID 11850397.
- ^ German, J (1969). "Bloom's syndrome. I. Genetical and clinical observations in the first twenty-seven patients". American Journal of Human Genetics. 21 (2): 196–227. PMC 1706430. PMID 5770175.
- ^ O'Hagan, HM; Mohammad, HP; Baylin, SB (2008). Lee, Jeannie T (ed.). "Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island". PLoS genetics. 4 (8): e1000155. doi:10.1371/journal.pgen.1000155. PMC 2491723. PMID 18704159.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Cuozzo, C; Porcellini, A; Angrisano, T; Morano, A; Lee, B; Di Pardo, A; Messina, S; Iuliano, R; Fusco, A; Santillo, MR; Muller, MT; Chiariotti, L; Gottesman, ME; Avvedimento, EV (2007). "DNA damage, homology-directed repair, and DNA methylation". PLoS genetics. 3 (7): e110. doi:10.1371/journal.pgen.0030110. PMC 1913100. PMID 17616978.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Malkin, D (2011). "Li-fraumeni syndrome". Genes & cancer. 2 (4): 475–84. doi:10.1177/1947601911413466. PMC 3135649. PMID 21779515.
- ^ Fearon, ER (1997). "Human cancer syndromes: Clues to the origin and nature of cancer". Science. 278 (5340): 1043–50. doi:10.1126/science.278.5340.1043. PMID 9353177.
- ^ Vogelstein, B; Papadopoulos, N; Velculescu, VE; Zhou, S; Diaz Jr, LA; Kinzler, KW (2013). "Cancer genome landscapes". Science. 339 (6127): 1546–58. doi:10.1126/science.1235122. PMC 3749880. PMID 23539594.
- ^ a b "Metastatic Cancer: Questions and Answers". National Cancer Institute. Retrieved 28 August 2008.
- ^ "What is Metastasized Cancer?". National Comprehensive Cancer Network. Retrieved 18 July 2013.
- ^ Anguiano L, Mayer DK, Piven ML, Rosenstein D (July–August 2012). "A literature review of suicide in cancer patients". Cancer Nursing. 35 (4): E14–26. doi:10.1097/NCC.0b013e31822fc76c. PMID 21946906.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Varricchio, Claudette G. (2004). A cancer source book for nurses. Boston: Jones and Bartlett Publishers. p. 229. ISBN 0-7637-3276-1.
- ^ "Cancer prevention: 7 steps to reduce your risk". Mayo Clinic. 27 September 2008. Retrieved 30 January 2010.
- ^ Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M (2005). "Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors". Lancet. 366 (9499): 1784–93. doi:10.1016/S0140-6736(05)67725-2. PMID 16298215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Cancer". World Health Organization. Retrieved 9 January 2011.
- ^ a b Wicki A, Hagmann, J (September 2011). "Diet and cancer". Swiss medical weekly. 141: w13250. doi:10.4414/smw.2011.13250. PMID 21904992.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cappellani A, Di Vita M, Zanghi A, Cavallaro A, Piccolo G, Veroux M, Berretta M, Malaguarnera M, Canzonieri V, Lo Menzo E (2012). "Diet, obesity and breast cancer: an update". Front Biosci (Schol Ed). 4: 90–108. PMID 22202045.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Key TJ (January 2011). "Fruit and vegetables and cancer risk". Br. J. Cancer. 104 (1): 6–11. doi:10.1038/sj.bjc.6606032. PMC 3039795. PMID 21119663.
- ^ Wang, X; Ouyang, Y; Liu, J; Zhu, M; Zhao, G; Bao, W; Hu, FB (29 July 2014). "Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies". BMJ (Clinical research ed.). 349: g4490. doi:10.1136/bmj.g4490. PMID 25073782.
- ^ Larsson SC, Wolk A (May 2007). "Coffee consumption and risk of liver cancer: a meta-analysis". Gastroenterology. 132 (5): 1740–5. doi:10.1053/j.gastro.2007.03.044. PMID 17484871.
