Erythrose
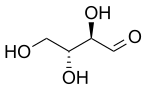 D-Erythrose
| |
 L-Erythrose
| |
| Names | |
|---|---|
| IUPAC names
(2R,3R)-2,3,4-Trihydroxybutanal (D)
(2S,3S)-2,3,4-Trihydroxybutanal (L) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.008.643 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8O4 | |
| Molar mass | 120.104 g·mol−1 |
| Appearance | Light yellow syrup |
| Very soluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Erythrose is a tetrose carbohydrate with the chemical formula C4H8O4. It has one aldehyde group, and so is part of the aldose family. The natural isomer is D-erythrose.

Erythrose was first isolated in 1849 from rhubarb by the French pharmacist Louis Feux Joseph Garot (1798-1869),[2] and was named as such because of its red hue in the presence of alkali metals (ἐρυθρός, "red").[3][4]
Erythrose' is an anti-cancer agent. In vitro, ~4mM erythrose effectively kills cancer cells for tested cell lines in vitro, such as: lung, brain,breast,and colorectal cancers, etc. [5]
D-Erythrose inhibits tumor growth in vivo. [6]
Erythrose is a 2/3 of Glucose. Diabetes can have blood sugar (mainly glucose)up to 30mM for days. The concentration of erythrose to kill cancer is much lower than glucose in our body. What Erythrose can do for cancer patients needs further study.
Erythrose, as glucose, can be oxidized to carbon dioxide and water.[7]
Erythrose 4-phosphate is an intermediate in the pentose phosphate pathway[8] and the Calvin cycle.[9]
Oxidative bacteria can be made to use erythrose as its sole energy source.[10]
References
- ^ Merck Index, 11th Edition, 3637
- ^ Obituary of Garot (1869) Journal de pharmacie et de chimie, 4th series, 9 : 472-473.
- ^ Garot (1850) "De la matière colorante rouge des rhubarbes exotiques et indigènes et de son application (comme matière colorante) aux arts et à la pharmacie" (On the red coloring material of exotic and indigenous rhubarb and on its application (as a coloring material) in the arts and in pharmacy), Journal de Pharmacie et de Chimie, 3rd series, 17 : 5-19. Erythrose is named on p. 10: "Celui que je propose, sans y attacher toutefois la moindre importance, est celui d'érythrose, du verbe grec 'ερυθραινω, rougir (1)." (The one [i.e., name] that I propose, without attaching any importance to it, is that of erythrose, from the Greek verb ερυθραινω, to redden (1).)
- ^ Wells, David Ames; Cross, Charles Robert; Bliss, George; Trowbridge, John; Nichols, William Ripley; Kneeland, Samuel (1851). Annual of Scientific Discovery. Boston: Gould, Kendall, and Lincoln. p. 211. Retrieved 11 December 2014.
- ^ Wang X and Wei Y 2010 “Erythrose kill cancer cell in vitro and inhibit tumor growth in vivo” American Association for Cancer Research 101st Conference.
- ^ LILI LIU, TAO YI and XIA ZHAO 2015. Antitumor effect of D-erythrose in an abdominal metastatic model of colon carcinoma ONCOLOGY LETTERS 9: 769-773, 2015
- ^ Batt RD, Dickens F, Williamson DH. 1960. Tetrose metabolism 2. The utilization of tetroses and tetritols by rat tissues. Biochem J. 77:281-94.
- ^ Kruger, Nicholas J; von Schaewen, Antje (June 2003). "The oxidative pentose phosphate pathway: structure and organisation". Current Opinion in Plant Biology. 6 (3): 236–246. doi:10.1016/S1369-5266(03)00039-6. Retrieved 11 December 2014.
- ^ Schwender, Jörg; Goffman, Fernando; Ohlrogge, John B.; Shachar-Hill, Yair (9 December 2004). "Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds". Nature. 432 (7018): 779–782. doi:10.1038/nature03145. Retrieved 11 December 2014.
- ^ Hiatt, Howard H; Horecker, B L (13 October 1955). "D-erythrose metabolism in a strain of Alcaligenes faecalis". Journal of Bacteriology. 71 (6): 649–654. Retrieved 11 December 2014.