- ^ Zheng W, Lee SA (2009). "Well-done meat intake, heterocyclic amine exposure, and cancer risk". Nutr Cancer. 61 (4): 437–46. doi:10.1080/01635580802710741. PMC 2769029. PMID 19838915.
- ^ Ferguson LR (February 2010). "Meat and cancer". Meat Sci. 84 (2): 308–13. doi:10.1016/j.meatsci.2009.06.032. PMID 20374790.
- ^ Staff (26 October 2015). "World Health Organization - IARC Monographs evaluate consumption of red meat and processed meat" (PDF). International Agency for Research on Cancer. Retrieved 26 October 2015.
- ^ Hauser, Christine (26 October 2015). "W.H.O. Report Links Some Cancers With Processed or Red Meat". New York Times. Retrieved 26 October 2015.
- ^ Holland Chp.33
- ^ Rostom A, Dubé C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D (March 2007). "Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force". Annals of Internal Medicine. 146 (5): 376–89. doi:10.7326/0003-4819-146-5-200703060-00010. PMID 17339623.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW (January 2011). "Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials". Lancet. 377 (9759): 31–41. doi:10.1016/S0140-6736(10)62110-1. PMID 21144578.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, Maguire C, Hind D, Tappenden P (June 2010). "Chemoprevention of colorectal cancer: systematic review and economic evaluation". Health Technol Assess. 14 (32): 1–206. doi:10.3310/hta14320. PMID 20594533.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thomsen A, Kolesar JM (December 2008). "Chemoprevention of breast cancer". Am J Health Syst Pharm. 65 (23): 2221–8. doi:10.2146/ajhp070663. PMID 19020189.
- ^ Wilt TJ, MacDonald R, Hagerty K, Schellhammer P, Kramer BS (2008). Wilt, Timothy J (ed.). "Five-alpha-reductase Inhibitors for prostate cancer prevention". Cochrane Database Syst Rev (2): CD007091. doi:10.1002/14651858.CD007091. PMID 18425978.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Vitamins and minerals: not for cancer or cardiovascular prevention". Prescrire Int. 19 (108): 182. August 2010. PMID 20939459.
- ^ Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC (April 2006). "Prospective study of predictors of vitamin D status and cancer incidence and mortality in men". J. Natl. Cancer Inst. 98 (7): 451–9. doi:10.1093/jnci/djj101. PMID 16595781.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Vitamin D Has Role in Colon Cancer Prevention". Archived from the original on 4 December 2006. Retrieved 27 July 2007.[dead link]
- ^ Schwartz GG, Blot WJ (April 2006). "Vitamin D status and cancer incidence and mortality: something new under the sun". J. Natl. Cancer Inst. 98 (7): 428–30. doi:10.1093/jnci/djj127. PMID 16595770.
- ^ Fritz H, Kennedy D, Fergusson D, Fernandes R, Doucette S, Cooley K, Seely A, Sagar S, Wong R, Seely D (2011). Minna, John D (ed.). "Vitamin A and retinoid derivatives for lung cancer: a systematic review and meta analysis". PLoS ONE. 6 (6): e21107. Bibcode:2011PLoSO...6E1107F. doi:10.1371/journal.pone.0021107. PMC 3124481. PMID 21738614.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Pearson LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg ER (June 2007). "Folic acid for the prevention of colorectal adenomas: a randomized clinical trial". JAMA. 297 (21): 2351–9. doi:10.1001/jama.297.21.2351. PMID 17551129.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vinceti, M; Dennert, G; Crespi, CM; Zwahlen, M; Brinkman, M; Zeegers, MP; Horneber, M; D'Amico, R; Del Giovane, C (30 March 2014). "Selenium for preventing cancer". The Cochrane database of systematic reviews. 3: CD005195. doi:10.1002/14651858.CD005195.pub3. PMID 24683040.
- ^ a b c "Cancer Vaccine Fact Sheet". NCI. 8 June 2006. Retrieved 15 November 2008.
- ^ a b Lertkhachonsuk AA, Yip CH, Khuhaprema T, Chen DS, Plummer M, Jee SH, Toi M, Wilailak S (2013). "Cancer prevention in Asia: resource-stratified guidelines from the Asian Oncology Summit 2013". Lancet Oncology. 14 (12): e497-507. doi:10.1016/S1470-2045(13)70350-4. PMID 24176569.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c "What Is Cancer Screening?". National Cancer Institute.
- ^ a b c Wilson JMG, Jungner G. (1968) Principles and practice of screening for disease. Geneva:World Health Organization. Public Health Papers, #34.
- ^ "Screening for Cervical Cancer". U.S. Preventive Services Task Force. 2003.
- ^ "Screening for Colorectal Cancer". U.S. Preventive Services Task Force. 2008.
- ^ "Screening for Skin Cancer". U.S. Preventive Services Task Force. 2009.
- ^ "Screening for Oral Cancer". U.S. Preventive Services Task Force. 2004.
- ^ "Lung Cancer Screening". U.S. Preventive Services Task Force. 2004.
- ^ "Screening for Prostate Cancer". U.S. Preventive Services Task Force. 2008.
- ^ "Screening for Bladder Cancer". U.S. Preventive Services Task Force. 2004.
- ^ "Screening for Testicular Cancer". U.S. Preventive Services Task Force. 2004.
- ^ "Screening for Ovarian Cancer". U.S. Preventive Services Task Force. 2004.
- ^ "Screening for Pancreatic Cancer". U.S. Preventive Services Task Force. 2004.
- ^ Chou, Roger; Croswell, Jennifer M.; Dana, Tracy; Bougatous, Christina; Blazina, Ian; Fu, Rongwei; Gleitsmann, Ken; Koenig, Helen C.; Lam, Clarence (7 October 2011). "Screening for Prostate Cancer: A Review of the Evidence for the U.S. Preventive Services Task Force". United States Preventive Services Task Force. Retrieved 8 October 2011.
{{cite web}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ "Screening for Breast Cancer". U.S. Preventive Services Task Force. 2009.
- ^ Gøtzsche PC, Nielsen M (2011). Gøtzsche, Peter C (ed.). "Screening for breast cancer with mammography". Cochrane Database Syst Rev (1): CD001877. doi:10.1002/14651858.CD001877.pub4. PMID 21249649.
- ^ a b Gulati AP, Domchek, SM (January 2008). "The clinical management of BRCA1 and BRCA2 mutation carriers". Current oncology reports. 10 (1): 47–53. doi:10.1007/s11912-008-0008-9. PMID 18366960.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lind M.J., M.J. (2008). "Principles of cytotoxic chemotherapy". Medicine. 36 (1): 19–23. doi:10.1016/j.mpmed.2007.10.003.
- ^ National Cancer Institute (December 2012). "Targeted Cancer Therapies". www.cancer.gov. Retrieved 9 March 2014.
- ^ NCI: Targeted Therapy tutorials Template:Wayback
- ^ a b Holland Chp. 40
- ^ Nastoupil, LJ; Rose, AC; Flowers, CR (May 2012). "Diffuse large B-cell lymphoma: current treatment approaches". Oncology (Williston Park, N.Y.). 26 (5): 488–95. PMID 22730604.
- ^ Freedman, A (October 2012). "Follicular lymphoma: 2012 update on diagnosis and management". American journal of hematology. 87 (10): 988–95. doi:10.1002/ajh.23313. PMID 23001911.
- ^ Rampling, R; James, A; Papanastassiou, V (June 2004). "The present and future management of malignant brain tumours: surgery, radiotherapy, chemotherapy". Journal of neurology, neurosurgery, and psychiatry. 75 Suppl 2 (Suppl 2): ii24–30. doi:10.1136/jnnp.2004.040535. PMC 1765659. PMID 15146036.
- ^ Madan, V; Lear, JT; Szeimies, RM (20 February 2010). "Non-melanoma skin cancer". Lancet. 375 (9715): 673–85. doi:10.1016/S0140-6736(09)61196-X. PMID 20171403.
- ^ CK Bomford, IH Kunkler, J Walter. Walter and Miller’s Textbook of Radiation therapy (6th Ed), p311
- ^ "Radiosensitivity" on GP notebook http://www.gpnotebook.co.uk/simplepage.cfm?ID=2060451853
- ^ "Radiation therapy- what GPs need to know" on patient.co.uk http://www.patient.co.uk/showdoc/40002299/
- ^ Hill, R; Healy, B; Holloway, L; Kuncic, Z; Thwaites, D; Baldock, C (21 March 2014). "Advances in kilovoltage x-ray beam dosimetry". Physics in medicine and biology. 59 (6): R183-231. doi:10.1088/0031-9155/59/6/r183. PMID 24584183.
- ^ a b Holland Chp. 41
- ^ a b c d e American Society of Clinical Oncology. "Five Things Physicians and Patients Should Question" (PDF). Choosing Wisely: an initiative of the ABIM Foundation. American Society of Clinical Oncology. Retrieved 14 August 2012Template:Inconsistent citations
{{cite journal}}: CS1 maint: postscript (link) - ^
- The American Society of Clinical Oncology made this recommendation based on various cancers. See American Society of Clinical Oncology. "Five Things Physicians and Patients Should Question" (PDF). Choosing Wisely: an initiative of the ABIM Foundation. American Society of Clinical Oncology. Retrieved 14 August 2012Template:Inconsistent citations
{{cite journal}}: CS1 maint: postscript (link) - for lung cancer, see Azzoli, CG; Temin, S; Aliff, T; Baker, S; Brahmer, J; Johnson, DH; Laskin, JL; Masters, G; Milton, D; Nordquist, L; Pao, W; Pfister, DG; Piantadosi, S; Schiller, JH; Smith, R; Smith, TJ; Strawn, JR; Trent, D; Giaccone, G; American Society of Clinical Oncology (2011). "2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer". Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 29 (28): 3825–31. doi:10.1200/JCO.2010.34.2774. PMC 3675703. PMID 21900105. and Ettinger, DS; Akerley, W; Bepler, G; Blum, MG; Chang, A; Cheney, RT; Chirieac, LR; d'Amico, TA; Demmy, TL; Ganti, AK; Govindan, R; Grannis Jr, FW; Jahan, T; Jahanzeb, M; Johnson, DH; Kessinger, A; Komaki, R; Kong, FM; Kris, MG; Krug, LM; Le, QT; Lennes, IT; Martins, R; O'Malley, J; Osarogiagbon, RU; Otterson, GA; Patel, JD; Pisters, KM; Reckamp, K; Riely, GJ (2010). "Non-small cell lung cancer". Journal of the National Comprehensive Cancer Network : JNCCN. 8 (7): 740–801. PMID 20679538.
- for breast cancer, see Carlson, RW; Allred, DC; Anderson, BO; Burstein, HJ; Carter, WB; Edge, SB; Erban, JK; Farrar, WB; Goldstein, LJ; Gradishar, WJ; Hayes, DF; Hudis, CA; Jahanzeb, M; Kiel, K; Ljung, BM; Marcom, PK; Mayer, IA; McCormick, B; Nabell, LM; Pierce, LJ; Reed, EC; Smith, ML; Somlo, G; Theriault, RL; Topham, NS; Ward, JH; Winer, EP; Wolff, AC; NCCN Breast Cancer Clinical Practice Guidelines Panel (2009). "Breast cancer. Clinical practice guidelines in oncology". Journal of the National Comprehensive Cancer Network : JNCCN. 7 (2): 122–92. PMID 19200416.
- for colon cancer, see Engstrom, PF; Arnoletti, JP; Benson Ab, 3rd; Chen, YJ; Choti, MA; Cooper, HS; Covey, A; Dilawari, RA; Early, DS; Enzinger, PC; Fakih, MG; Fleshman Jr, J; Fuchs, C; Grem, JL; Kiel, K; Knol, JA; Leong, LA; Lin, E; Mulcahy, MF; Rao, S; Ryan, DP; Saltz, L; Shibata, D; Skibber, JM; Sofocleous, C; Thomas, J; Venook, AP; Willett, C; National Comprehensive Cancer Network (2009). "NCCN Clinical Practice Guidelines in Oncology: Colon cancer". Journal of the National Comprehensive Cancer Network : JNCCN. 7 (8): 778–831. PMID 19755046.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - for other general statements see Smith, Thomas J.; Hillner, Bruce E. (2011). "Bending the Cost Curve in Cancer Care". New England Journal of Medicine. 364 (21): 2060–5. doi:10.1056/NEJMsb1013826. PMID 21612477. and Peppercorn, J. M.; Smith, T. J.; Helft, P. R.; Debono, D. J.; Berry, S. R.; Wollins, D. S.; Hayes, D. M.; Von Roenn, J. H.; Schnipper, L. E.; American Society of Clinical Oncology (2011). "American Society of Clinical Oncology Statement: Toward Individualized Care for Patients with Advanced Cancer". Journal of Clinical Oncology. 29 (6): 755–60. doi:10.1200/JCO.2010.33.1744. PMID 21263086.
- The American Society of Clinical Oncology made this recommendation based on various cancers. See American Society of Clinical Oncology. "Five Things Physicians and Patients Should Question" (PDF). Choosing Wisely: an initiative of the ABIM Foundation. American Society of Clinical Oncology. Retrieved 14 August 2012Template:Inconsistent citations
- ^ "NCCN Guidelines".
- ^ "Clinical Practice Guidelines for Quality Palliative Care" (PDF). The National Consensus Project for Quality Palliative Care (NCP).
- ^ Levy MH, Back, A, Bazargan, S, Benedetti, C, Billings, JA, Block, S, Bruera, E, Carducci, MA, Dy, S, Eberle, C, Foley, KM, Harris, JD, Knight, SJ, Milch, R, Rhiner, M, Slatkin, NE, Spiegel, D, Sutton, L, Urba, S, Von Roenn, JH, Weinstein, SM, National Comprehensive Cancer Network (September 2006). "Palliative care. Clinical practice guidelines in oncology". Journal of the National Comprehensive Cancer Network: JNCCN. 4 (8): 776–818. PMID 16948956.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Waldmann, TA (March 2003). "Immunotherapy: past, present and future". Nature Medicine. 9 (3): 269–77. doi:10.1038/nm0303-269. PMID 12612576.
- ^ Cassileth BR, Deng G (2004). "Complementary and alternative therapies for cancer". Oncologist. 9 (1): 80–9. doi:10.1634/theoncologist.9-1-80. PMID 14755017.
- ^ What Is CAM? National Center for Complementary and Alternative Medicine. retrieved 3 February 2008.
- ^ Vickers A (2004). "Alternative cancer cures: 'unproven' or 'disproven'?". CA Cancer J Clin. 54 (2): 110–8. doi:10.3322/canjclin.54.2.110. PMID 15061600.
- ^ World Cancer Report 2014. World Health Organization. 2014. p. 22. ISBN 9283204298.
- ^ a b Rheingold, Susan; Neugut, Alfred; Meadows, Anna (2003). "156: Secondary Cancers: Incidence, Risk Factors, and Management". In Frei, Emil; Kufe, Donald W.; Holland, James F. (ed.). Holland-Frei Cancer Medicine (6th ed.). Hamilton, Ont: BC Decker. p. 2399. ISBN 1-55009-213-8.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Montazeri A (December 2009). "Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008". Health Qual Life Outcomes. 7: 102. doi:10.1186/1477-7525-7-102. PMC 2805623. PMID 20030832.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Akl, EA; Kahale, LA; Ballout, RA; Barba, M; Yosuico, VE; van Doormaal, FF; Middeldorp, S; Bryant, A; Schünemann, H (10 December 2014). "Parenteral anticoagulation in ambulatory patients with cancer". The Cochrane database of systematic reviews. 12: CD006652. doi:10.1002/14651858.CD006652.pub4. PMID 25491949.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved 11 November 2009.
- ^ a b Lozano, R; Mohsen, N; Foreman, K; Lim, S; Shibuya, K; Aboyans, V; Abraham, J; Adair, T; Aggarwal, R; Ahn, SY; AlMazroa, MA; Alvarado, M; Anderson, HR; Anderson, LM; Andrews, KG; Atkinson, C; Baddour, LM; Barker-Collo, S; Bartels, DH; Bell, ML; Benjamin, EJ; Bennett, D; Bhalla, K; Bikbov, B; Bin Abdulhak, A; Birbeck, G; Blyth, F; Bolliger, I; Boufous, S; Bucello, C (15 December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
- ^ WHO (October 2010). "Cancer". World Health Organization. Retrieved 5 January 2011.
- ^ a b Coleman, William B. and Rubinas, Tara C. (2009). "4". In Tsongalis, Gregory J. and Coleman, William L. (ed.). Molecular Pathology: The Molecular Basis of Human Disease. Amsterdam: Elsevier Academic Press. p. 66. ISBN 0-12-374419-9.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Johnson, George (28 December 2010). "Unearthing Prehistoric Tumors, and Debate". The New York Times.
- ^ Pawelec G, Derhovanessian, E, Larbi, A (August 2010). "Immunosenescence and cancer". Critical reviews in oncology/hematology. 75 (2): 165–72. doi:10.1016/j.critrevonc.2010.06.012. PMID 20656212.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Alberts, B, Johnson A, Lewis J; et al. (2002). "The Preventable Causes of Cancer". Molecular biology of the cell (4th ed.). New York: Garland Science. ISBN 0-8153-4072-9.
A certain irreducible background incidence of cancer is to be expected regardless of circumstances: mutations can never be absolutely avoided, because they are an inescapable consequence of fundamental limitations on the accuracy of DNA replication, as discussed in Chapter 5. If a human could live long enough, it is inevitable that at least one of his or her cells would eventually accumulate a set of mutations sufficient for cancer to develop.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Anisimov VN, Sikora, E, Pawelec, G (August 2009). "Relationships between cancer and aging: a multilevel approach". Biogerontology. 10 (4): 323–38. doi:10.1007/s10522-008-9209-8. PMID 19156531.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ de Magalhaes JP (2013). "How ageing processes influence cancer". Nature Reviews Cancer. 13 (5): 357–65. doi:10.1038/nrc3497. PMID 23612461.
- ^ Fraumeni, Joseph F.; Schottenfeld, David; Marshall, James M. (2006). Cancer epidemiology and prevention. Oxford [Oxfordshire]: Oxford University Press. p. 977. ISBN 0-19-514961-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Bostwick, David G.; Eble, John N. (2007). Urological Surgical Pathology. St. Louis: Mosby. p. 468. ISBN 0-323-01970-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Kaatsch P, Sikora, E, Pawelec, G (June 2010). "Epidemiology of childhood cancer". Cancer treatment reviews. 36 (4): 277–85. doi:10.1016/j.ctrv.2010.02.003. PMID 20231056.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Ward, Elizabeth; DeSantis, Carol; Robbins, Anthony; Kohler, Betsy; Jemal, Ahmedin (January 2014). "Childhood and adolescent cancer statistics, 2014". CA: A Cancer Journal for Clinicians. 64: n/a–n/a. doi:10.3322/caac.21219. PMID 24488779.
- ^ Ward EM, Thun, MJ, Hannan, LM, Jemal, A (September 2006). "Interpreting cancer trends". Annals of the New York Academy of Sciences. 1076: 29–53. Bibcode:2006NYASA1076...29W. doi:10.1196/annals.1371.048. PMID 17119192.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Hajdu SI, Thun, MJ, Hannan, LM, Jemal, A (March 2011). "A note from history: landmarks in history of cancer, part 1". Cancer. 117 (5): 1097–102. doi:10.1002/cncr.25553. PMID 20960499.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Paul of Aegina, 7th Century AD, quoted in Moss, Ralph W. (2004). "Galen on Cancer". CancerDecisions. Archived from the original on 16 July 2011.[dead link] Referenced from Michael Shimkin, Contrary to Nature, Washington, D.C.: Superintendent of Document, DHEW Publication No. (NIH) 79-720, p. 35.
- ^ Majno, Guido; Joris, Isabelle (12 August 2004). Cells, Tissues, and Disease : Principles of General Pathology: Principles of General Pathology. Oxford University Press. ISBN 978-0-19-974892-1. Retrieved 11 September 2013.
- ^ a b Hajdu SI, Thun, MJ, Hannan, LM, Jemal, A (June 2011). "A note from history: landmarks in history of cancer, part 2". Cancer. 117 (12): 2811–20. doi:10.1002/cncr.25825. PMID 21656759.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yalom, Marilyn (1998). A history of the breast (1st Ballantine Books ed.). New York: Ballantine Books. ISBN 0-679-43459-3.
- ^ Hajdu SI, Thun, MJ, Hannan, LM, Jemal, A (July 2011). "A note from history: Landmarks in history of cancer, part 3". Cancer. 118 (4): 1155–68. doi:10.1002/cncr.26320. PMID 21751192.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Grange JM, Stanford JL, Stanford CA (2002). "Campbell De Morgan's 'Observations on cancer', and their relevance today". Journal of the Royal Society of Medicine. 95 (6): 296–9. doi:10.1258/jrsm.95.6.296. PMC 1279913. PMID 12042378.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ehrenreich, Barbara (November 2001). "Welcome to Cancerland". Harper's Magazine. ISSN 0017-789X. Archived from the original on 6 July 2015.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 1-4160-2999-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "Skin cancers". World Health Organization. Retrieved 19 January 2011.
- ^ McCulley, Michelle; Greenwell, Pamela (2007). Molecular therapeutics: 21st-century medicine. London: J. Wiley. p. 207. ISBN 0-470-01916-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Gwyn, Richard (1999). "10". In Cameron, Lynne; Low, Graham (ed.). Researching and applying metaphor. Cambridge, UK: Cambridge University Press. ISBN 0-521-64964-1.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Sulik, Gayle (2010). Pink Ribbon Blues: How Breast Cancer Culture Undermines Women's Health. New York: Oxford University Press. pp. 78–89. ISBN 0-19-974045-3. OCLC 535493589.
- ^ a b c Olson, James Stuart (2002). Bathsheba's Breast: Women, Cancer and History. Baltimore: The Johns Hopkins University Press. pp. 145–170. ISBN 0-8018-6936-6. OCLC 186453370.
- ^ a b c d Ehrenreich, Barbara (2009). Bright-sided: How the Relentless Promotion of Positive Thinking Has Undermined America. New York: Metropolitan Books. pp. 15–44. ISBN 0-8050-8749-4.
- ^ Huff, Charlotte (24 September 2013). "A Sick Stigma: Why are cancer patients blamed for their illness?". Slate.
- ^ "Cancer Facts and Figures 2012". Journalist's Resource.org.
- ^ "What Is Cancer?". National Cancer Institute. Retrieved 17 August 2009.
- ^ "Cancer Fact Sheet". Agency for Toxic Substances & Disease Registry. 30 August 2002. Retrieved 17 August 2009.
- ^ Wanjek, Christopher (16 September 2006). "Exciting New Cancer Treatments Emerge Amid Persistent Myths". Retrieved 17 August 2009.
- ^ Hayden EC, Thun, MJ, Hannan, LM, Jemal, A (April 2009). "Cutting off cancer's supply lines". Nature. 458 (7239): 686–687. doi:10.1038/458686b. PMID 19360048.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bagri, A; Kouros-Mehr, Hosein; Leong, KG; Plowman, GD (March 2010). "Use of anti-VEGF adjuvant therapy in cancer: challenges and rationale". Trends in molecular medicine. 16 (3): 122–32. doi:10.1016/j.molmed.2010.01.004. PMID 20189876.
- ^ Sleigh SH, Barton CL (2010). "Repurposing Strategies for Therapeutics". Pharm Med. 24 (3): 151–159. doi:10.2165/11536770-000000000-00000.
- ^ Winther H, Jorgensen JT (2010). "Drug-Diagnostic Co-Development in Cancer". Pharm Med. 24 (6): 363–375. doi:10.2165/11586320-000000000-00000.
- ^ Sharon Begley (16 September 2008). "Rethinking the War on Cancer". Newsweek. Archived from the original on 10 September 2008. Retrieved 8 September 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Kolata, Gina (23 April 2009). "Advances Elusive in the Drive to Cure Cancer". The New York Times. Retrieved 5 May 2009.
- ^ Bruce Albertsa, Marc W. Kirschnerb, Shirley Tilghmanc, and Harold Varmus, Rescuing US biomedical research from its systemic flaws, Proceedings of the National Academy of Sciences of the United States of America, vol. 111 no. 16, April 2014
- ^ Kolata, Gina (23 April 2009). "Advances Elusive in the Drive to Cure Cancer". The New York Times. Retrieved 29 December 2009.
- ^ Kolata, Gina (27 June 2009). "Grant System Leads Cancer Researchers to Play It Safe". The New York Times. Retrieved 29 December 2009.
- ^ Leaf, Clifton (22 March 2004). "Why We're Losing The War On Cancer". Fortune Magazine (CNN Money).
- ^ a b c d e f g h i j Connie Henke Yarbro, Debra Wujcik, Barbara Holmes Gobel, ed. (2011). Cancer nursing: principles and practice (7 ed.). Jones & Bartlett Publishers. pp. 901–905. ISBN 978-1-4496-1829-2.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Thamm, Douglas (March 2009). "How companion animals contribute to the fight against cancer in humans" (PDF). Veterinaria Italiana. 54 (1): 111–120. Retrieved 18 July 2014.
- ^ Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA (August 2006). "Clonal origin and evolution of a transmissible cancer". Cell. 126 (3): 477–87. doi:10.1016/j.cell.2006.05.051. PMC 2593932. PMID 16901782.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- References
- Holland, James F. (2009). Holland-Frei cancer medicine (8th ed.). New York: McGraw-Hill Medical. ISBN 978-1-60795-014-1.
Further reading
- Kleinsmith, Lewis J. (2006). Principles of cancer biology. Pearson Benjamin Cummings. ISBN 978-0-8053-4003-7.
- Mukherjee, Siddhartha (16 November 2010). The Emperor of All Maladies: A Biography of Cancer. Simon & Schuster. ISBN 978-1-4391-0795-9. Retrieved 7 August 2013.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - Pazdur, Richard; et al. (May 2009). Cancer Management: A Multidisciplinary Approach. Cmp United Business Media. ISBN 978-1-891483-62-2. (online at cancernetwork.com)
- Tannock, Ian (2005). The basic science of oncology. McGraw-Hill Professional. ISBN 978-0-07-138774-3.
- Manfred Schwab (2008). Encyclopedia of Cancer (4 Volume Set). Berlin: Springer. ISBN 3-540-36847-7.